Rotavirus A during the COVID-19 Pandemic in Brazil, 2020–2022: Emergence of G6P[8] Genotype
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Stool Collection
2.2. Ethics Statements
2.3. Nucleic Acid Extraction
2.4. RVA Detection, Genotyping, and Sequencing
2.5. Phylogenetic Analysis
2.6. Statistical Analysis
3. Results
3.1. Rotavirus A Epidemiology
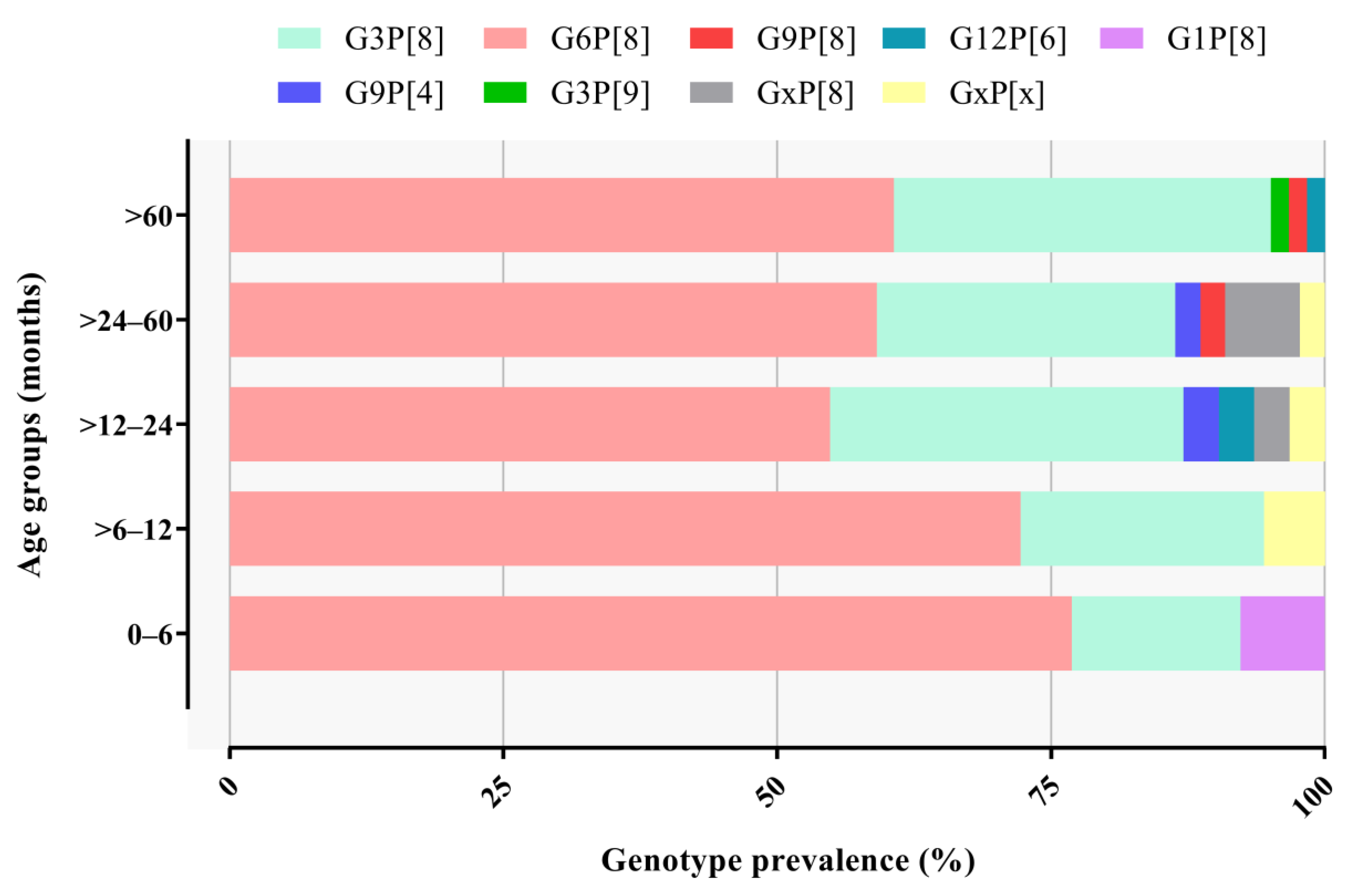
3.2. RVA Genotyping
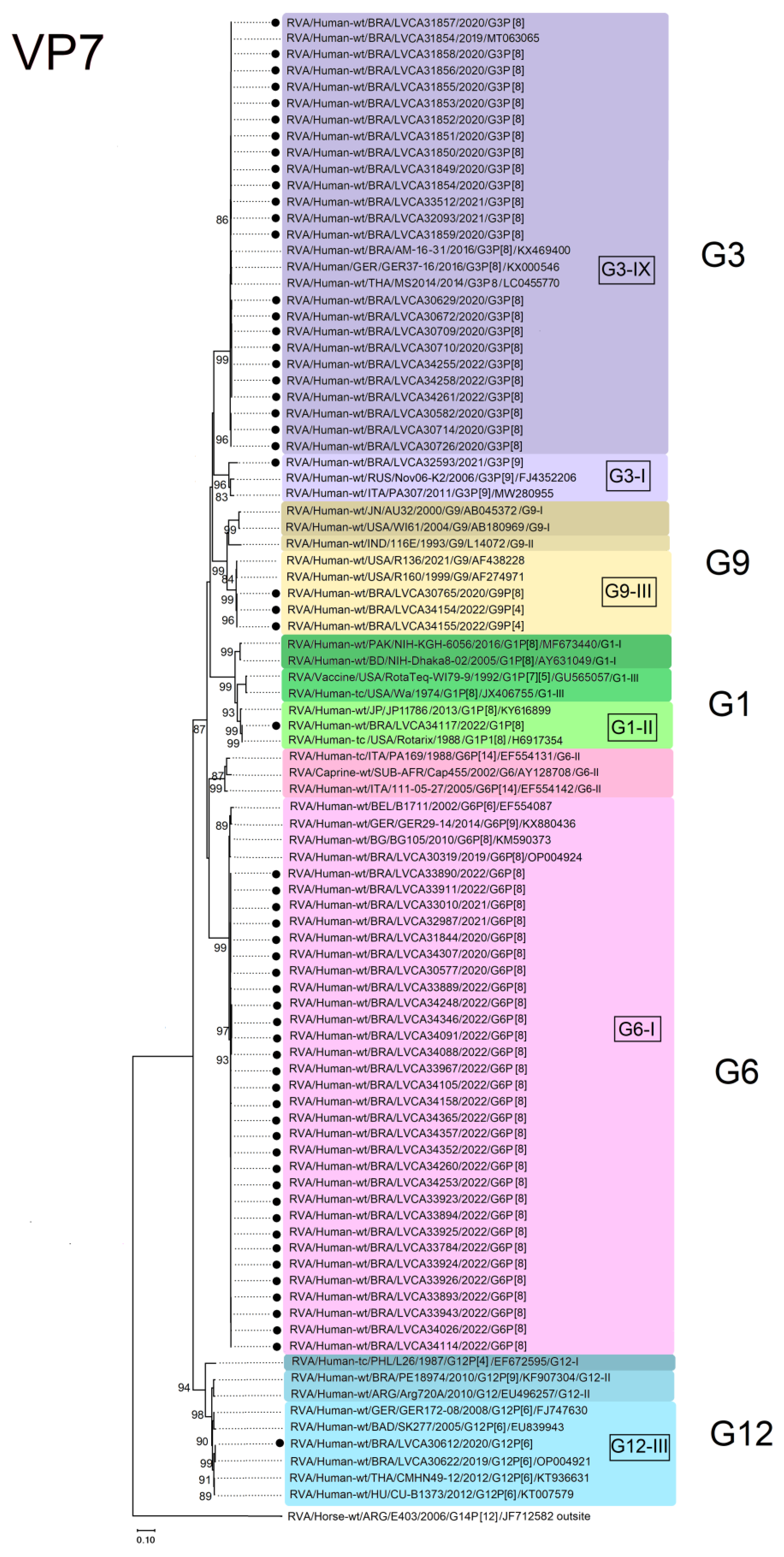
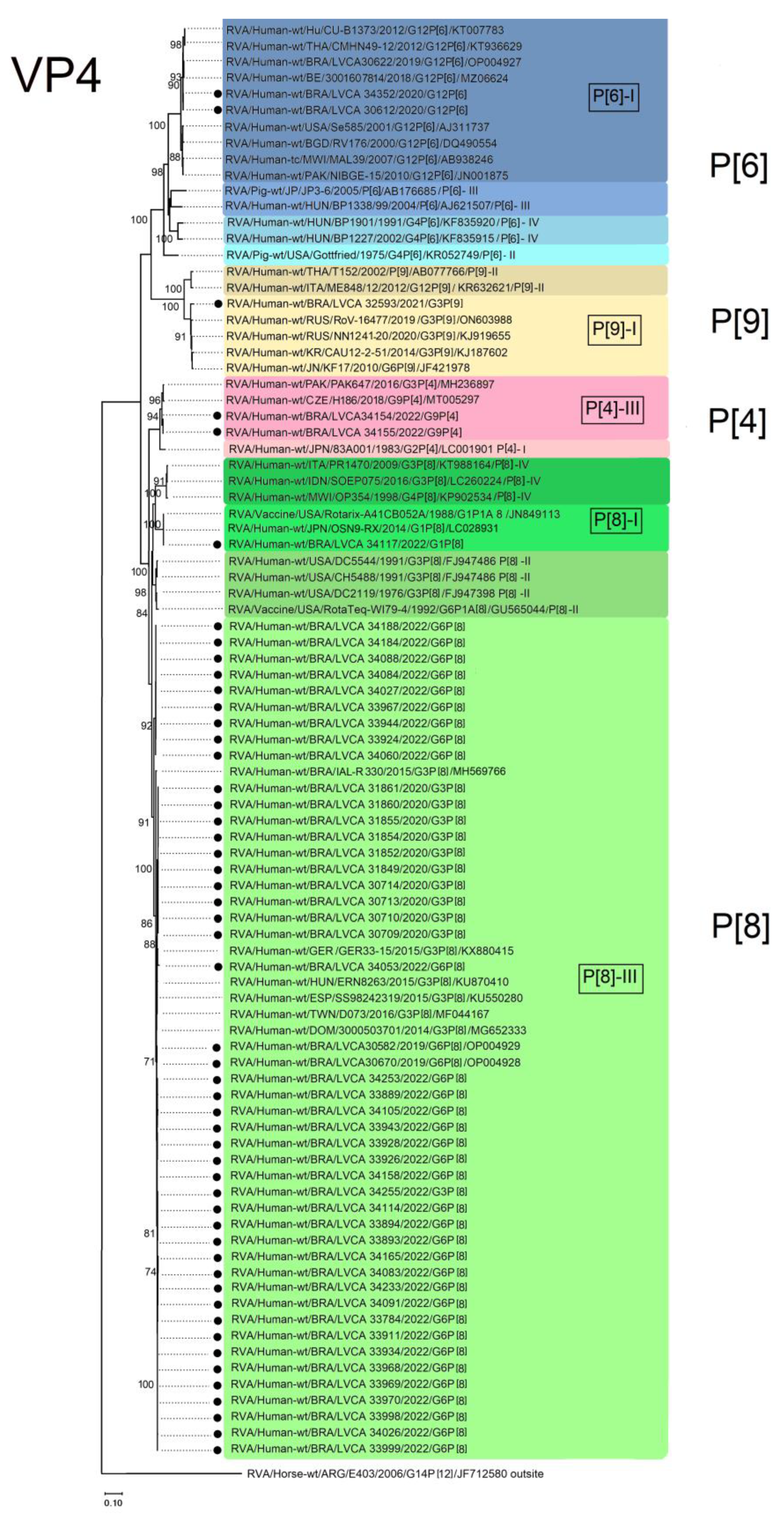
3.3. VP7 and VP4 Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Reiner, R.C.; Fullman, N.; Thompson, R.L.; Abajobir, A.; Ahmed, M.; et al. Estimates of Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoeal Diseases: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 909–948. [Google Scholar] [CrossRef]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea among Children Younger than 5 Years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Martinón-Torres, F.; Posiuniene, I.; Benninghoff, B.; Oh, K.-B.; Poelaert, D. The Value of Rotavirus Vaccination in Europe: A Call for Action. Infect. Dis. Ther. 2023, 12, 9–29. [Google Scholar] [CrossRef]
- Greenberg, H.B.; Estes, M.K. Rotaviruses: From Pathogenesis to Vaccination. Gastroenterology 2009, 136, 1939–1951. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; Heiman, E.; Arijs, I.; Delbeke, T.; McDonald, S.M.; Palombo, E.A.; Iturriza-Gómara, M.; Maes, P.; Patton, J.T.; et al. Full Genome-Based Classification of Rotaviruses Reveals a Common Origin between Human Wa-Like and Porcine Rotavirus Strains and Human DS-1-like and Bovine Rotavirus Strains. J. Virol. 2008, 82, 3204–3219. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; McDonald, S.M.; Attoui, H.; Bányai, K.; Brister, J.R.; Buesa, J.; Esona, M.D.; Estes, M.K.; Gentsch, J.R.; et al. Uniformity of Rotavirus Strain Nomenclature Proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 2011, 156, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Dóró, R.; László, B.; Martella, V.; Leshem, E.; Gentsch, J.; Parashar, U.; Bányai, K. Review of Global Rotavirus Strain Prevalence Data from Six Years Post Vaccine Licensure Surveillance: Is There Evidence of Strain Selection from Vaccine Pressure? Infect. Genet. Evol. 2014, 28, 446–461. [Google Scholar] [CrossRef]
- Gentsch, J.R.; Laird, A.R.; Bielfelt, B.; Griffin, D.D.; Banyai, K.; Ramachandran, M.; Jain, V.; Cunliffe, N.A.; Nakagomi, O.; Kirkwood, C.D.; et al. Serotype Diversity and Reassortment between Human and Animal Rotavirus Strains: Implications for Rotavirus Vaccine Programs. J. Infect. Dis. 2005, 192 (Suppl. S1), S146–S159. [Google Scholar] [CrossRef]
- Iturriza-Gómara, M.; Dallman, T.; Bányai, K.; Böttiger, B.; Buesa, J.; Diedrich, S.; Fiore, L.; Johansen, K.; Koopmans, M.; Korsun, N.; et al. Rotavirus Genotypes Co-Circulating in Europe between 2006 and 2009 as Determined by EuroRotaNet, a Pan-European Collaborative Strain Surveillance Network. Epidemiol. Infect. 2011, 139, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, R.Q.; Alvarez, A.D.J.; Rodrigues, A.; Ribeiro, R.R.; Dolabella, S.S.; Da Mota, N.L.; Santos, V.S.; Iturriza-Gomara, M.; Cunliffe, N.A.; Cuevas, L.E. Incidence of Rotavirus and Circulating Genotypes in Northeast Brazil during 7 Years of National Rotavirus Vaccination. PLoS ONE 2014, 9, e110217. [Google Scholar] [CrossRef][Green Version]
- Clark, A.; Black, R.; Tate, J.; Roose, A.; Kotloff, K.; Lam, D.; Blackwelder, W.; Parashar, U.; Lanata, C.; Kang, G.; et al. Estimating Global, Regional and National Rotavirus Deaths in Children Aged <5 Years: Current Approaches, New Analyses and Proposed Improvements. PLoS ONE 2017, 12, e0183392. [Google Scholar] [CrossRef]
- Saha, D.; Ota, M.O.C.; Pereira, P.; Buchy, P.; Badur, S. Rotavirus Vaccines Performance: Dynamic Interdependence of Host, Pathogen and Environment. Expert Rev. Vaccines 2021, 20, 945–957. [Google Scholar] [CrossRef]
- Henschke, N.; Bergman, H.; Hungerford, D.; Cunliffe, N.A.; Grais, R.F.; Kang, G.; Parashar, U.D.; Wang, S.A.; Neuzil, K.M. The Efficacy and Safety of Rotavirus Vaccines in Countries in Africa and Asia with High Child Mortality. Vaccine 2022, 40, 1707–1711. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.M.; Tate, J.E.; Kirkwood, C.D.; Steele, A.D.; Parashar, U.D. Current and New Rotavirus Vaccines. Curr. Opin. Infect. Dis. 2019, 32, 435–444. [Google Scholar] [CrossRef]
- Kiely, M.; Mansour, T.; Brousseau, N.; Rafferty, E.; Paudel, Y.R.; Sadarangani, M.; Svenson, L.W.; Robinson, J.L.; Gagneur, A.; Driedger, S.M.; et al. COVID-19 Pandemic Impact on Childhood Vaccination Coverage in Quebec, Canada. Hum. Vaccines Immunother. 2022, 18, 2007707. [Google Scholar] [CrossRef] [PubMed]
- SeyedAlinaghi, S.; Karimi, A.; Mojdeganlou, H.; Alilou, S.; Mirghaderi, S.P.; Noori, T.; Shamsabadi, A.; Dadras, O.; Vahedi, F.; Mohammadi, P.; et al. Impact of COVID-19 Pandemic on Routine Vaccination Coverage of Children and Adolescents: A Systematic Review. Health Sci. Rep. 2022, 5, e00516. [Google Scholar] [CrossRef]
- Bramer, C.A.; Kimmins, L.M.; Swanson, R.; Kuo, J.; Vranesich, P.; Jacques-Carroll, L.A.; Shen, A.K. Decline in Child Vaccination Coverage during the COVID-19 Pandemic—Michigan Care Improvement Registry, May 2016–May 2020. Am. J. Transplant. 2020, 20, 1930–1931. [Google Scholar] [CrossRef]
- Saxena, S.; Skirrow, H.; Bedford, H. Routine Vaccination during COVID-19 Pandemic Response. BMJ 2020, 369, m2392. [Google Scholar] [CrossRef]
- Barros, L.L.; Barros, L.L.; do Carmo, R.F.; Santos, M.B.; da Costa Armstrong, A.; de Vasconcelos, R.A.; de Souza, C.D.F. Change in Rotavirus Vaccine Coverage in Brazil from before (2015–2019) through the COVID-19 Pandemic Period (2020–2021). Viruses 2023, 15, 292. [Google Scholar] [CrossRef]
- Procianoy, G.S.; Junior, F.R.; Lied, A.F.; Jung, L.F.P.P.; de Souza, M.C.S.C. Impact of the COVID-19 Pandemic on the Vaccination of Children 12 Months of Age and under: An Ecological Study. Cienc. Saude Coletiva 2022, 27, 969–978. [Google Scholar] [CrossRef]
- Zeng, S.Q.; Halkosalo, A.; Salminen, M.; Szakal, E.D.; Puustinen, L.; Vesikari, T. One-Step Quantitative RT-PCR for the Detection of Rotavirus in Acute Gastroenteritis. J. Virol. Methods 2008, 153, 238–240. [Google Scholar] [CrossRef]
- Gentsch, J.R.; Glass, R.I.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.K.; Bhan, M.K. Identification of Group A Rotavirus Gene 4 Types by Polymerase Chain Reaction. J. Clin. Microbiol. 1992, 30, 1365–1373. [Google Scholar] [CrossRef]
- Iturriza-Gómara, M.; Isherwood, B.; Desselberger, U.; Gray, J. Reassortment in Vivo: Driving Force for Diversity of Human Rotavirus Strains Isolated in the United Kingdom between 1995 and 1999. J. Virol. 2001, 75, 3696–3705. [Google Scholar] [CrossRef]
- Esona, M.D.; Gautam, R. Rotavirus. Clin. Lab. Med. 2015, 35, 363–391. [Google Scholar] [CrossRef] [PubMed]
- Bonkoungou, I.J.O.; Ouédraogo, N.; Tamini, L.; Teguera, R.K.; Yaméogo, P.; Drabo, M.K.; Medah, I.; Barro, N.; Sharma, S.; Svensson, L.; et al. Rotavirus and Norovirus in Children with Severe Diarrhea in Burkina Faso before Rotavirus Vaccine Introduction. J. Med. Virol. 2018, 90, 1453–1460. [Google Scholar] [CrossRef]
- Iturriza-Gómara, M.; Kang, G.; Gray, J. Rotavirus Genotyping: Keeping up with an Evolving Population of Human Rotaviruses. J. Clin. Virol. 2004, 31, 259–265. [Google Scholar] [CrossRef]
- Gouvea, V.; Glass, R.I.; Woods, P.; Taniguchi, K.; Clark, H.F.; Forrester, B.; Fang, Z.Y. Polymerase Chain Reaction Amplification and Typing of Rotavirus Nucleic Acid from Stool Specimens. J. Clin. Microbiol. 1990, 28, 276–282. [Google Scholar] [CrossRef]
- Pickett, B.E.; Greer, D.S.; Zhang, Y.; Stewart, L.; Zhou, L.; Sun, G.; Gu, Z.; Kumar, S.; Zaremba, S.; Larsen, C.N.; et al. Virus Pathogen Database and Analysis Resource (ViPR): A Comprehensive Bioinformatics Database and Analysis Resource for the Coronavirus Research Community. Viruses 2012, 4, 3209–3226. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- da Silva Soares, L.; de Fátima Dos Santos Guerra, S.; do Socorro Lima de Oliveira, A.; da Silva Dos Santos, F.; de Fátima Costa de Menezes, E.M.; Mascarenhas, J.; d’Arc, P.; Linhares, A.C. Diversity of Rotavirus Strains Circulating in Northern Brazil after Introduction of a Rotavirus Vaccine: High Prevalence of G3P[6] Genotype. J. Med. Virol. 2014, 86, 1065–1072. [Google Scholar] [CrossRef]
- Justino, M.C.A.; Campos, E.A.; Mascarenhas, J.D.P.; Soares, L.S.; Guerra, S.D.F.S.; Furlaneto, I.P.; Pavão, M.J.C.; Maciel, T.S.; Farias, F.P.; Bezerra, O.M.; et al. Rotavirus Antigenemia as a Common Event among Children Hospitalised for Severe, Acute Gastroenteritis in Belém, Northern Brazil. BMC Pediatr. 2019, 19, 193. [Google Scholar] [CrossRef]
- Luchs, A.; da Costa, A.C.; Cilli, A.; Komninakis, S.C.V.; Carmona, R.d.C.C.; Boen, L.; Morillo, S.G.; Sabino, E.C.; Timenetsky, M.d.C.S.T. Spread of the Emerging Equine-like G3P[8] DS-1-like Genetic Backbone Rotavirus Strain in Brazil and Identification of Potential Genetic Variants. J. Gen. Virol. 2019, 100, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Costa, F.A.; de Assis, R.M.S.; Fialho, A.M.; Araújo, I.T.; Silva, M.F.; Gómez, M.M.; Andrade, J.S.; Rose, T.L.; Fumian, T.M.; Volotão, E.M.; et al. The Evolving Epidemiology of Rotavirus A Infection in Brazil a Decade after the Introduction of Universal Vaccination with Rotarix®. BMC Pediatr. 2019, 19, 42. [Google Scholar] [CrossRef]
- Gutierrez, M.B.; Fialho, A.M.; Maranhão, A.G.; Malta, F.C.; Andrade, J.d.S.R.d.; de Assis, R.M.S.; Mouta, S.d.S.e.; Miagostovich, M.P.; Leite, J.P.G.; Fumian, T.M. Rotavirus A in Brazil: Molecular Epidemiology and Surveillance during 2018–2019. Pathogens 2020, 9, E515. [Google Scholar] [CrossRef] [PubMed]
- Lo Moro, G.; Scaioli, G.; Nicolino, S.; Sinigaglia, T.; DE Vito, E.; Bert, F.; Siliquini, R. Risk Perception, Knowledge about SARS-CoV-2, and Perception towards Preventive Measures in Italy: A Nationwide Cross-Sectional Study. J. Prev. Med. Hyg. 2023, 64, E9–E12. [Google Scholar] [CrossRef] [PubMed]
- Roczo-Farkas, S.; Thomas, S.; Bogdanovic-Sakran, N.; Donato, C.M.; Lyons, E.A.; Bines, J. Australian Rotavirus Surveillance Group Australian Rotavirus Surveillance Program: Annual Report, 2021. Commun. Dis. Intell. (2018) 2022, 46. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.-W. Return of Norovirus and Rotavirus Activity in Winter 2020–21 in City with Strict COVID-19 Control Strategy, China. Emerg. Infect. Dis. 2022, 28, 713–716. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Lou, J.; Chen, J.; Xie, X.; Mao, J. Rotavirus and Adenovirus Infections in Children during COVID-19 Outbreak in Hangzhou, China. Transl. Pediatr. 2021, 10, 2281–2286. [Google Scholar] [CrossRef]
- Kuitunen, I.; Artama, M.; Haapanen, M.; Renko, M. Noro- and Rotavirus Detections in Children during COVID-19 Pandemic-A Nationwide Register Study in Finland. Acta Paediatr. 2022, 111, 1978–1980. [Google Scholar] [CrossRef]
- Okitsu, S.; Khamrin, P.; Hikita, T.; Thongprachum, A.; Pham, N.T.K.; Hoque, S.A.; Hayakawa, S.; Maneekarn, N.; Ushijima, H. Changing Distribution of Rotavirus A Genotypes Circulating in Japanese Children with Acute Gastroenteritis in Outpatient Clinic, 2014–2020. J. Infect. Public Health 2022, 15, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Burnett, E.; Parashar, U.D.; Winn, A.; Tate, J.E. Trends in Rotavirus Laboratory Detections and Internet Search Volume Before and After Rotavirus Vaccine Introduction and in the Context of the Coronavirus Disease 2019 Pandemic-United States, 2000–2021. J. Infect. Dis. 2022, 226, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Chan-It, W.; Chanta, C.; Ushijima, H. Predominance of DS-1-like G8P[8] Rotavirus Reassortant Strains in Children Hospitalized with Acute Gastroenteritis in Thailand, 2018–2020. J. Med. Virol. 2023, 95, e28870. [Google Scholar] [CrossRef] [PubMed]
- Luchs, A.; Cilli, A.; Morillo, S.G.; Carmona, R.d.C.C.; Timenetsky, M.d.C.S.T. Rotavirus Genotypes Circulating in Brazil, 2007–2012: Implications for the Vaccine Program. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Contreras, S.; Dehning, J.; Linden, M.; Iftekhar, E.; Mohr, S.B.; Olivera-Nappa, A.; Priesemann, V. Relaxing Restrictions at the Pace of Vaccination Increases Freedom and Guards against Further COVID-19 Waves. PLoS Comput. Biol. 2021, 17, e1009288. [Google Scholar] [CrossRef]
- O’Reilly, K.M.; Sandman, F.; Allen, D.; Jarvis, C.I.; Gimma, A.; Douglas, A.; Larkin, L.; Wong, K.L.; Baguelin, M.; Baric, R.S.; et al. Predicted Norovirus Resurgence in 2021-2022 Due to the Relaxation of Nonpharmaceutical Interventions Associated with COVID-19 Restrictions in England: A Mathematical Modelling Study. medRxiv 2021. [Google Scholar] [CrossRef]
- Lappe, B.L.; Wikswo, M.E.; Kambhampati, A.K.; Mirza, S.A.; Tate, J.E.; Kraay, A.N.M.; Lopman, B.A. Predicting Norovirus and Rotavirus Resurgence in the United States following the COVID-19 Pandemic: A Mathematical Modelling Study. BMC Infect. Dis. 2023, 23, 254. [Google Scholar] [CrossRef]
- Knudsen, P.K.; Lind, A.; Klundby, I.; Dudman, S. The Incidence of Infectious Diseases and Viruses Other than SARS-CoV-2 amongst Hospitalised Children in Oslo, Norway during the COVID-19 Pandemic 2020–2021. J. Clin. Virol. Plus 2022, 2, 100060. [Google Scholar] [CrossRef]
- Leite, J.P.G.; Carvalho-Costa, F.A.; Linhares, A.C. Group A Rotavirus Genotypes and the Ongoing Brazilian Experience: A Review. Mem. Inst. Oswaldo Cruz 2008, 103, 745–753. [Google Scholar] [CrossRef]
- Linhares, A.C.; Velázquez, F.R.; Pérez-Schael, I.; Sáez-Llorens, X.; Abate, H.; Espinoza, F.; López, P.; Macías-Parra, M.; Ortega-Barría, E.; Rivera-Medina, D.M.; et al. Efficacy and Safety of an Oral Live Attenuated Human Rotavirus Vaccine against Rotavirus Gastroenteritis during the First 2 Years of Life in Latin American Infants: A Randomised, Double-Blind, Placebo-Controlled Phase III Study. Lancet 2008, 371, 1181–1189. [Google Scholar] [CrossRef]
- De Jesus, M.C.S.; Santos, V.S.; Storti-Melo, L.M.; De Souza, C.D.F.; Barreto, Í.D.D.C.; Paes, M.V.C.; Lima, P.A.S.; Bohland, A.K.; Berezin, E.N.; Machado, R.L.D.; et al. Impact of a Twelve-Year Rotavirus Vaccine Program on Acute Diarrhea Mortality and Hospitalization in Brazil: 2006–2018. Expert Rev. Vaccines 2020, 19, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Cowley, D.; Donato, C.M.; Roczo-Farkas, S.; Kirkwood, C.D. Emergence of a Novel Equine-like G3P[8] Inter-Genogroup Reassortant Rotavirus Strain Associated with Gastroenteritis in Australian Children. J. Gen. Virol. 2016, 97, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Dóró, R.; Marton, S.; Bartókné, A.H.; Lengyel, G.; Agócs, Z.; Jakab, F.; Bányai, K. Equine-like G3 Rotavirus in Hungary, 2015—Is It a Novel Intergenogroup Reassortant Pandemic Strain? Acta Microbiol. Immunol. Hung. 2016, 63, 243–255. [Google Scholar] [CrossRef]
- Pietsch, C.; Liebert, U.G. Molecular Characterization of Different Equine-like G3 Rotavirus Strains from Germany. Infect. Genet. Evol. 2018, 57, 46–50. [Google Scholar] [CrossRef]
- Arana, A.; Montes, M.; Jere, K.C.; Alkorta, M.; Iturriza-Gómara, M.; Cilla, G. Emergence and Spread of G3P[8] Rotaviruses Possessing an Equine-like VP7 and a DS-1-like Genetic Backbone in the Basque Country (North of Spain), 2015. Infect. Genet. Evol. 2016, 44, 137–144. [Google Scholar] [CrossRef]
- Komoto, S.; Ide, T.; Negoro, M.; Tanaka, T.; Asada, K.; Umemoto, M.; Kuroki, H.; Ito, H.; Tanaka, S.; Ito, M.; et al. Characterization of Unusual DS-1-like G3P[8] Rotavirus Strains in Children with Diarrhea in Japan. J. Med. Virol. 2018, 90, 890–898. [Google Scholar] [CrossRef]
- Roczo-Farkas, S.; Cowley, D.; Bines, J.E.; The Australian Rotavirus Surveillance Group. Australian Rotavirus Surveillance Program: Annual Report, 2017. Commun. Dis. Intell. (2018) 2019, 43, 1–21. [Google Scholar] [CrossRef]
- Esposito, S.; Camilloni, B.; Bianchini, S.; Ianiro, G.; Polinori, I.; Farinelli, E.; Monini, M.; Principi, N. First Detection of a Reassortant G3P[8] Rotavirus A Strain in Italy: A Case Report in an 8-Year-Old Child. Virol. J. 2019, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.; Bostan, N.; Bokhari, H.; Matthijnssens, J.; Yinda, K.C.; Raza, S.; Nawaz, T. Molecular Characterization of Human Group A Rotavirus Genotypes Circulating in Rawalpindi, Islamabad, Pakistan during 2015–2016. PLoS ONE 2019, 14, e0220387. [Google Scholar] [CrossRef]
- Gutierrez, M.B.; de Figueiredo, M.R.; Fialho, A.M.; Cantelli, C.P.; Miagostovich, M.P.; Fumian, T.M. Nosocomial Acute Gastroenteritis Outbreak Caused by an Equine-like G3P[8] DS-1-like Rotavirus and GII.4 Sydney[P16] Norovirus at a Pediatric Hospital in Rio de Janeiro, Brazil, 2019. Hum. Vaccines Immunother. 2021, 17, 4654–4660. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Rahman, M.; Van Ranst, M. Two out of the 11 Genes of an Unusual Human G6P[6] Rotavirus Isolate Are of Bovine Origin. J. Gen. Virol. 2008, 89, 2630–2635. [Google Scholar] [CrossRef]
- Pietsch, C.; Liebert, U.G. Evidence for Presumable Feline Origin of Sporadic G6P[9] Rotaviruses in Humans. Infect. Genet. Evol. 2018, 63, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Mladenova, Z.; Nawaz, S.; Ganesh, B.; Iturriza-Gomara, M. Increased Detection of G3P[9] and G6P[9] Rotavirus Strains in Hospitalized Children with Acute Diarrhea in Bulgaria. Infect. Genet. Evol. 2015, 29, 118–126. [Google Scholar] [CrossRef]
- Gutierrez, M.B.; de Assis, R.M.S.; Arantes, I.; Fumian, T.M. Full Genotype Constellations Analysis of Unusual DS-1-like G12P[6] and G6P[8] Rotavirus Strains Detected in Brazil, 2019. Virology 2022, 577, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.M.; Esona, M.D.; Betrapally, N.S.; De La Cruz De Leon, L.A.; Neira, Y.R.; Rey, G.J.; Bowen, M.D. Whole-Gene Analysis of Inter-Genogroup Reassortant Rotaviruses from the Dominican Republic: Emergence of Equine-like G3 Strains and Evidence of Their Reassortment with Locally-Circulating Strains. Virology 2019, 534, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Bányai, K.; Gentsch, J.R.; Griffin, D.D.; Holmes, J.L.; Glass, R.I.; Szücs, G. Genetic Variability among Serotype G6 Human Rotaviruses: Identification of a Novel Lineage Isolated in Hungary. J. Med. Virol. 2003, 71, 124–134. [Google Scholar] [CrossRef]
- Gerna, G.; Sarasini, A.; Parea, M.; Arista, S.; Miranda, P.; Brüssow, H.; Hoshino, Y.; Flores, J. Isolation and Characterization of Two Distinct Human Rotavirus Strains with G6 Specificity. J. Clin. Microbiol. 1992, 30, 9–16. [Google Scholar] [CrossRef]
- Heylen, E.; Zeller, M.; Ciarlet, M.; Lawrence, J.; Steele, D.; Van Ranst, M.; Matthijnssens, J. Human P[6] Rotaviruses From Sub-Saharan Africa and Southeast Asia Are Closely Related to Those of Human P[4] and P[8] Rotaviruses Circulating Worldwide. J. Infect. Dis. 2016, 214, 1039–1049. [Google Scholar] [CrossRef]
- Griffin, D.D.; Nakagomi, T.; Hoshino, Y.; Nakagomi, O.; Kirkwood, C.D.; Parashar, U.D.; Glass, R.I.; Gentsch, J.R.; National Rotavirus Surveillance System. Characterization of Nontypeable Rotavirus Strains from the United States: Identification of a New Rotavirus Reassortant (P2A[6],G12) and Rare P3[9] Strains Related to Bovine Rotaviruses. Virology 2002, 294, 256–269. [Google Scholar] [CrossRef][Green Version]
- Palombo, E.A.; Bishop, R.F. Genetic and Antigenic Characterization of a Serotype G6 Human Rotavirus Isolated in Melbourne, Australia. J. Med. Virol. 1995, 47, 348–354. [Google Scholar] [CrossRef]
- Boom, J.A.; Sahni, L.C.; Payne, D.C.; Gautam, R.; Lyde, F.; Mijatovic-Rustempasic, S.; Bowen, M.D.; Tate, J.E.; Rench, M.A.; Gentsch, J.R.; et al. Symptomatic Infection and Detection of Vaccine and Vaccine-Reassortant Rotavirus Strains in 5 Children: A Case Series. J. Infect. Dis. 2012, 206, 1275–1279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Afrad, M.H.; Matthijnssens, J.; Moni, S.; Kabir, F.; Ashrafi, A.; Rahman, M.Z.; Faruque, A.S.G.; Azim, T.; Rahman, M. Genetic Characterization of a Rare Bovine-like Human VP4 Mono-Reassortant G6P[8] Rotavirus Strain Detected from an Infant in Bangladesh. Infect. Genet. Evol. 2013, 19, 120–126. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, D.C.; Fuller, T.; Cantelli, C.P.; de Moraes, M.T.B.; Leite, J.P.G.; Carvalho-Costa, F.A.; Brasil, P. Circulation of Vaccine-Derived Rotavirus G1P[8] in a Vulnerable Child Cohort in Rio de Janeiro. Pediatr. Infect. Dis. J. 2023, 42, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Than, V.T.; Thanh, H.D.; Kim, W. Evidence of Multiple Reassortment Events of Feline-to-Human Rotaviruses Based on a Rare Human G3P[9] Rotavirus Isolated from a Patient with Acute Gastroenteritis. Comp. Immunol. Microbiol. Infect. Dis. 2016, 46, 53–59. [Google Scholar] [CrossRef]
- Bezerra, D.A.M.; Guerra, S.F.S.; Serra, A.C.S.; Fecury, P.C.M.S.; Bandeira, R.S.; Penha, E.T.; Lobo, P.S.; Sousa, E.C.; Linhares, A.C.; Soares, L.S.; et al. Analysis of a Genotype G3P[9] Rotavirus a Strain That Shows Evidence of Multiple Reassortment Events between Animal and Human Rotaviruses. J. Med. Virol. 2017, 89, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Reslan, L.; El-Husseini, M.; Raoof, H.; Finianos, M.; Guo, C.; Thakkar, R.; Inati, A.; Dbaibo, G.; Lipkin, W.I.; et al. Full Genome Characterization of Human G3P[6] and G3P[9] Rotavirus Strains in Lebanon. Infect. Genet. Evol. 2020, 78, 104133. [Google Scholar] [CrossRef]
- Yamamoto, S.P.; Kaida, A.; Ono, A.; Kubo, H.; Iritani, N. Detection and Characterization of a Human G9P[4] Rotavirus Strain in Japan. J. Med. Virol. 2015, 87, 1311–1318. [Google Scholar] [CrossRef]
- Ianiro, G.; Recanatini, C.; D’Errico, M.M.; Monini, M.; RotaNet-Italy Study Group. Uncommon G9P[4] Group A Rotavirus Strains Causing Dehydrating Diarrhea in Young Children in Italy. Infect. Genet. Evol. 2018, 64, 57–64. [Google Scholar] [CrossRef]
- Degiuseppe, J.I.; Stupka, J.A.; Argentinean Rotavirus Surveillance Network. Emergence of Unusual Rotavirus G9P[4] and G8P[8] Strains during Post Vaccination Surveillance in Argentina, 2017–2018. Infect. Genet. Evol. 2021, 93, 104940. [Google Scholar] [CrossRef]
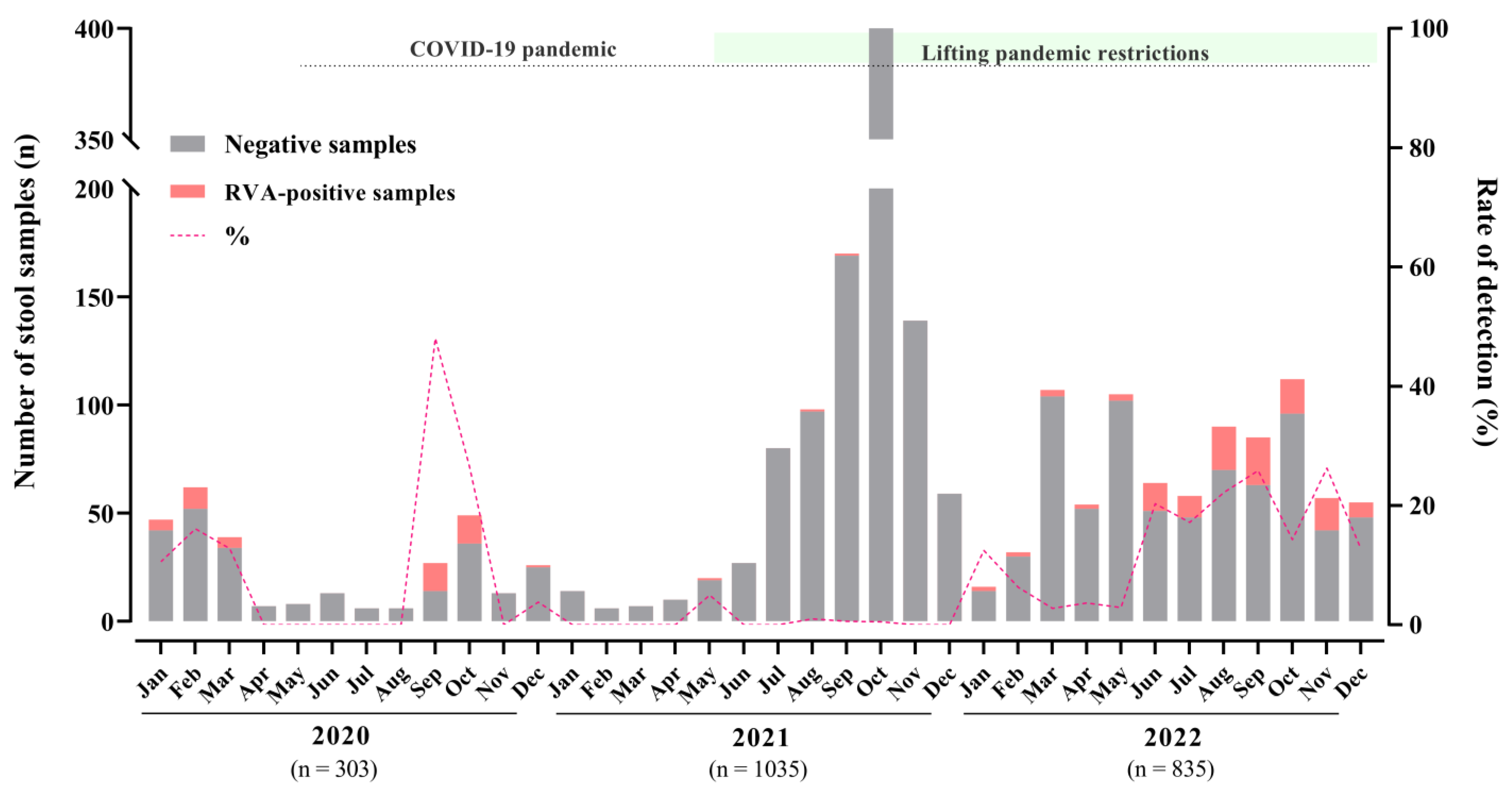
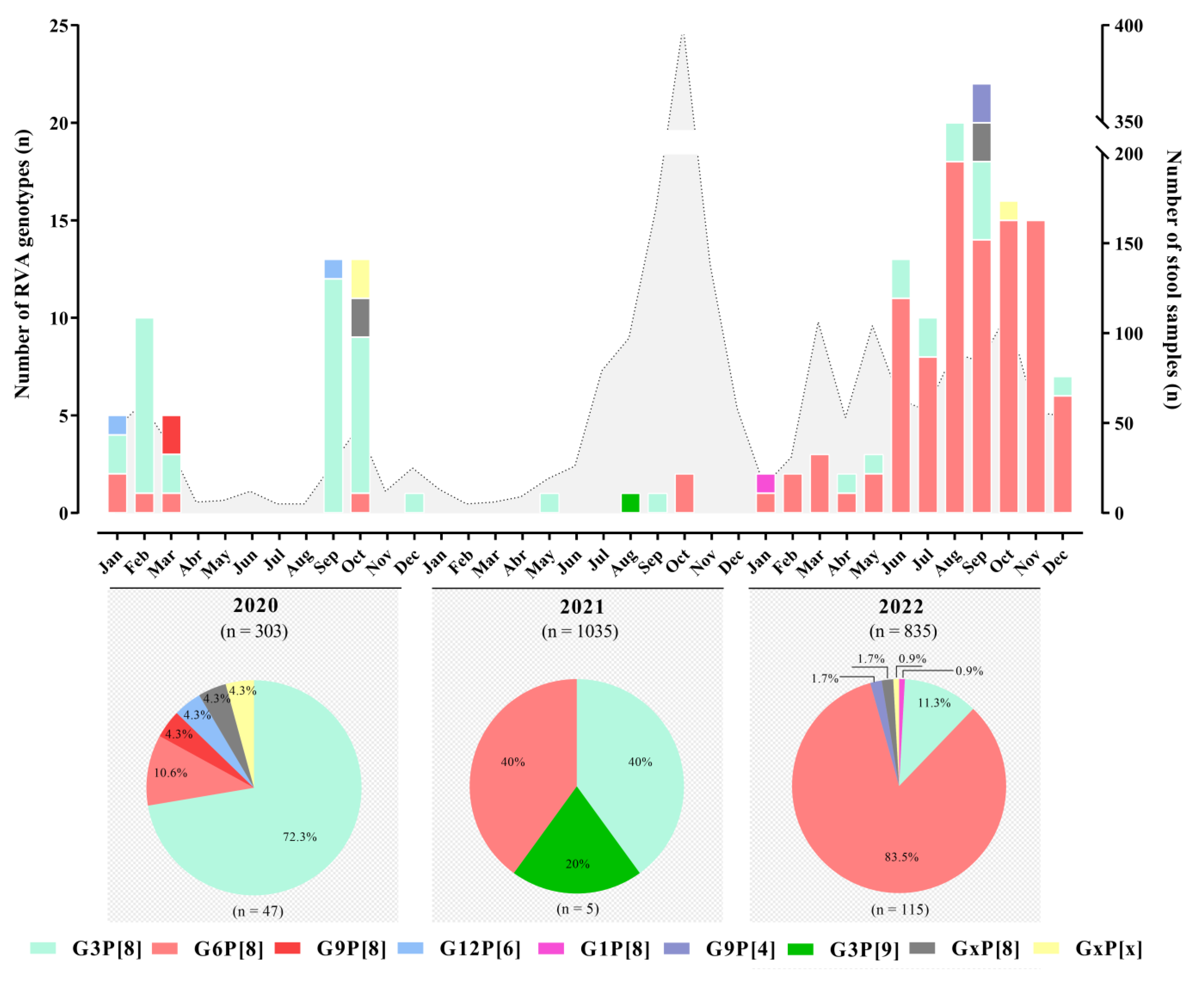
| No. of Fecal Samples—Positive/Tested (%) | |||||
|---|---|---|---|---|---|
| 2020 | 2021 | 2022 | Total | p-Value | |
| Region/State | |||||
| Northeastern | 25/106 (23.6) | 3/108 (2.8) | 34/108 (31.5) | 62/322 (19.3) | <0.0001 |
| Bahia (BA) | 24/105 | 1/92 | 10/20 | ||
| Paraíba (PB) | 0/0 | 0/0 | 4/6 | ||
| Pernambuco (PE) | 1/1 | 2/15 | 19/81 | ||
| Sergipe (SE) | 0/0 | 0/1 | 1/1 | ||
| Southeastern | 9/61 (14.8) | 1/142 (0.7) | 17/240 (7.1) | 27/443 (6.1) | 0.1590 |
| Minas Gerais (MG) | 0/18 | 1/79 | 13/101 | ||
| Rio de Janeiro (RJ) | 9/30 | 0/24 | 4/48 | ||
| Southern | 13/136 (9.6) | 1/785 (0.1) | 64/487(13.1) | 78/1408 (5.5) | <0.0001 |
| Rio Grande do Sul (RS) | 4/94 | 1/598 | 34/312 | ||
| Santa Catarina (SC) | 9/42 | 0/187 | 30/175 | ||
| Age groups (months) | |||||
| 0 to 6 | 3/33 (9.1) | 0/52 | 10/48 (20.8) | 13/133 (9.8) | - |
| >6 to 12 | 4/36 (11.1) | 0/102 | 15/85 (17.6) | 19/223 (8.5) | 0.6212 |
| >12 to 24 | 11/52 (21.2) | 1/246 (0.4) | 19/170 (11.2) | 31/468 (6.6) | 0.3305 |
| >24 to 60 | 10/56 (17.9) | 1/242 (0.4) | 33/205 (16.1) | 44/503 (8.7) | 0.3076 |
| >60 | 19/126 (15.1) | 3/393 (0.8) | 38/327(11.6) | 60/846 (7.1) | 0.4073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez, M.B.; de Assis, R.M.S.; Andrade, J.d.S.R.d.; Fialho, A.M.; Fumian, T.M. Rotavirus A during the COVID-19 Pandemic in Brazil, 2020–2022: Emergence of G6P[8] Genotype. Viruses 2023, 15, 1619. https://doi.org/10.3390/v15081619
Gutierrez MB, de Assis RMS, Andrade JdSRd, Fialho AM, Fumian TM. Rotavirus A during the COVID-19 Pandemic in Brazil, 2020–2022: Emergence of G6P[8] Genotype. Viruses. 2023; 15(8):1619. https://doi.org/10.3390/v15081619
Chicago/Turabian StyleGutierrez, Meylin Bautista, Rosane Maria Santos de Assis, Juliana da Silva Ribeiro de Andrade, Alexandre Madi Fialho, and Tulio Machado Fumian. 2023. "Rotavirus A during the COVID-19 Pandemic in Brazil, 2020–2022: Emergence of G6P[8] Genotype" Viruses 15, no. 8: 1619. https://doi.org/10.3390/v15081619
APA StyleGutierrez, M. B., de Assis, R. M. S., Andrade, J. d. S. R. d., Fialho, A. M., & Fumian, T. M. (2023). Rotavirus A during the COVID-19 Pandemic in Brazil, 2020–2022: Emergence of G6P[8] Genotype. Viruses, 15(8), 1619. https://doi.org/10.3390/v15081619






