Seroprevalence of Alphaviruses (Togaviridae) among Urban Population in Nouakchott, Mauritania, West Africa
Abstract
1. Introduction
2. Materials and Methods
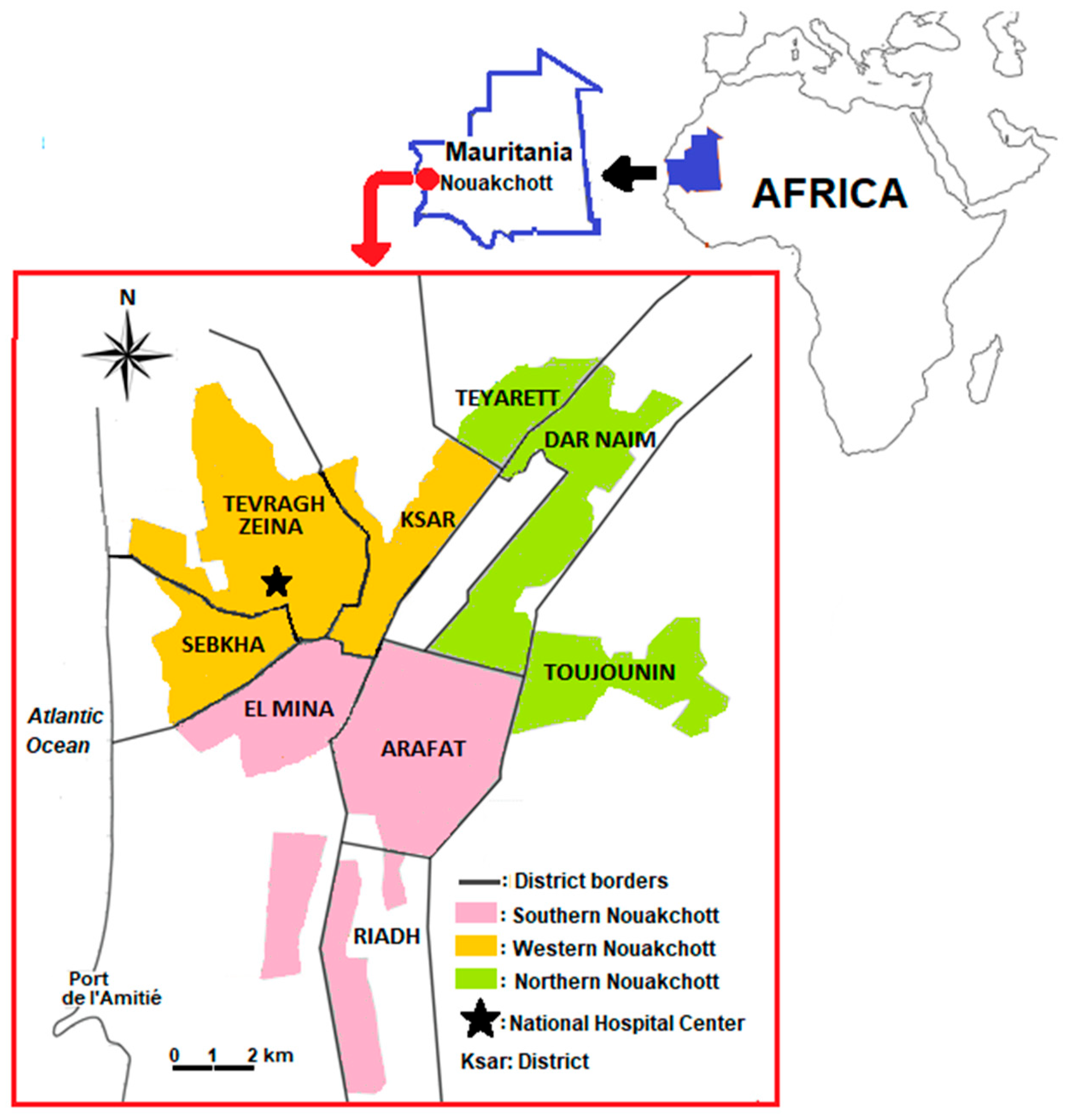
2.1. Study Site
2.2. Study Design and Sample Collection
2.3. IgG Antibody Testing
2.4. Serum Neutralization Assay
2.5. Statistical Analysis
3. Results
3.1. Seroprevalence of Anti-CHIKV IgG
3.2. Serum Neutralization Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Guidelines on Clinical Management of Chikungunya Fever; World Health Organization: Geneva, Switzerland, 2008; Available online: https://www.who.int/publications/i/item/guidelines-on-clinical-management-of-chikungunya-fever (accessed on 15 June 2023).
- Li, Z.; Wang, J.; Cheng, X.; Hu, H.; Guo, C.; Huang, J.; Chen, Z.; Lu, J. The Worldwide Seroprevalence of DENV, CHIKV and ZIKV Infection: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009337. [Google Scholar] [CrossRef]
- Rezza, G.; Chen, R.; Weaver, S.C. O’nyong-Nyong Fever: A Neglected Mosquito-Borne Viral Disease. Pathog. Glob. Health 2017, 111, 271–275. [Google Scholar] [CrossRef]
- Hozé, N.; Diarra, I.; Sangaré, A.K.; Pastorino, B.; Pezzi, L.; Kouriba, B.; Sagara, I.; Dabo, A.; Djimdé, A.; Thera, M.A.; et al. Model-Based Assessment of Chikungunya and O’nyong-Nyong Virus Circulation in Mali in a Serological Cross-Reactivity Context. Nat. Commun. 2021, 12, 6735. [Google Scholar] [CrossRef]
- Puntasecca, C.J.; King, C.H.; LaBeaud, A.D. Measuring the Global Burden of Chikungunya and Zika Viruses: A Systematic Review. PLoS Negl. Trop. Dis. 2021, 15, e0009055. [Google Scholar] [CrossRef]
- Powers, A.M.; Brault, A.C.; Tesh, R.B.; Weaver, S.C. Re-Emergence of Chikungunya and O’nyong-Nyong Viruses: Evidence for Distinct Geographical Lineages and Distant Evolutionary Relationships. J. Gen. Virol. 2000, 81, 471–479. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Akande, A.O.; Muhammed, Y.; Rogo, L.D.; Oderinde, B.S. Prevalence Pattern of Chikungunya Virus Infection in Nigeria: A Four Decade Systematic Review and Meta-Analysis. Pathog. Glob. Health 2020, 114, 111–116. [Google Scholar] [CrossRef]
- Diallo, M.; Thonnon, J.; Traore-Lamizana, M.; Fontenille, D. Vectors of Chikungunya Virus in Senegal: Current Data and Transmission Cycles. Am. J. Trop. Med. Hyg. 1999, 60, 281–286. [Google Scholar] [CrossRef]
- Althouse, B.M.; Guerbois, M.; Cummings, D.A.T.; Diop, O.M.; Faye, O.; Faye, A.; Diallo, D.; Sadio, B.D.; Sow, A.; Faye, O.; et al. Role of Monkeys in the Sylvatic Cycle of Chikungunya Virus in Senegal. Nat. Commun. 2018, 9, 1046. [Google Scholar] [CrossRef]
- Ngoagouni, C.; Kamgang, B.; Kazanji, M.; Paupy, C.; Nakouné, E. Potential of Aedes aegypti and Aedes albopictus Populations in the Central African Republic to Transmit Enzootic Chikungunya Virus Strains. Parasit. Vectors 2017, 10, 164. [Google Scholar] [CrossRef]
- World Health Organization. Chikungunya Emergencies. 2019. Available online: https://www.who.int/emergencies/diseases/chikungunya/en/ (accessed on 23 July 2022).
- Sow, A.; Faye, O.; Diallo, M.; Diallo, D.; Chen, R.; Faye, O.; Diagne, C.T.; Guerbois, M.; Weidmann, M.; Ndiaye, Y.; et al. Chikungunya Outbreak in Kedougou, Southeastern Senegal in 2009–2010. Open Forum Infect. Dis. 2018, 5, ofx259. [Google Scholar] [CrossRef] [PubMed]
- Pezzi, L.; LaBeaud, A.D.; Reusken, C.B.; Drexler, J.F.; Vasilakis, N.; Diallo, M.; Simon, F.; Jaenisch, T.; Gallian, P.; Sall, A.; et al. GloPID-R Report on Chikungunya, O’nyong-Nyong and Mayaro Virus, Part 2: Epidemiological Distribution of o’nyong-Nyong Virus. Antivir. Res. 2019, 172, 104611. [Google Scholar] [CrossRef]
- Johnston, R.E.; Peters, C.J. Alphaviruses. In Fields Virology, 3rd ed.; Fields, B.N., Ed.; Lip-Pincott-Raven Publishers: Philadelphia, PA, USA, 1996; pp. 843–898. [Google Scholar]
- Thiberville, S.-D.; Moyen, N.; Dupuis-Maguiraga, L.; Nougairede, A.; Gould, E.A.; Roques, P.; de Lamballerie, X. Chikungunya Fever: Epidemiology, Clinical Syndrome, Pathogenesis and Therapy. Antivir. Res. 2013, 99, 345–370. [Google Scholar] [CrossRef]
- Navarro, J.C.; Carrera, J.P.; Liria, J.; Auguste, A.J.; Weaver, S.C. Alphaviruses in Latin America and the Introduction of Chikungunya Virus. In Human Virology in Latin America; Ludert, J., Pujol, F., Arbiza, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 169–192. [Google Scholar]
- European Center for Disease Prevention and Control (ECDPC). Communicable Disease Threats Report. Week 11, 12–18 March 2023. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/communicable-disease-threats-report-16-jun-2023.pdf (accessed on 16 June 2023).
- Simon, F.; Javelle, E.; Gasque, P. Chikungunya Virus Infections. N. Engl. J. Med. 2015, 373, 93–94. [Google Scholar] [CrossRef]
- Thonnon, J.; Spiegel, A.; Diallo, M.; Diallo, A.; Fontenille, D. Chikungunya virus outbreak in Senegal in 1996 and 1997. Bull. Soc. Pathol. Exot. 1999, 92, 79–82. [Google Scholar]
- CDC. Chikungunya Virus: Geographic Distribution [Internet]. Centers for Disease Control and Prevention. [Cited 2022 July 2]. 2022; pp. 3–5. Available online: http://www.cdc.gov/chikungunya/geo/index.html (accessed on 15 June 2023).
- Pezzi, L.; Reusken, C.B.; Weaver, S.C.; Drexler, J.F.; Busch, M.; LaBeaud, A.D.; Diamond, M.S.; Vasilakis, N.; Drebot, M.A.; Siqueira, A.M.; et al. GloPID-R Report on Chikungunya, O’nyong-Nyong and Mayaro Virus, Part I: Biological Diagnostics. Antivir. Res. 2019, 166, 66–81. [Google Scholar] [CrossRef]
- Zeller, H.; Van Bortel, W.; Sudre, B. Chikungunya: Its History in Africa and Asia and Its Spread to New Regions in 2013-2014. J. Infect. Dis. 2016, 214, S436–S440. [Google Scholar] [CrossRef]
- Safronetz, D.; Sacko, M.; Sogoba, N.; Rosenke, K.; Martellaro, C.; Traoré, S.; Cissé, I.; Maiga, O.; Boisen, M.; Nelson, D.; et al. Vectorborne Infections, Mali. Emerg. Infect. Dis. 2016, 22, 340–342. [Google Scholar] [CrossRef]
- Fourié, T.; El Bara, A.; Dubot-Pérès, A.; Grard, G.; Briolant, S.; Basco, L.K.; Ouldabdallahi Moukah, M.; Leparc-Goffart, I. Emergence of Dengue Virus Serotype 2 in Mauritania and Molecular Characterization of Its Circulation in West Africa. PLoS Negl. Trop. Dis. 2021, 15, e0009829. [Google Scholar] [CrossRef]
- Lekweiry, K.M.; Salem, M.S.O.A.; Basco, L.K.; Briolant, S.; Hafid, J.; Boukhary, A.O.M.S. Malaria in Mauritania: Retrospective and Prospective Overview. Malar. J. 2015, 14, 100. [Google Scholar] [CrossRef]
- Mint Mohamed Lemine, A.; Ould Lemrabott, M.A.; Hasni Ebou, M.; Mint Lekweiry, K.; Ould Ahmedou Salem, M.S.; Ould Brahim, K.; Ouldabdallahi Moukah, M.; Ould Bouraya, I.N.; Brengues, C.; Trape, J.-F.; et al. Mosquitoes (Diptera: Culicidae) in Mauritania: A Review of Their Biodiversity, Distribution and Medical Importance. Parasit. Vectors 2017, 10, 35. [Google Scholar] [CrossRef]
- Parreira, R.; Centeno-Lima, S.; Lopes, A.; Portugal-Calisto, D.; Constantino, A.; Nina, J. Dengue Virus Serotype 4 and Chikungunya Virus Coinfection in a Traveller Returning from Luanda, Angola, January 2014. Eurosurveillance 2014, 19, 20730. [Google Scholar] [CrossRef]
- Jain, J.; Dubey, S.K.; Shrinet, J.; Sunil, S. Dengue Chikungunya Co-Infection: A Live-in Relationship?? Biochem. Biophys. Res. Commun. 2017, 492, 608–616. [Google Scholar] [CrossRef]
- National Statistical Office. Annuaire Statistique, 2018. Ministry of Economy and Industry. 2020. Available online: https://ansade.mr/fr/annuaire-statistique-2020/ (accessed on 23 July 2022).
- Mint Lekweiry, K.; Ould Ahmedou Salem, M.S.; Ould Brahim, K.; Ould Lemrabott, M.A.; Brengues, C.; Faye, O.; Simard, F.; Ould Mohamed Salem Boukhary, A. Aedes aegypti (Diptera: Culicidae) in Mauritania: First Report on the Presence of the Arbovirus Mosquito Vector in Nouakchott. J. Med. Entomol. 2015, 52, 730–733. [Google Scholar] [CrossRef]
- El Moustapha, I.; Deida, J.; Dadina, M.; El Ghassem, A.; Begnoug, M.; Hamdinou, M.; Mint Lekweiry, K.; Ould Ahmedou Salem, M.S.; Khalef, Y.; Semane, A.; et al. Changing Epidemiology of Plasmodium vivax Malaria in Nouakchott, Mauritania: A Six-Year (2015-2020) Prospective Study. Malar. J. 2023, 22, 18. [Google Scholar] [CrossRef]
- Choplin, A. Nouakchott: ériger des tours et éradiquer les bidonvilles, ou comment faire rentrer dans la compétition les périphéries du monde. In Métropoles en Débat: (dé)Constructions de la Ville Compétitive; Le Blanc, A., Piermay, J.L., Gervais-Lambony, P., Eds.; Presses Universitaires de Paris Nanterre, OpenEdition Books: Nanterre, France, 2014; pp. 255–272. [Google Scholar] [CrossRef]
- LaBeaud, A.D.; Banda, T.; Brichard, J.; Muchiri, E.M.; Mungai, P.L.; Mutuku, F.M.; Borland, E.; Gildengorin, G.; Pfeil, S.; Teng, C.Y.; et al. High Rates of O’nyong Nyong and Chikungunya Virus Transmission in Coastal Kenya. PLoS Negl. Trop. Dis. 2015, 9, e0003436. [Google Scholar] [CrossRef]
- Bob, N.S.; Bâ, H.; Fall, G.; Ishagh, E.; Diallo, M.Y.; Sow, A.; Sembene, P.M.; Faye, O.; El Kouri, B.; Sidi, M.L.; et al. Detection of the Northeastern African Rift Valley Fever Virus Lineage During the 2015 Outbreak in Mauritania. Open Forum. Infect. Dis. 2017, 4, ofx087. [Google Scholar] [CrossRef]
- Boushab, B.M.; Kelly, M.; Kébé, H.; Bollahi, M.A.; Basco, L.K. Crimean-Congo Hemorrhagic Fever, Mauritania. Emerg. Infect. Dis. 2020, 26, 817–818. [Google Scholar] [CrossRef]
- Caminade, C.; Ndione, J.A.; Diallo, M.; MacLeod, D.A.; Faye, O.; Ba, Y.; Dia, I.; Morse, A.P. Rift Valley Fever Outbreaks in Mauritania and Related Environmental Conditions. Int. J. Environ. Res. Public. Health 2014, 11, 903–918. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; LeGuenno, B.; Guillaud, M.; Wilson, M.L. A Fatal Case of Crimean-Congo Haemorrhagic Fever in Mauritania: Virological and Serological Evidence Suggesting Epidemic Transmission. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 573–576. [Google Scholar] [CrossRef]
- Jouan, A.; Le Guenno, B.; Digoutte, J.P.; Philippe, B.; Riou, O.; Adam, F. An RVF Epidemic in Southern Mauritania. Ann. Inst. Pasteur Virol. 1988, 139, 307–308. [Google Scholar] [CrossRef]
- Nabeth, P.; Kane, Y.; Abdalahi, M.O.; Diallo, M.; Ndiaye, K.; Ba, K.; Schneegans, F.; Sall, A.A.; Mathiot, C. Rift Valley Fever Outbreak, Mauritania, 1998: Seroepidemiologic, Virologic, Entomologic, and Zoologic Investigations. Emerg. Infect. Dis. 2001, 7, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Nabeth, P.; Cheikh, D.O.; Lo, B.; Faye, O.; Vall, I.O.M.; Niang, M.; Wague, B.; Diop, D.; Diallo, M.; Diallo, B.; et al. Crimean-Congo Hemorrhagic Fever, Mauritania. Emerg. Infect. Dis. 2004, 10, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Saluzzo, J.F.; Digoutte, J.P.; Camicas, J.L.; Chauvancy, G. Crimean-Congo Haemorrhagic Fever and Rift Valley Fever in South-Eastern Mauritania. Lancet 1985, 1, 116. [Google Scholar] [CrossRef] [PubMed]
- Stoek, F.; Barry, Y.; Ba, A.; Schulz, A.; Rissmann, M.; Wylezich, C.; Sadeghi, B.; Beyit, A.D.; Eisenbarth, A.; N’diaye, F.B.; et al. Mosquito Survey in Mauritania: Detection of Rift Valley Fever Virus and Dengue Virus and the Determination of Feeding Patterns. PLoS Negl. Trop. Dis. 2022, 16, e0010203. [Google Scholar] [CrossRef]
- Seck, M.C.; Badiane, A.S.; Thwing, J.; Moss, D.; Fall, F.B.; Gomis, J.F.; Deme, A.B.; Diongue, K.; Sy, M.; Mbaye, A.; et al. Serological Data Shows Low Levels of Chikungunya Exposure in Senegalese Nomadic Pastoralists. Pathogens 2019, 8, 113. [Google Scholar] [CrossRef]
- Adusei, J.A.; Narkwa, P.W.; Owusu, M.; Domfeh, S.A.; Alhassan, M.; Appau, E.; Salam, A.; Mutocheluh, M. Evidence of Chikungunya Virus Infections among Febrile Patients at Three Secondary Health Facilities in the Ashanti and the Bono Regions of Ghana. PLoS Negl. Trop. Dis. 2021, 15, e0009735. [Google Scholar] [CrossRef]
- Proesmans, S.; Katshongo, F.; Milambu, J.; Fungula, B.; Muhindo Mavoko, H.; Ahuka-Mundeke, S.; Inocêncio da Luz, R.; Van Esbroeck, M.; Ariën, K.K.; Cnops, L.; et al. Dengue and Chikungunya among Outpatients with Acute Undifferentiated Fever in Kinshasa, Democratic Republic of Congo: A Cross-Sectional Study. PLoS Negl. Trop. Dis. 2019, 13, e0007047. [Google Scholar] [CrossRef]
- Chisenga, C.C.; Bosomprah, S.; Musukuma, K.; Mubanga, C.; Chilyabanyama, O.N.; Velu, R.M.; Kim, Y.C.; Reyes-Sandoval, A.; Chilengi, R. Sero-Prevalence of Arthropod-Borne Viral Infections among Lukanga Swamp Residents in Zambia. PLoS ONE 2020, 15, e0235322. [Google Scholar] [CrossRef]
- Ekong, P.S.; Aworh, M.K.; Grossi-Soyster, E.N.; Wungak, Y.S.; Maurice, N.A.; Altamirano, J.; Ekong, M.J.; Olugasa, B.O.; Nwosuh, C.I.; Shamaki, D.; et al. A Retrospective Study of the Seroprevalence of Dengue Virus and Chikungunya Virus Exposures in Nigeria, 2010–2018. Pathogens 2022, 11, 762. [Google Scholar] [CrossRef]
- Omatola, C.A.; Onoja, B.A.; Fassan, P.K.; Osaruyi, S.A.; Iyeh, M.; Samuel, M.A.; Haruna, P.U. Seroprevalence of Chikungunya Virus Infection in Five Hospitals within Anyigba, Kogi State of Nigeria. Braz. J. Infect. Dis. 2020, 24, 1–6. [Google Scholar] [CrossRef]
- Masika, M.M.; Korhonen, E.M.; Smura, T.; Uusitalo, R.; Ogola, J.; Mwaengo, D.; Jääskeläinen, A.J.; Alburkat, H.; Gwon, Y.-D.; Evander, M.; et al. Serological Evidence of Exposure to O’nyong-Nyong and Chikungunya Viruses in Febrile Patients of Rural Taita-Taveta County and Urban Kibera Informal Settlement in Nairobi, Kenya. Viruses 2022, 14, 1286. [Google Scholar] [CrossRef]
- Mwanyika, G.O.; Sindato, C.; Rugarabamu, S.; Rumisha, S.F.; Karimuribo, E.D.; Misinzo, G.; Rweyemamu, M.M.; Abdel Hamid, M.M.; Haider, N.; Vairo, F.; et al. Seroprevalence and Associated Risk Factors of Chikungunya, Dengue, and Zika in Eight Districts in Tanzania. Int. J. Infect. Dis. 2021, 111, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Asebe, G.; Michlmayr, D.; Mamo, G.; Abegaz, W.E.; Endale, A.; Medhin, G.; Larrick, J.W.; Legesse, M. Seroprevalence of Yellow Fever, Chikungunya, and Zika Virus at a Community Level in the Gambella Region, South West Ethiopia. PLoS ONE 2021, 16, e0253953. [Google Scholar] [CrossRef] [PubMed]
- Endale, A.; Michlmayr, D.; Abegaz, W.E.; Asebe, G.; Larrick, J.W.; Medhin, G.; Legesse, M. Community-Based Sero-Prevalence of Chikungunya and Yellow Fever in the South Omo Valley of Southern Ethiopia. PLoS Negl. Trop. Dis. 2020, 14, e0008549. [Google Scholar] [CrossRef] [PubMed]
- Ferede, G.; Tiruneh, M.; Abate, E.; Wondimeneh, Y.; Gadisa, E.; Howe, R.; Aseffa, A.; Tessema, B. Evidence of Chikungunya Virus Infection among Febrile Patients in Northwest Ethiopia. Int. J. Infect. Dis. 2021, 104, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Simo, F.B.N.; Bigna, J.J.; Well, E.A.; Kenmoe, S.; Sado, F.B.Y.; Weaver, S.C.; Moundipa, P.F.; Demanou, M. Chikungunya Virus Infection Prevalence in Africa: A Contemporaneous Systematic Review and Meta-Analysis. Public Health 2019, 166, 79–88. [Google Scholar] [CrossRef]
- Delynn, M.M.; Matthew, T.W.; Anna, N.C.; Victoria, T.; Seydou, D.; Christin, H.G.; Stevan, B.; Ryan, E.W.; Matthew, C.F.; Patrick, J.L.; et al. Serological Evidence of Dengue and Chikungunya Exposures in Malian Children by Multiplex Bead Assay. Int. J. Trop. Dis. 2018, 1, 7. [Google Scholar] [CrossRef]
- Rashid, M.D.H.O.; Patwary, M.H.; Imtiaz, A.; Abdullah, S.A.; Zahedur, R.; Ibrahim, K.; Zzaman, M.T. Seroprevalence of Chikungunya during Outbreak in Dhaka, Bangladesh in 2017. J. Virol. Antivir. Res. 2018, 7, 2. [Google Scholar] [CrossRef]
- Rodríguez-Barraquer, I.; Solomon, S.S.; Kuganantham, P.; Srikrishnan, A.K.; Vasudevan, C.K.; Iqbal, S.H.; Balakrishnan, P.; Solomon, S.; Mehta, S.H.; Cummings, D.A.T. The Hidden Burden of Dengue and Chikungunya in Chennai, India. PLoS Negl. Trop. Dis. 2015, 9, e0003906. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and Future Spread of the Arbovirus Vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Doumbe-Belisse, P.; Kopya, E.; Ngadjeu, C.S.; Sonhafouo-Chiana, N.; Talipouo, A.; Djamouko-Djonkam, L.; Awono-Ambene, H.P.; Wondji, C.S.; Njiokou, F.; Antonio-Nkondjio, C. Urban Malaria in Sub-Saharan Africa: Dynamic of the Vectorial System and the Entomological Inoculation Rate. Malar. J. 2021, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Salem, O.A.S.M.; Khadijetou, M.L.; Moina, M.H.; Lassana, K.; Sébastien, B.; Ousmane, F.; Ali, O.M.S.B. Characterization of Anopheline (Diptera: Culicidae) Larval Habitats in Nouakchott, Mauritania. J. Vector Borne Dis. 2013, 50, 302–306. [Google Scholar] [PubMed]
- Mfueni Bikundi, E.; Coppieters, Y. Prediction Ability of Vector Species, Environmental Characteristics and Socio-Economic Factors for Malaria Risk in Sub-Saharan African Countries. Int. J. Environ. Health Res. 2022, 32, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Mathiot, C.C.; Grimaud, G.; Garry, P.; Bouquety, J.C.; Mada, A.; Daguisy, A.M.; Georges, A.J. An Outbreak of Human Semliki Forest Virus Infections in Central African Republic. Am. J. Trop. Med. Hyg. 1990, 42, 386–393. [Google Scholar] [CrossRef]
| Variable | No. Tested (%) | Anti-CHIKV IgG Positive n (%; 95% CI) | cOR (95% CI) | p Value |
|---|---|---|---|---|
| Overall | 1300 (100.0) | 37 (2.8; 2.0–3.9) | - | - |
| Sex Female Male | 691 (53.1) 609 (46.8) | 18 (2.6; 1.6–4.1) 19 (3.1; 1.9–4.8) | 1 1.2 (0.6–2.3) | 0.58 |
| Age (yr) 0–<20 20–<35 35–<50 ≥50 | 141 (10.8) 448 (34.5) 345 (26.5) 366 (28.1) | 3 (2.1; 0.4–6.1) 13 (2.9; 1.6–4.9) 6 (1.7; 0.6–3.8) 15 (4.1; 2.3–6.7) | 1 1.4 (0.4–4.9) 1.2 (0.3–5.0) 2.0 (0.6–6.9) | 0.62 0.77 0.29 |
| Residence Northern Nouakchott (NN) Teyarett Dar Naim Toujounin Total in NN Western Nouakchott (WN) Sebkha Tevragh Zeina Ksar Total in WN Southern Nouakchott (SN) Riadh Arafat El Mina Total in SN | 129 96 97 322 144 160 73 377 176 241 184 601 | 2 (1.6; 0.2–5.5) 3 (3.1; 0.7–8.9) 2 (2.1; 0.3–7.3) 7 (2.2; 2.0–7.5) 5 (3.5; 1.1–7.9) 2 (1.3; 0.2–4.4) 2 (2.7; 0.3–9.6) 9 (2.4; 0.7–8.9) 8 (4.5; 2.0–8.8) 10 (4.1; 2.0–7.5) 3 (1.6; 0.3–4.7) 21 (3.5; 0.3–4.7) | 1 1.1 (0.4–3.0) 1.6 (0.7–3.9) | 0.85 0.27 |
| Use of impregnated bed net No Yes | 1050 (80.7) 250 (19.2) | 31 (3.0; 2.0–4.2) 5 (2.4; 0.9–5.2) | 1 1.2 (0.6–2.3) | 0.64 |
| Sleeping under bed net Never Always | 758 (58.3) 542 (41.7) | 19 (2.5; 1.5–3.9) 18 (3.3; 2.0–5.2) | 1 0.8 (0.4–1.4) | 0.39 |
| Repellent use Never Always | 1073 (82.5) 227 (17.5) | 34 (3.2; 2.2–4.4) 3 (1.3; 0.3–3.8) | 1 0.4 (0.1–1.3) | 0.14 |
| Sample No. | District | CHIKV | ONNV | SFV | |||
|---|---|---|---|---|---|---|---|
| Titre | Interpretation | Titre | Interpretation | Titre | Interpretation | ||
| 72 | El Mina | <1:20 | − | 1:80 | + | 1:40 | + |
| 352 | El Mina | 1:640 | + | 1:160 | − | 1:20 | + |
| 1197 | El Mina | <1:20 | − | 1:20 | + | <1:20 | − |
| 212 | Sebkha | <1:20 | − | 1:160 | + | <1:20 | − |
| 232 | Sebkha | <1:20 | − | 1:20 | + | <1:20 | − |
| 235 | Sebkha | 1:20 | − | 1:80 | + | 1:40 | + |
| 1040 | Sebkha | 1:20 | − | 1:80 | + | <1:20 | − |
| 1065 | Sebkha | <1:20 | − | 1:20 | + | <1:20 | − |
| 224 | Riadh | 1:160 | Eq | 1:160 | Eq | <1:20 | − |
| 265 | Riadh | 1:20 | − | 1:160 | + | <1:20 | − |
| 453 | Riadh | 1:1280 | + | 1:160 | − | 1:80 | + |
| 657 | Riadh | 1:80 | Eq | 1:160 | Eq | 1:40 | + |
| 690 | Riadh | 1:160 | Eq | 1:160 | Eq | <1:20 | − |
| 759 | Riadh | 1:80 | Eq | 1:80 | Eq | 1:40 | + |
| 824 | Riadh | 1:40 | − | 1:160 | + | <1:20 | − |
| 1111 | Riadh | 1:320 | Eq | 1:160 | Eq | <1:20 | − |
| 252 | Teyarett | <1:20 | − | <1:20 | − | 1:20 | + |
| 834 | Teyarett | 1:40 | Eq | 1:80 | Eq | <1:20 | − |
| 342 | Arafat | 1:20 | − | 1:160 | + | 1:20 | + |
| 380 | Arafat | 1:160 | Eq | 1:160 | Eq | 1:160 | + |
| 463 | Arafat | <1:20 | − | <1:20 | − | 1:80 | + |
| 599 | Arafat | 1:40 | − | 1:160 | + | 1:40 | + |
| 632 | Arafat | <1:20 | − | 1:40 | + | <1:20 | − |
| 780 | Arafat | 1:40 | Eq | 1:80 | Eq | <1:20 | − |
| 1066 | Arafat | <1:20 | − | 1:20 | + | 1:20 | + |
| 1214 | Arafat | 1:20 | − | 1:80 | + | 1:40 | + |
| 40,487 | Arafat | 1:80 | Eq | 1:160 | Eq | 1:20 | + |
| 584 | Ksar | <1:20 | − | <1:20 | − | 1:20 | + |
| 1295 | Ksar | <1:20 | − | <1:20 | − | 1:40 | + |
| 624 | Tevragh Zeina | <1:20 | − | 1:160 | + | 1:20 | + |
| 873 | Tevragh Zeina | 1:160 | Eq | 1:160 | Eq | 1:80 | + |
| 703 | Toujounin | 1:80 | Eq | 1:160 | Eq | <1:20 | − |
| 949 | Toujounin | <1:20 | − | 1:40 | + | <1:20 | − |
| 943 | Dar Naim | <1:20 | − | 1:40 | + | <1:20 | − |
| 1038 | Dar Naim | <1:20 | − | 1:40 | + | <1:20 | − |
| 1165 | Dar Naim | <1:20 | − | 1:40 | + | <1:20 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdoullah, B.; Durand, G.A.; Basco, L.K.; El Bara, A.; Bollahi, M.A.; Bosio, L.; Geulen, M.; Briolant, S.; Boukhary, A.O.M.S. Seroprevalence of Alphaviruses (Togaviridae) among Urban Population in Nouakchott, Mauritania, West Africa. Viruses 2023, 15, 1588. https://doi.org/10.3390/v15071588
Abdoullah B, Durand GA, Basco LK, El Bara A, Bollahi MA, Bosio L, Geulen M, Briolant S, Boukhary AOMS. Seroprevalence of Alphaviruses (Togaviridae) among Urban Population in Nouakchott, Mauritania, West Africa. Viruses. 2023; 15(7):1588. https://doi.org/10.3390/v15071588
Chicago/Turabian StyleAbdoullah, Bedia, Guillaume André Durand, Leonardo K. Basco, Ahmed El Bara, Mohamed Abdallahi Bollahi, Laurent Bosio, Manon Geulen, Sébastien Briolant, and Ali Ould Mohamed Salem Boukhary. 2023. "Seroprevalence of Alphaviruses (Togaviridae) among Urban Population in Nouakchott, Mauritania, West Africa" Viruses 15, no. 7: 1588. https://doi.org/10.3390/v15071588
APA StyleAbdoullah, B., Durand, G. A., Basco, L. K., El Bara, A., Bollahi, M. A., Bosio, L., Geulen, M., Briolant, S., & Boukhary, A. O. M. S. (2023). Seroprevalence of Alphaviruses (Togaviridae) among Urban Population in Nouakchott, Mauritania, West Africa. Viruses, 15(7), 1588. https://doi.org/10.3390/v15071588






