Virus-Specific T-Cell Therapy for Viral Infections of the Central Nervous System: A Review
Abstract
1. Introduction
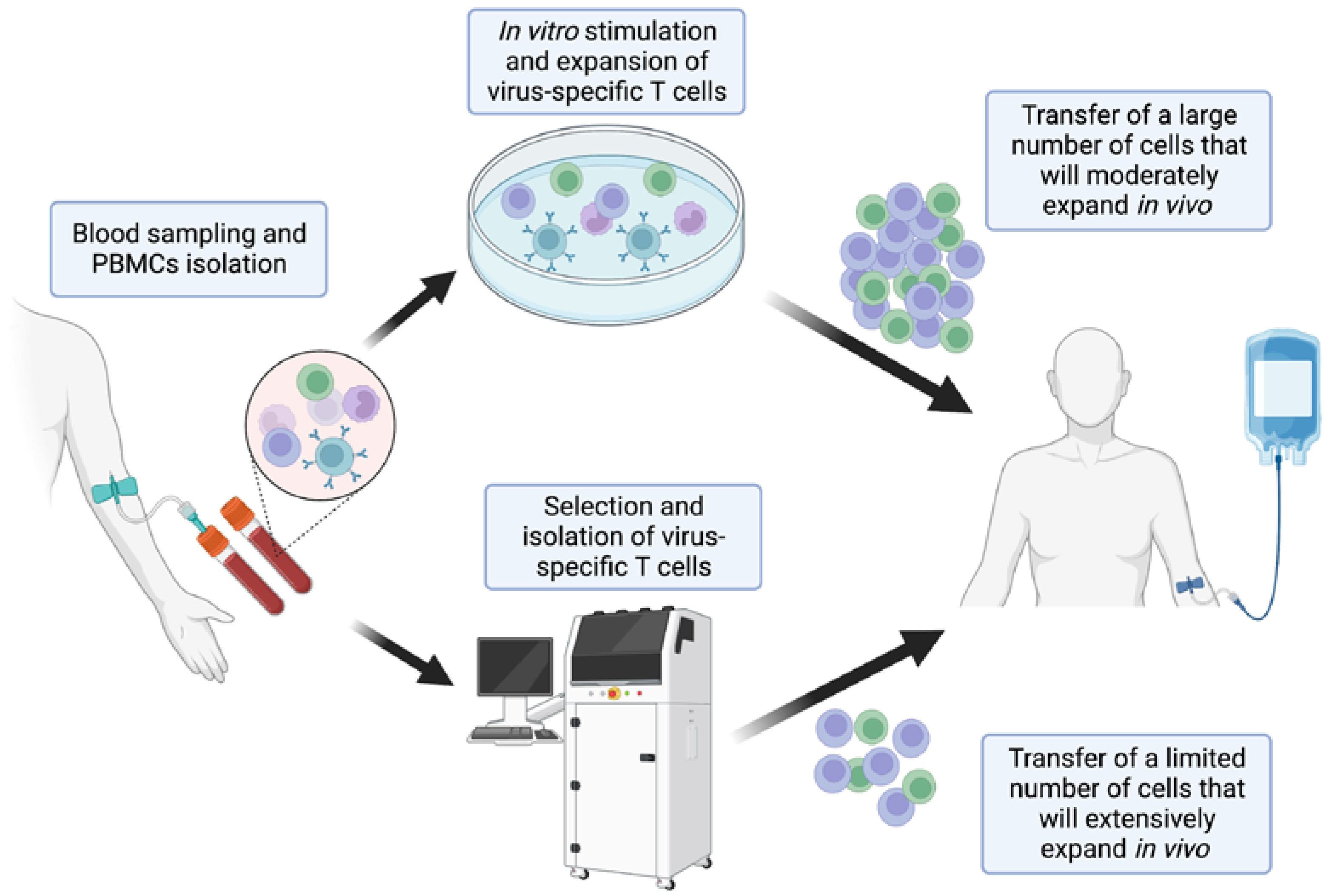
2. Development and Selection of Virus-Specific T-Cells
3. Clinical Experience in CNS Infections
3.1. Progressive Multifocal Leukoencephalopathy
3.2. Human Adenovirus Infection
3.3. Cytomegalovirus Infection
4. EBV-Specific T-Cell Therapy for Multiple Sclerosis
5. Safety
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pruitt, A.A. Central Nervous System Infections in Immunocompromised Patients. Curr. Neurol. Neurosci. Rep. 2021, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Sonneville, R.; Magalhaes, E.; Meyfroidt, G. Central nervous system infections in immunocompromised patients. Curr. Opin. Crit. Care 2017, 23, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Joly, M.; Conte, C.; Cazanave, C.; Le Moing, V.; Tattevin, P.; Delobel, P.; Sommet, A.; Martin-Blondel, G. Progressive multifocal leukoencephalopathy: Epidemiology and spectrum of predisposing conditions. Brain 2023, 146, 349–358. [Google Scholar] [CrossRef]
- Nath, A.; Tyler, K.L. Novel approaches and challenges to treatment of central nervous system viral infections. Ann. Neurol. 2013, 74, 412–422. [Google Scholar] [CrossRef]
- Lambert, N.; El Moussaoui, M.; Maquet, P. Immune checkpoint inhibitors for progressive multifocal leukoencephalopathy: Identifying relevant outcome factors. Eur. J. Neurol. 2021, 28, 3814–3819. [Google Scholar] [CrossRef] [PubMed]
- Mapunda, J.A.; Tibar, H.; Regragui, W.; Engelhardt, B. How Does the Immune System Enter the Brain? Front. Immunol. 2022, 13, 805657. [Google Scholar] [CrossRef]
- Sallusto, F.; Impellizzieri, D.; Basso, C.; Laroni, A.; Uccelli, A.; Lanzavecchia, A.; Engelhardt, B. T-cell trafficking in the central nervous system. Immunol. Rev. 2012, 248, 216–227. [Google Scholar] [CrossRef]
- Korn, T.; Kallies, A. T cell responses in the central nervous system. Nat. Rev. Immunol. 2017, 17, 179–194. [Google Scholar] [CrossRef]
- Papadopoulos, E.B.; Ladanyi, M.; Emanuel, D.; Mackinnon, S.; Boulad, F.; Carabasi, M.H.; Castro-Malaspina, H.; Childs, B.H.; Gillio, A.P.; Small, T.N. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N. Engl. J. Med. 1994, 330, 1185–1191. [Google Scholar] [CrossRef]
- Leen, A.M.; Tripic, T.; Rooney, C.M. Challenges of T cell therapies for virus-associated diseases after hematopoietic stem cell transplantation. Expert Opin. Biol. Ther. 2010, 10, 337–351. [Google Scholar] [CrossRef]
- Walter, E.A.; Greenberg, P.D.; Gilbert, M.J.; Finch, R.J.; Watanabe, K.S.; Thomas, E.D.; Riddell, S.R. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 1995, 333, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Riddell, S.R.; Watanabe, K.S.; Goodrich, J.M.; Li, C.R.; Agha, M.E.; Greenberg, P.D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 1992, 257, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Riddell, S.R.; Greenberg, P.D. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J. Immunol. Methods 1990, 128, 189–201. [Google Scholar] [CrossRef]
- Einsele, H.; Roosnek, E.; Rufer, N.; Sinzger, C.; Riegler, S.; Löffler, J.; Grigoleit, U.; Moris, A.; Rammensee, H.G.; Kanz, L. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 2002, 99, 3916–3922. [Google Scholar] [CrossRef]
- Peggs, K.; Verfuerth, S.; Mackinnon, S. Induction of cytomegalovirus (CMV)-specific T-cell responses using dendritic cells pulsed with CMV antigen: A novel culture system free of live CMV virions. Blood 2001, 97, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Manz, R.; Assenmacher, M.; Pflüger, E.; Miltenyi, S.; Radbruch, A. Analysis and sorting of live cells according to secreted molecules, relocated to a cell-surface affinity matrix. Proc. Natl. Acad. Sci. USA 1995, 92, 1921–1925. [Google Scholar] [CrossRef]
- Brosterhus, H.; Brings, S.; Leyendeckers, H.; Manz, R.A.; Miltenyi, S.; Radbruch, A.; Assenmacher, M.; Schmitz, J. Enrichment and detection of live antigen-specific CD4(+) and CD8(+) T cells based on cytokine secretion. Eur. J. Immunol. 1999, 29, 4053–4059. [Google Scholar] [CrossRef]
- Neill, L.; Peggs, K. Cell therapy for cytomegalovirus infection. Expert Opin. Biol. Ther. 2021, 21, 649–659. [Google Scholar] [CrossRef]
- Gottschalk, S.; Rooney, C.M. Adoptive T-Cell Immunotherapy. In Epstein Barr Virus Volume 2; Springer: Berlin/Heidelberg, Germany, 2015; Volume 391, pp. 427–454. [Google Scholar] [CrossRef]
- Tzannou, I.; Papadopoulou, A.; Naik, S.; Leung, K.; Martinez, C.A.; Ramos, C.A.; Carrum, G.; Sasa, G.; Lulla, P.; Watanabe, A.; et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2017, 35, 3547–3557. [Google Scholar] [CrossRef]
- Olson, A.; Lin, R.; Marin, D.; Rafei, H.; Bdaiwi, M.H.; Thall, P.F.; Basar, R.; Abudayyeh, A.; Banerjee, P.; Aung, F.M.; et al. Third-Party BK Virus-Specific Cytotoxic T Lymphocyte Therapy for Hemorrhagic Cystitis Following Allotransplantation. J. Clin. Oncol. 2021, 39, 2710–2719. [Google Scholar] [CrossRef]
- Peterson, C.W.; Kiem, H.P. Cell and Gene Therapy for HIV Cure. Curr. Top Microbiol. Immunol. 2018, 417, 211–248. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.M.; Saleh, R.; Sasidharan Nair, V.; Taha, R.Z.; Elkord, E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology 2021, 162, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Leen, A.M.; Sun, J.; Nakazawa, Y.; Yvon, E.; Heslop, H.E.; Brenner, M.K.; Rooney, C.M. Exploiting cytokine secretion to rapidly produce multivirus-specific T cells for adoptive immunotherapy. J. Immunother. 2008, 31, 665–674. [Google Scholar] [CrossRef]
- Gerdemann, U.; Vera, J.F.; Rooney, C.M.; Leen, A.M. Generation of multivirus-specific T cells to prevent/treat viral infections after allogeneic hematopoietic stem cell transplant. J. Vis. Exp. 2011, 51, 2736. [Google Scholar] [CrossRef]
- Kállay, K.; Kassa, C.; Réti, M.; Karászi, É.; Sinkó, J.; Goda, V.; Stréhn, A.; Csordás, K.; Horváth, O.; Szederjesi, A.; et al. Early Experience With CliniMACS Prodigy CCS (IFN-gamma) System in Selection of Virus-specific T Cells From Third-party Donors for Pediatric Patients With Severe Viral Infections After Hematopoietic Stem Cell Transplantation. J. Immunother. 2018, 41, 158–163. [Google Scholar] [CrossRef]
- Cortese, I.; Reich, D.S.; Nath, A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat. Rev. Neurol. 2021, 17, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Boumaza, X.; Bonneau, B.; Roos-Weil, D.; Pinnetti, C.; Rauer, S.; Nitsch, L.; Del Bello, A.; Jelcic, I.; Sühs, K.W.; Gasnault, J.; et al. Progressive Multifocal Leukoencephalopathy Treated by Immune Checkpoint Inhibitors. Ann. Neurol. 2023, 93, 257–270. [Google Scholar] [CrossRef]
- Lambert, N.; Dauby, S.; Dive, D.; Sadzot, B.; Maquet, P. Atezolizumab Treatment for Progressive Multifocal Leukoencephalopathy. Emerg. Infect. Dis. 2022, 28, 253–256. [Google Scholar] [CrossRef]
- Lambert, N.; El Moussaoui, M.; Ritacco, C.; Ritacco, C.; Moïse, M.; Paulus, A.; Delvenne, P.; Baron, F.; Sadzot, B.; Maquet, P. Killing Two Birds With One Stone: Effective Control of Both Non-Small Cell Lung Cancer and Progressive Multifocal Leukoencephalopathy with Atezolizumab, A Case Report. Front. Immunol. 2022, 13, 889148. [Google Scholar] [CrossRef]
- Cortese, I.; Muranski, P.; Enose-Akahata, Y.; Ha, S.K.; Smith, B.; Monaco, M.; Ryschkewitsch, C.; Majof, E.O.; Ohayon, J.; Schindler, M.; et al. Pembrolizumab Treatment for Progressive Multifocal Leukoencephalopathy. N. Engl. J. Med. 2019, 380, 1597–1605. [Google Scholar] [CrossRef]
- Lajaunie, R.; Mainardi, I.; Gasnault, J.; Rousseau, V.; Tarantino, A.G.; Sommet, A.; Cinque, P.; Martin-Blondel, G.; PML study group. Outcome of Progressive Multifocal Leukoencephalopathy Treated by Interleukin-7. Ann. Neurol. 2022, 91, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Möhn, N.; Grote-Levi, L.; Hopfner, F.; Eiz-Vesper, B.; Maecker-Kolhoff, B.; Warnke, C.; Sühs, K.W.; Wattjes, M.P.; Höglinger, G.U.; Skripuletz, T. Innovative therapeutic concepts of progressive multifocal leukoencephalopathy. J. Neurol. 2022, 269, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Balduzzi, A.; Lucchini, G.; Hirsch, H.H.; Basso, S.; Cioni, M.; Rovelli, A.; Zincone, A.; Grimaldi, M.; Corti, P.; Bonanomi, S.; et al. Polyomavirus JC-targeted T-cell therapy for progressive multiple leukoencephalopathy in a hematopoietic cell transplantation recipient. Bone Marrow Transpl. 2011, 46, 987–992. [Google Scholar] [CrossRef]
- Muftuoglu, M.; Olson, A.; Marin, D.; Ahmed, S.; Mulanovich, V.; Tummala, S.; Chi, T.L.; Ferrajoli, A.; Kaur, I.; Li, L.; et al. Allogeneic BK Virus-Specific T Cells for Progressive Multifocal Leukoencephalopathy. N. Engl. J. Med. 2018, 379, 1443–1451. [Google Scholar] [CrossRef]
- Krymskaya, L.; Sharma, M.C.; Martinez, J.; Haq, W.; Huang, E.C.; Limaye, A.P.; Diamond, D.J.; Lacey, S.F. Cross-reactivity of T lymphocytes recognizing a human cytotoxic T-lymphocyte epitope within BK and JC virus VP1 polypeptides. J. Virol. 2005, 79, 11170–11178. [Google Scholar] [CrossRef] [PubMed]
- Cantalupo, P.; Doering, A.; Sullivan, C.S.; Pal, A.; Peden, K.W.; Lewis, A.M.; Pipas, J.M. Complete nucleotide sequence of polyomavirus SA12. J. Virol. 2005, 79, 13094–13104. [Google Scholar] [CrossRef]
- Li, J.; Melenhorst, J.; Hensel, N.; Rezvani, K.; Sconocchia, G.; Kilical, Y.; Hou, J.; Curfman, B.; Major, E.; Barrett, A.J. T-cell responses to peptide fragments of the BK virus T antigen: Implications for cross-reactivity of immune response to JC virus. J. Gen. Virol. 2006, 87 Pt 10, 2951–2960. [Google Scholar] [CrossRef]
- Koralnik, I.J.; Du Pasquier, R.A.; Kuroda, M.J.; Schmitz, J.E.; Dang, X.; Zheng, Y.; Lifton, M.; Letvin, N.L. Association of prolonged survival in HLA-A2+ progressive multifocal leukoencephalopathy patients with a CTL response specific for a commonly recognized JC virus epitope. J. Immunol. 2002, 168, 499–504. [Google Scholar] [CrossRef]
- Cortese, I.; Beck, E.S.; Al-Louzi, O.; Ohayon, J.; Andrada, F.; Osuorah, I.; Dwyer, J.; Billioux, B.J.; Dargah-Zada, N.; Schindler, M.K.; et al. BK virus-specific T cells for immunotherapy of progressive multifocal leukoencephalopathy: An open-label, single-cohort pilot study. Lancet Neurol. 2021, 20, 639–652. [Google Scholar] [CrossRef]
- Berzero, G.; Basso, S.; Stoppini, L.; Palermo, A.; Pichiecchio, A.; Paoletti, M.; Lucev, F.; Gerevini, S.; Rossi, A.; Vegezzi, E.; et al. Adoptive Transfer of JC Virus-Specific T Lymphocytes for the Treatment of Progressive Multifocal Leukoencephalopathy. Ann. Neurol. 2021, 89, 769–779. [Google Scholar] [CrossRef]
- Wicklein, R.; Heidegger, S.; Verbeek, M.; Eiz-Vesper, B.; Maecker-Kolhoff, B.; Kirschke, J.S.; Page, A.; Korn, T.; Hemmer, B.; Deschauer, M. Combined Treatment With Pembrolizumab and Allogenic BK Virus-Specific T Cells in Progressive Multifocal Leukoencephalopathy: A Case Report. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1042. [Google Scholar] [CrossRef]
- Hopfner, F.; Möhn, N.; Eiz-Vesper, B.; Ahmed, S.; Mulanovich, V.; Tummala, S.; Chi, T.L.; Ferrajoli, A.; Kaur, I.; Li, L.; et al. Allogeneic BK Virus-Specific T-Cell Treatment in 2 Patients With Progressive Multifocal Leukoencephalopathy. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1020. [Google Scholar] [CrossRef]
- Steinhardt, M.J.; Wiercinska, E.; Pham, M.; Grigoleit, G.U.; Mazzoni, A.; Da-Via, M.; Zhou, X.; Meckel, K.; Nickel, K.; Duell, J.; et al. Progressive multifocal leukoencephalopathy in a patient post allo-HCT successfully treated with JC virus specific donor lymphocytes. J. Transl. Med. 2020, 18, 177. [Google Scholar] [CrossRef]
- Rubinstein, J.D.; Jodele, S.; Heyenbruch, D.; Wilhelm, J.; Thomas, S.; Lutzko, C.; Zhu, X.; Leemhuis, T.; Cancelas, J.A.; Keller, M.; et al. Off-the-Shelf Third-Party Virus-Specific T Cell Therapy to Treat JC Polyomavirus Infection in Hematopoietic Stem Cell Transplantation Recipients. Transpl. Cell Ther. 2022, 28, e1–e116. [Google Scholar] [CrossRef]
- Peghin, M.; Castaldo, N.; Tascini, C.; Bassetti, M.; Graziano, E.; Givone, F.; Savignano, C.; De Colle, M.C.; Bove, T.; Pipan, C.; et al. Successful JC virus-targeted T-cell therapy for progressive multifocal leukoencephalopathy in a lung transplant recipient. J. Heart Lung Transpl. 2022, 41, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Jiang, Z.; Ma, Q.; Liu, Q.; Lu, X.; Liu, W.; Liao, X.; Zhou, R.; Su, X.; Luo, Q. Prevalence of neutralizing antibodies to common respiratory viruses in intravenous immunoglobulin and in healthy donors in southern China. J. Thorac. Dis. 2016, 8, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Lion, T. Adenovirus persistence, reactivation, and clinical management. FEBS Lett. 2019, 593, 3571–3582. [Google Scholar] [CrossRef]
- Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef]
- Sedláček, P.; Petterson, T.; Robin, M.; Sivaprakasam, P.; Vainorius, E.; Brundage, T.; Chandak, A.; Mozaffari, E.; Nichols, G.; Voigt, S. Incidence of Adenovirus Infection in Hematopoietic Stem Cell Transplantation Recipients: Findings from the Advance Study. Biol. Blood Marrow Transpl. 2019, 25, 810–818. [Google Scholar] [CrossRef]
- Cesaro, S.; Porta, F. Adenovirus Infection in Pediatric Hematopoietic Cell Transplantation: A Challenge Still Open for Survival. J. Clin. Med. 2022, 11, 4827. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, A.M.; Champlin, R.E.; Mirza, N.; Gajewski, J.; Giralt, S.; Rolston, K.V.; Raad, I.; Jacobson, K.; Kontoyiannis, D.; Elting, L.; et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin. Infect. Dis. 2001, 32, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.L.; Richardson, S.E.; MacGregor, D.; Mahant, S.; Raghuram, K.; Bitnun, A. Adenovirus-Associated Central Nervous System Disease in Children. J. Pediatr. 2019, 205, 130–137. [Google Scholar] [CrossRef]
- Campbell, D.; Wong, G.S.; Park, H.; McLeod, G. An Adult Case of Adenovirus-Associated Acute Disseminated Encephalomyelitis. Case Rep. Infect Dis. 2023, 2023, 5528198. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, S.L.; Chen, S.P.; Huang, Y.L.; Huang, C.G.; Tsao, K.C.; Lin, T.Y. Adenovirus infection associated with central nervous system dysfunction in children. J. Clin. Virol. 2013, 57, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Matthes-Martin, S.; Boztug, H.; Lion, T. Diagnosis and treatment of adenovirus infection in immunocompromised patients. Expert Rev. Anti. Infect Ther. 2013, 11, 1017–1028. [Google Scholar] [CrossRef]
- Kampmann, B.; Cubitt, D.; Walls, T.; Naik, P.; Depala, M.; Samarasinghe, S.; Robson, D.; Hassan, A.; Rao, K.; Gaspar, H.; et al. Improved outcome for children with disseminated adenoviral infection following allogeneic stem cell transplantation. Br. J. Haematol. 2005, 130, 595–603. [Google Scholar] [CrossRef]
- Hiwarkar, P.; Kosulin, K.; Cesaro, S.; Mikulska, M.; Styczynski, J.; Wynn, R.; Lion, T. Management of adenovirus infection in patients after haematopoietic stem cell transplantation: State-of-the-art and real-life current approach: A position statement on behalf of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation. Rev. Med. Virol. 2018, 28, e1980. [Google Scholar] [CrossRef]
- Lopez, S.M.C.; Michaels, M.G.; Green, M. Adenovirus infection in pediatric transplant recipients: Are effective antiviral agents coming our way? Curr. Opin. Organ Transpl. 2018, 23, 395–399. [Google Scholar] [CrossRef]
- Studahl, M.; Lindquist, L.; Eriksson, B.M.; Günther, G.; Bengner, M.; Franzen-Röhl, E.; Fohlman, J.; Bergström, T.; Aurelius, E. Acute viral infections of the central nervous system in immunocompetent adults: Diagnosis and management. Drugs 2013, 73, 131–158. [Google Scholar] [CrossRef]
- Hiwarkar, P.; Amrolia, P.; Sivaprakasam, P.; Lum, S.H.; Doss, H.; O’Rafferty, C.; Petterson, T.; Patrick, K.; Silva, J.; Slatter, M.; et al. Brincidofovir is highly efficacious in controlling adenoviremia in pediatric recipients of hematopoietic cell transplant. Blood 2017, 129, 2033–2037. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Nichols, W.G.; Marty, F.M.; Papanicolaou, G.A.; Brundage, T.M.; Lanier, R.; Zerr, D.M.; Boeckh, M.J. Oral brincidofovir decreases the incidence of HHV-6B viremia after allogeneic HCT. Blood 2020, 135, 1447–1451. [Google Scholar] [CrossRef]
- Hromas, R.; Clark, C.; Blanke, C.; Tricot, G.; Cornetta, K.; Hedderman, A.; Broun, E.R. Failure of ribavirin to clear adenovirus infections in T cell-depleted allogeneic bone marrow transplantation. Bone Marrow Transpl. 1994, 14, 663–664. [Google Scholar]
- Howard, D.S.; Phillips, I.I.G.L.; Reece, D.E.; Munn, R.K.; Henslee-Downey, J.; Pittard, M.; Barker, M.; Pomeroy, C. Adenovirus infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 1999, 29, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Mautner, V.; Osman, H.; Collingham, K.E.; Fegan, C.D.; Klapper, P.E.; Moss, P.A.H.; Milligan, D.W. Adenovirus infections following allogeneic stem cell transplantation: Incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood 2002, 100, 1619–1627. [Google Scholar] [CrossRef]
- Leen, A.M.; Rooney, C.M. Adenovirus as an emerging pathogen in immunocompromised patients. Br. J. Haematol. 2005, 128, 135–144. [Google Scholar] [CrossRef]
- Feuchtinger, T.; Lang, P.; Hamprecht, K.; Schumm, M.; Greil, J.; Jahn, G.; Niethammer, D.; Einsele, H. Isolation and expansion of human adenovirus-specific CD4+ and CD8+ T cells according to IFN-gamma secretion for adjuvant immunotherapy. Exp. Hematol. 2004, 32, 282–289. [Google Scholar] [CrossRef]
- Feuchtinger, T.; Matthes-Martin, S.; Richard, C.; Lion, T.; Fuhrer, M.; Hamprecht, K.; Handgretinger, R.; Peters, C.; Schuster, F.R.; Beck, R.; et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br. J. Haematol. 2006, 134, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Feucht, J.; Opherk, K.; Lang, P.; Kayser, S.; Hartl, L.; Bethge, W.; Matthes-Martin, S.; Bader, P.; Albert, M.H.; Maecker-Kolhoff, B.; et al. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after, HSCT. Blood 2015, 125, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Campidelli, A.; Wang, Y.; Cai, H.; Venard, V.; Jeulin, H.; Dalle, J.H.; Pochon, C.; D’aveni, M.; Bruno, B.; et al. Curative or pre-emptive adenovirus-specific T cell transfer from matched unrelated or third party haploidentical donors after HSCT, including UCB transplantations: A successful phase I/II multicenter clinical trial. J. Hematol. Oncol. 2017, 10, 102. [Google Scholar] [CrossRef]
- Rubinstein, J.D.; Zhu, X.; Leemhuis, T.; Pham, G.; Ray, L.; Emberesh, S.; Jodele, S.; Thomas, S.; Cancelas, J.A.; Bollard, C.M.; et al. Virus-specific T cells for adenovirus infection after stem cell transplantation are highly effective and class II HLA restricted. Blood Adv. 2021, 5, 3309–3321. [Google Scholar] [CrossRef]
- Bollard, C.M.; Rooney, C.M.; Heslop, H.E. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat. Rev. Clin. Oncol. 2012, 9, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Leen, A.M.; Bollard, C.M.; Mendizabal, A.M.; Shpall, E.J.; Szabolcs, P.; Antin, J.H.; Kapoor, N.; Pai, S.-Y.; Rowley, S.D.; Kebriaei, P.; et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 2013, 121, 5113–5123. [Google Scholar] [CrossRef]
- Ip, W.; Silva, J.M.F.; Gaspar, H.; Mitra, A.; Patel, S.; Rao, K.; Chiesa, R.; Amrolia, P.; Gilmour, K.; Ahsan, G.; et al. Multicenter phase 1/2 application of adenovirus-specific T cells in high-risk pediatric patients after allogeneic stem cell transplantation. Cytotherapy 2018, 20, 830–838. [Google Scholar] [CrossRef]
- Creidy, R.; Moshous, D.; Touzot, F.; Elie, C.; Neven, B.; Gabrion, A.; Leruez-Ville, M.; Maury, S.; Ternaux, B.; Nisoy, J.; et al. Specific T cells for the treatment of cytomegalovirus and/or adenovirus in the context of hematopoietic stem cell transplantation. J. Allergy Clin. Immunol. 2016, 138, 920–924.e3. [Google Scholar] [CrossRef] [PubMed]
- Humar, A.; Snydman, D. AST Infectious Diseases Community of Practice. Cytomegalovirus in solid organ transplant recipients. Am. J. Transpl. 2009, 9 (Suppl. S4), S78–S86. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.; Briggs, D.; Craddock, C.; Mahendra, P.; Milligan, D.; Fegan, C.; Darbyshire, P.; Lawson, S.; Boxall, E.; Moss, P.; et al. Donor KIR genotype has a major influence on the rate of cytomegalovirus reactivation following T-cell replete stem cell transplantation. Blood 2006, 107, 1230–1232. [Google Scholar] [CrossRef]
- Schwarcz, L.; Chen, M.J.; Vittinghoff, E.; Hsu, L.; Schwarcz, S. Declining incidence of AIDS-defining opportunistic illnesses: Results from 16 years of population-based AIDS surveillance. AIDS 2013, 27, 597–605. [Google Scholar] [CrossRef]
- Bowen, E.F.; Wilson, P.; Cope, A.; Sabin, C.; Griffiths, P.; Davey, C.; Johnson, M.; Emery, V. Cytomegalovirus retinitis in AIDS patients: Influence of cytomegaloviral load on response to ganciclovir, time to recurrence and survival. AIDS 1996, 10, 1515–1520. [Google Scholar] [CrossRef]
- Einsele, H.; Ljungman, P.; Boeckh, M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood 2020, 135, 1619–1629. [Google Scholar] [CrossRef]
- Ueda Oshima, M.; Xie, H.; Zamora, D.; Flowers, M.E.; Hill, G.R.; Mielcarek, M.B.; Sandmaier, B.M.; Gooley, T.A.; Boeckh, M.J. Impact of GVHD prophylaxis on CMV reactivation and disease after HLA-matched peripheral blood stem cell transplantation. Blood Adv. 2023, 7, 1394–1403. [Google Scholar] [CrossRef]
- Roemhild, A.; Reinke, P. Virus-specific T-cell therapy in solid organ transplantation. Transpl. Int. 2016, 29, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.M.; Winston, D.J.; Territo, M.C.; Schiller, G.J. CMV central nervous system disease in stem-cell transplant recipients: An increasing complication of drug-resistant CMV infection and protracted immunodeficiency. Bone Marrow Transpl. 2010, 45, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Maschke, M.; Kastrup, O.; Diener, H.C. CNS manifestations of cytomegalovirus infections: Diagnosis and treatment. CNS Drugs 2002, 16, 303–315. [Google Scholar] [CrossRef]
- Schmidt-Hieber, M.; Schwender, J.; Heinz, W.J.; Zabelina, T.; Kühl, J.S.; Mousset, S.; Schüttrumpf, S.; Junghanss, C.; Silling, G.; Basara, N.; et al. Viralence-phalitis after allogeneic stem cell transplantation: A rare complication with distinct characteristics of different causative agents. Haematologica 2011, 96, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Wang, S.C.; Chen, Y.C. Antiviral Agents as Therapeutic Strategies Against Cytomegalovirus Infections. Viruses 2019, 12, 21. [Google Scholar] [CrossRef]
- Baghban, A.; Malinis, M. Ganciclovir and foscarnet dual-therapy for cytomegalovirus encephalitis: A case report and review of the literature. J. Neurol. Sci. 2018, 15, 28–36. [Google Scholar] [CrossRef]
- Limaye, A.P.; Babu, T.M.; Boeckh, M. Progress and Challenges in the Prevention, Diagnosis, and Management of Cytomegalovirus Infection in Transplantation. Clin. Microbiol. Rev. 2020, 34, e00043-19. [Google Scholar] [CrossRef]
- Shigle, T.L.; Handy, V.W.; Chemaly, R.F. Letermovir and its role in the prevention of cytomegalovirus infection in seropositive patients receiving an allogeneic hematopoietic cell transplant. Ther. Adv. Hematol. 2020, 11, 2040620720937150. [Google Scholar] [CrossRef]
- Hantz, S.; Garnier-Geoffroy, F.; Mazeron, M.C.; Garrigue, I.; Merville, P.; Mengelle, C.; Rostaing, L.; Saint Marcoux, F.; Essig, M.; Rerolle, J.-P.; et al. Drug-resistant cytomegalovirus in transplant recipients: A French cohort study. J. Antimicrob. Chemother. 2010, 65, 2628–2640. [Google Scholar] [CrossRef]
- Shmueli, E.; Or, R.; Shapira, M.Y.; Resnick, I.B.; Caplan, O.; Bdolah-Abram, T.; Wolf, D.G. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J. Infect. Dis. 2014, 209, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, C.P. Adoptive Immunotherapy for Prophylaxis and Treatment of Cytomegalovirus Infection. Viruses 2022, 14, 2370. [Google Scholar] [CrossRef]
- Feuchtinger, T.; Opherk, K.; Bethge, W.A.; Topp, M.S.; Schuster, F.R.; Weissinger, E.M.; Mohty, M.; Or, R.; Maschan, M.; Schumm, M.; et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood 2010, 116, 4360–4367. [Google Scholar] [CrossRef]
- Pei, X.Y.; Zhao, X.Y.; Liu, X.F.; Mo, X.-D.; Lv, M.; Xu, L.-P.; Wang, Y.; Chang, Y.-J.; Zhang, X.-H.; Liu, K.-Y.; et al. Adoptive therapy with cytomegalovirus-specific T cells for cytomegalovirus infection after haploidentical stem cell transplantation and factors affecting efficacy. Am. J. Hematol. 2022, 97, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, U.; Yang, C.; Wang, C.; Zhang, X.; Li, Y.; Li, C.; Wen, F.; Liu, S. Cytomegalovirus (CMV)-specific cytotoxic T lymphocyte therapy resolve CMV diseases and refractory CMV infections in paediatric recipients of allogeneic haematopoietic stem cell transplantation. Bone Marrow Transpl. 2022, 57, 271–275. [Google Scholar] [CrossRef]
- Withers, B.; Blyth, E.; Clancy, L.E.; Yong, A.; Fraser, C.; Burgess, J.; Simms, R.; Brown, R.; Kliman, D.; Dubosq, M.-C.; et al. Long-term control of recurrent or refractory viral infections after allogeneic HSCT with third-party virus-specific T cells. Blood Adv. 2017, 1, 2193–2205. [Google Scholar] [CrossRef]
- Smith, C.; Beagley, L.; Rehan, S.; Neller, M.A.; Crooks, P.; Solomon, M.; Holmes-Liew, C.-L.; Holmes, M.; McKenzie, S.C.; Hopkins, P.; et al. Autologous Adoptive T-cell Therapy for Recurrent or Drug-resistant Cytomegalovirus Complications in Solid Organ Transplant Recipients: A Single-arm Open-label Phase I Clinical Trial. Clin. Infect. Dis. 2019, 68, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Prockop, S.E.; Hasan, A.; Doubrovina, E.; Dahi, P.B.; Rodriguez-Sanchez, I.; Curry, M.; Mauguen, A.; Papanicolaou, G.A.; Su, Y.; Yao, J.; et al. Third-party cytomegalovirus-specific T cells improved survival in refractory cytomegalovirus viremia after hematopoietic transplant. J. Clin. Invest. 2023, 133, e165476. [Google Scholar] [CrossRef]
- Alonso, L.; Rudilla, F.; Gimeno, R.; Codinach, M.; Blanco, M.; Querol, S.; Diaz de Heredia, C. Successful treatment of post-transplant CMV meningoencephalitis with third-party CMV virus-specific T cells: Lessons learned. Pediatr. Transpl. 2019, 23, e13584. [Google Scholar] [CrossRef]
- Ke, P.; Bao, X.; Zhou, J.; Li, X.; Zhuang, J.; He, X.; Wu, D.; Zhang, X.; Ma, X. Donor CMV-specific cytotoxic T lymphocytes successfully treated drug-resistant cytomegalovirus encephalitis after allogeneic hematopoietic stem cell transplantation. Hematology 2020, 25, 43–47. [Google Scholar] [CrossRef]
- Micklethwaite, K.; Hansen, A.; Foster, A.; Snape, E.; Antonenas, V.; Sartor, M.; Shaw, P.; Bradstock, K.; Gottlieb, D. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 2007, 13, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, M.; Lidehall, A.K.; Totterman, T.H.; Herrman, B.; Eriksson, B.M.; Korsgren, O. Cellular responses to cytomegalovirus in immunosuppressed patients: Circulating CD8+ T cells recognizing CMVpp65 are present but display functional impairment. Clin. Exp. Immunol. 2003, 132, 96–104. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Soldan, S.S.; Lieberman, P.M. Epstein-Barr virus and multiple sclerosis. Nat. Rev. Microbiol. 2023, 21, 51–64. [Google Scholar] [CrossRef]
- Wong, Y.; Meehan, M.T.; Burrows, S.R.; Doolan, D.L.; Miles, J.J. Estimating the global burden of Epstein-Barr virus-related cancers. J. Cancer Res. Clin. Oncol. 2022, 148, 31–46. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Khanna, R. Adoptive T-cell therapy targeting Epstein-Barr virus as a treatment for multiple sclerosis. Clin. Transl. Immunol. 2023, 12, e1444. [Google Scholar] [CrossRef]
- Thorley-Lawson, D.A.; Hawkins, J.B.; Tracy, S.I.; Shapiro, M. The pathogenesis of Epstein-Barr virus persistent infection. Curr. Opin. Virol. 2013, 3, 227–232. [Google Scholar] [CrossRef]
- Arvin, A.; Campadelli-Fiume, G.; Mocarski, E.; Moore, P.S.; Roizman, B.; Whitley, R.; Yamanishi, K. (Eds.) Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Young, L.; Alfieri, C.; Hennessy, K.; Evans, H.; O’Hara, C.; Anderson, K.C.; Ritz, J.; Shapiro, R.S.; Rickinson, A.; Kieff, E. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N. Engl. J. Med. 1989, 321, 1080–1085. [Google Scholar] [CrossRef]
- Hislop, A.D.; Taylor, G.S.; Sauce, D.; Rickinson, A.B. Cellular responses to viral infection in humans: Lessons from Epstein-Barr virus. Ann. Rev. Immunol. 2007, 25, 587–617. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Burrows, S.R. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Ann. Rev. Microbiol. 2000, 54, 19–48. [Google Scholar] [CrossRef]
- Smith, C.; Beagley, L.; Khanna, R. Acquisition of polyfunctionality by Epstein-Barr virus-specific CD8+ T cells correlates with increased resistance to galectin-1-mediated suppression. J. Virol. 2009, 83, 6192–6198. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Serafini, B.; Rosicarelli, B.; Franciotta, D.; Magliozzi, R.; Reynolds, R.; Cinque, P.; Andreoni, L.; Trivedi, P.; Salvetti, M.; Faggioni, A.; et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 2007, 204, 2899–2912. [Google Scholar] [CrossRef]
- Aloisi, F.; Serafini, B.; Magliozzi, R.; Howell, O.W.; Reynolds, R. Detection of Epstein-Barr virus and B-cell follicles in the multiple sclerosis brain: What you find depends on how and where you look. Brain 2010, 133 Pt 12, e157. [Google Scholar] [CrossRef]
- Magliozzi, R.; Serafini, B.; Rosicarelli, B.; Chiappetta, G.; Veroni, C.; Reynolds, R.; Aloisi, F. B-cell enrichment and Epstein-Barr virus infection in inflammatory cortical lesions in secondary progressive multiple sclerosis. J. Neuropathol. Exp. Neurol. 2013, 72, 29–41. [Google Scholar] [CrossRef]
- Serafini, B.; Severa, M.; Columba-Cabezas, S.; Rosicarelli, B.; Veroni, C.; Chiappetta, G.; Magliozzi, R.; Reynolds, R.; Coccia, E.M.; Aloisi, F. Epstein-Barr virus latent infection and BAFF expression in B cells in the multiple sclerosis brain: Implications for viral persistence and intrathecal B-cell activation. J. Neuropathol. Exp. Neurol. 2010, 69, 677–693. [Google Scholar] [CrossRef]
- Hassani, A.; Corboy, J.R.; Al-Salam, S.; Khan, G. Epstein-Barr virus is present in the brain of most cases of multiple sclerosis and may engage more than just B cells. PLoS ONE 2018, 13, e0192109. [Google Scholar] [CrossRef]
- Moreno, M.A.; Or-Geva, N.; Aftab, B.T.; Khanna, R.; Croze, E.; Steinman, L.; Han, M.H. Molecular signature of Epstein-Barr virus infection in MS brain lesions. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e466. [Google Scholar] [CrossRef]
- Bar-Or, A.; Pender, M.P.; Khanna, R.; Steinman, L.; Hartung, H.-P.; Maniar, T.; Croze, E.; Aftab, B.T.; Giovannoni, G.; Joshi, M.A. Epstein-Barr Virus in Multiple Sclerosis: Theory and Emerging Immunotherapies. Trends Mol. Med. 2021, 27, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Pender, M.P.; Khanna, R. Epstein-Barr virus-specific adoptive immunotherapy: A new horizon for multiple sclerosis treatment? Immunotherapy 2014, 6, 659–661. [Google Scholar] [CrossRef]
- Smith, C.; Cooper, L.; Burgess, M.; Rist, M.; Webb, N.; Lambley, E.; Tellam, J.; Marlton, P.; Seymour, J.F.; Gandhi, M.; et al. Functional reversion of antigen-specific CD8+ T cells from patients with Hodgkin lymphoma following in vitro stimulation with recombinant polyepitope. J. Immunol. 2006, 177, 4897–4906. [Google Scholar] [CrossRef]
- Smith, C.; Økern, G.; Rehan, S.; Beagley, L.; Lee, S.K.; Aarvak, T.; Schjetne, K.W.; Khanna, R. Ex vivo expansion of human T cells for adoptive immunotherapy using the novel Xeno-free CTS Immune Cell Serum Replacement. Clin. Transl. Immunol. 2015, 4, e31. [Google Scholar] [CrossRef]
- Pender, M.P.; Csurhes, P.A.; Smith, C.; Douglas, N.L.; Neller, M.A.; Matthews, K.K.; Beagley, L.; Rehan, S.; Crooks, P.; Hopkins, T.J.; et al. Epstein-Barr virus-specific T cell therapy for progressive multiple sclerosis. JCI Insight 2018, 3, e124714, Corrected in JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Smith, C.; Lee, V.; Schuessler, A.; Beagley, L.; Rehan, S.; Tsang, J.; Li, V.; Tiu, R.; Smith, D.; Neller, M.A.; et al. Pre-emptive and therapeutic adoptive immunotherapy for nasopharyngeal carcinoma: Phenotype and effector function of T cells impact on clinical response. Oncoimmunology 2017, 6, e1273311. [Google Scholar] [CrossRef] [PubMed]
- Pender, M.P.; Csurhes, P.A.; Smith, C.; Beagley, L.; Hooper, K.D.; Raj, M.; Coulthard, A.; Burrows, S.R.; Khanna, R. Epstein-Barr virus-specific adoptive immunotherapy for progressive multiple sclerosis. Mult. Scler. 2014, 20, 1541–1544. [Google Scholar] [CrossRef]
- Ioannides, Z.A.; Csurhes, P.A.; Douglas, N.L.; Mackenroth, G.; Swayne, A.; Thompson, K.M.; Hopkins, T.J.; Green, K.A.; Blum, S.; Hooper, K.D.; et al. Sustained Clinical Improvement in a Subset of Patients With Progressive Multiple Sclerosis Treated With Epstein-Barr Virus-Specific T Cell Therapy. Front. Neurol. 2021, 12, 652811. [Google Scholar] [CrossRef]
- Pender, M.P.; Hodgkinson, S.J.; Broadley, S.; Lindsey, J.W.; Ioannides, Z.A.; Bagert, B.; Gamelin, L.; Liu, E.; Ye, W.; Willmer, J.; et al. Updated open-label extension clinical data and new magnetization transfer ratio imaging data from a phase I study of ATA188, an off-the-shelf, allogeneic Epstein-Barr virus-targeted T-cell immunotherapy for progressive multiple sclerosis. Mult. Scler. J. 2022, 28, 72. [Google Scholar]
- Simmons, H.Z.; Bazzell, A.F.; Dains, J.E. Adverse Effects of Virus-Specific T-Cell Therapy: An Integrative Review. J. Adv. Pract. Oncol. 2019, 10, 120–131. [Google Scholar]
- Cruz, C.R.; Hanley, P.J.; Liu, H.; Torrano, V.; Lin, Y.F.; Arce, J.A.; Gottschalk, S.; Savoldo, B.; Dotti, G.; Louis, C.U.; et al. Adverse events following infusion of T cells for adoptive immunotherapy: A 10-year experience. Cytotherapy 2010, 12, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Rooney, C.M.; Smith, C.A.; Ng, C.Y.; Loftin, S.; Li, C.; Krance, R.A.; Brenner, M.K.; Heslop, H.E. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virusrelated lymphoproliferation. Lancet 1995, 345, 9–13. [Google Scholar] [CrossRef]
- Peggs, K.S.; Verfuerth, S.; Pizzey, A.; Khan, N.; Guiver, M.; Moss, P.A.; Mackinnon, S. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet 2003, 362, 1375–1377. [Google Scholar] [CrossRef]
- Leen, A.M.; Christin, A.; Myers, G.D.; Liu, H.; Cruz, C.R.; Hanley, P.J.; Kennedy-Nasser, A.A.; Leung, K.S.; Gee, A.P.; Krance, R.A.; et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood 2009, 114, 4283–4292. [Google Scholar] [CrossRef] [PubMed]
- Houghtelin, A.; Bollard, C.M. Virus-Specific T Cells for the Immunocompromised Patient. Front. Immunol. 2017, 8, 1272. [Google Scholar] [CrossRef]
- Melenhorst, J.J.; Castillo, P.; Hanley, P.J.; Keller, M.D.; Krance, R.A.; Margolin, J.; Leen, A.M.; Heslop, H.E.; Barrett, A.J.; Rooney, C.M.; et al. Graft versus leukemia response without graft-versus-host disease elicited by adoptively transferred multivirus-specific T-cells. Mol. Ther. 2015, 23, 179–183. [Google Scholar] [CrossRef]
- Melenhorst, J.J.; Scheinberg, P.; Williams, A.; Ambrozak, D.R.; Keyvanfar, K.; Smith, M.; McCoy, J.P., Jr.; Hensel, N.F.; Douek, D.C.; Barrett, A.J. Alloreactivity across HLA barriers is mediated by both naïve and antigen-experienced T cells. Biol. Blood Marrow Transpl. 2011, 17, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Koehne, G.; Hasan, A.; Doubrovina, E.; Prockop, S.; Tyler, E.; Wasilewski, G.; O’Reilly, R.J. Immunotherapy with Donor T Cells Sensitized with Overlapping Pentadecapeptides for Treatment of Persistent Cytomegalovirus Infection or Viremia. Biol. Blood Marrow Transpl. 2015, 21, 1663–1678. [Google Scholar] [CrossRef] [PubMed]
- Peggs, K.S.; Thomson, K.; Samuel, E.; Dyer, G.; Armoogum, J.; Chakraverty, R.; Pang, K.; Mackinnon, S.; Lowdell, M.W. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin. Infect. Dis. 2011, 52, 49–57. [Google Scholar] [CrossRef]
- Fuji, S.; Kapp, M.; Einsele, H. Alloreactivity of virus-specific T cells: Possible implication of graft-versus-host disease and graft-versus-leukemia effects. Front. Immunol. 2013, 4, 330. [Google Scholar] [CrossRef]
- Melenhorst, J.J.; Leen, A.M.; Bollard, C.M.; Quigley, M.F.; Price, D.A.; Rooney, C.M.; Brenner, M.K.; Barrett, A.J.; Heslop, H.E. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood 2010, 116, 4700–4702. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Gerdemann, U.; Katari, U.L.; Tzannou, I.; Liu, H.; Martinez, C.; Leung, K.; Carrum, G.; Gee, A.P.; Vera, J.F.; et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci. Transl. Med. 2014, 6, 242ra83. [Google Scholar] [CrossRef] [PubMed]
- Gallot, G.; Vollant, S.; Saïagh, S.; Clémenceau, B.; Vivien, R.; Cerato, E.; Bignon, J.D.; Ferrand, C.; Jaccard, A.; Vigouroux, S.; et al. T-cell therapy using a bank of EBV-specific cytotoxic T cells: Lessons from a phase I/II feasibility and safety study. J. Immunother. 2014, 37, 170–179. [Google Scholar] [CrossRef]
- Barker, J.N.; Doubrovina, E.; Sauter, C.; Jaroscak, J.J.; Perales, M.A.; Doubrovin, M.; Prockop, S.E.; Koehne, G.; O’Reilly, R.J. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood 2010, 116, 5045–5049. [Google Scholar] [CrossRef]
- Foster, A.E.; Dotti, G.; Lu, A.; Khalil, M.; Brenner, M.K.; Heslop, H.E.; Rooney, C.M.; Bollard, C.M. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J. Immunother. 2008, 31, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef]
- Menger, L.; Gouble, A.; Marzolini, M.A.; Pachnio, A.; Bergerhoff, K.; Henry, J.Y.; Smith, J.; Pule, M.; Moss, P.; Riddell, S.R.; et al. TALEN-mediated genetic inactivation of the glucocorticoid receptor in cytomegalovirus-specific T cells. Blood 2015, 126, 2781–2789. [Google Scholar] [CrossRef]
- Ricciardelli, I.; Brewin, J.; Lugthart, G.; Albon, S.J.; Pule, M.; Amrolia, P.J. Rapid generation of EBV-specific cytotoxic T lymphocytes resistant to calcineurin inhibitors for adoptive immunotherapy. Am. J. Transpl. 2013, 13, 3244–3252. [Google Scholar] [CrossRef]
- De Angelis, B.; Dotti, G.; Quintarelli, C.; Huye, L.E.; Zhang, L.; Zhang, M.; Pane, F.; Heslop, H.E.; Brenner, M.K.; Rooney, C.M.; et al. Generation of Epstein-Barr virus-specific cytotoxic T lymphocytes resistant to the immunosuppressive drug tacrolimus (FK506). Blood 2009, 114, 4784–4791. [Google Scholar] [CrossRef]

| Article | Age (Years), Sex | Underlying Immune Deficiency | Virus Targeted by the Cells | Autologous or Allogeneic Cells | Number of Infusions | HLA Matching | Associated Treatments | Complications | Clinical Outcome Regarding PML |
|---|---|---|---|---|---|---|---|---|---|
| Balduzzi et al., 2011 [34] | 14, M | Acute lymphoblastic leukemia | HPyV2 | Allogeneic (HSCT donor) | 2 | 10/10 | Cidofovir, citalopram | None | Improvement |
| Muftuoglu et al., 2018 [35] | 32, F | Acute myeloblastic leukemia | HPyV1 | Allogeneic | 3 | 5/10 | Mirtazapine (stopped) | None | Improvement |
| Muftuoglu et al., 2018 [35] | 73, F | Polycythemia rubra | HPyV1 | Allogeneic | 2 | 4/10 | None | IRIS | Stabilization |
| Muftuoglu et al., 2018 [35] | 35, M | HIV | HPyV1 | Allogeneic | 5 | 5/10 | ART | None | Improvement |
| Steinhardt et al., 2020 [44] | 59, M | Multiple myeloma | HpyV2 | Allogeneic (HSCT donor) | 1 | 10/10 | Cidofovir, mirtazapine, DLI | None | Stabilization |
| Berzero et al., 2021 [41] | 59, F | Non-Hodgkin lymphoma | HpyV2 | Allogeneic | 4 | Unknown | None | None | Stabilization |
| Berzero et al., 2021 [41] | 55, M | Multiple myeloma | HpyV2 | Autologous | 3 | Not appropriate | Mirtazapine (stopped) | None | Improvement |
| Berzero et al., 2021 [41] | 70, F | Non-Hodgkin lymphoma | HpyV2 | Autologous | 1 | Not appropriate | None | None | Deterioration and death |
| Berzero et al., 2021 [41] | 50, M | Hodgkin lymphoma | HpyV2 | Allogeneic | 3 | Unknown | Cidofovir (stopped) | None | Unknown (death due to VZV infection) |
| Berzero et al., 2021 [41] | 68, M | Non-Hodgkin lymphoma | HpyV2 | Autologous | 6 | Not appropriate | None | None | Improvement |
| Berzero et al., 2021 [41] | 54, M | Non-Hodgkin lymphoma | HpyV2 | Allogeneic | 2 | Unknown | None | None | Deterioration and death |
| Berzero et al., 2021 [41] | 66, M | Chronic lymphocytic leukemia | HpyV2 | Allogeneic | 4 | Unknown | None | None | Deterioration and death |
| Berzero et al., 2021 [41] | 54, F | Idiopathic CD4 lymphocytopenia | HpyV2 | Autologous | 6 | Not appropriate | None | None | Improvement |
| Berzero et al., 2021 [41] | 17, M | Wiskott–Aldrich syndrome | HpyV2 | Allogeneic (HSCT donor) | 5 | Unknown | None | None | Improvement |
| Hopfner et al., 2021 [43] | 55, F | Hodgkin lymphoma | HPyV1 | Allogeneic | 4 | 6/10 | None | None | Improvement |
| Hopfner et al., 2021 [43] | 71, F | Breast cancer, dermatomyositis | HPyV1 | Allogeneic | 3 | 6/10 | None | None | Improvement |
| Cortese et al., 2021 [40] | 62, F | Idiopathic lymphocytopenia, cyclic neutropenia | HPyV1 | Allogeneic (two different donors) | 3 | 6/10 (1st) 1/10 (2nd) | Pembrolizumab (stopped), mefloquine | None severe | Deterioration and death |
| Cortese et al., 2021 [40] | 61, F | Microscopic polyangeitis | HPyV1 | Allogeneic | 3 | 5/10 | Mirtazapine | None severe | Improvement |
| Cortese et al., 2021 [40] | 53, F | Chronic lymphocytic leukemia | HPyV1 | Allogeneic | 2 | 10/10 | Mirtazapine | None severe | Deterioration and death |
| Cortese et al., 2021 [40] | 71, M | Non-Hodgkin lymphoma | HPyV1 | Allogeneic | 2 | 5/10 | Cidofovir (stopped), mirtazapine | None severe | Improvement |
| Cortese et al., 2021 [40] | 40, F | Systemic lupus erythrematosus | HPyV1 | Allogeneic | 3 | 5/10 | None | None severe | Improvement |
| Cortese et al., 2021 [40] | 40, F | Non-Hodgkin lymphoma | HPyV1 | Allogeneic | 3 | 6/10 | Mirtazapine | None severe | Deterioration and death |
| Cortese et al., 2021 [40] | 60, M | B and D hepatitis | HPyV1 | Allogeneic | 3 | 5/10 | None | None severe | Stabilization |
| Cortese et al., 2021 [40] | 71, M | Chronic lymphocytic leukemia | HPyV1 | Allogeneic | 1 | 2/10 | Mirtazapine, mefloquine | None severe | Unknown (withdrew from study) |
| Cortese et al., 2021 [40] | 40, F | Severe combined immunodeficiency | HPyV1 | Allogeneic | 1 | 5/10 | None | None severe | Deterioration and death |
| Cortese et al., 2021 [40] | 35, F | Common variable immunodeficiency | HPyV1 | Allogeneic | 2 | 5/10 | Interleukin-7 (stopped), mirtazapine, mefloquine | None severe | Deterioration and death |
| Cortese et al., 2021 [40] | 57, M | Chronic lymphocytic leukemia | HPyV1 | Allogeneic | 3 | 10/10 | Mirtazapine | None severe | Improvement |
| Cortese et al., 2021 [40] | 72, M | Chronic lymphocytic leukemia | HPyV1 | Allogeneic | 3 | 7/10 | Mirtazapine | None severe | Stabilization |
| Wicklein et al., 2021 [42] | 57, M | Non-Hodgkin lymphoma | HPyV1 | Allogeneic | 2 | 5/10 | Pembrolizumab | None | Improvement |
| Rubinstein et al., 2022 [45] | 64, F | Acute myeloblastic leukemia | HPyV1 | Allogeneic | 6 | 3/10 | None | None | Improvement |
| Rubinstein et al., 2022 [45] | 59, M | Non-Hodgkin lymphoma | HPyV1 | Allogeneic (two different donors) | 3 | 2/10 (1st) 3/10 (2nd) | Pembrolizumab (stopped) | None | Deterioration and death |
| Rubinstein et al., 2022 [45] | 62, M | Non-Hodgkin lymphoma | HPyV1 | Allogeneic | 2 | 4/10 | Unknown | IRIS | Deterioration and death |
| Rubinstein et al., 2022 [45] | 67, F | Non-Hodgkin lymphoma | HPyV1 | Allogeneic | 1 | 5/10 | Unknown | None | Deterioration and death |
| Peghin et al., 2022 [46] | 29, F | Lung transplantation | HPyV2 | Allogeneic | Unknown | 5/10 | Mirtazapine, mefloquine | None | Improvement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambert, N.; El Moussaoui, M.; Baron, F.; Maquet, P.; Darcis, G. Virus-Specific T-Cell Therapy for Viral Infections of the Central Nervous System: A Review. Viruses 2023, 15, 1510. https://doi.org/10.3390/v15071510
Lambert N, El Moussaoui M, Baron F, Maquet P, Darcis G. Virus-Specific T-Cell Therapy for Viral Infections of the Central Nervous System: A Review. Viruses. 2023; 15(7):1510. https://doi.org/10.3390/v15071510
Chicago/Turabian StyleLambert, Nicolas, Majdouline El Moussaoui, Frédéric Baron, Pierre Maquet, and Gilles Darcis. 2023. "Virus-Specific T-Cell Therapy for Viral Infections of the Central Nervous System: A Review" Viruses 15, no. 7: 1510. https://doi.org/10.3390/v15071510
APA StyleLambert, N., El Moussaoui, M., Baron, F., Maquet, P., & Darcis, G. (2023). Virus-Specific T-Cell Therapy for Viral Infections of the Central Nervous System: A Review. Viruses, 15(7), 1510. https://doi.org/10.3390/v15071510






