Abstract
The clinical evolution of patients infected with the Severe Acute Respiratory Coronavirus type 2 (SARS-CoV-2) depends on the complex interplay between viral and host factors. The evolution to less aggressive but better-transmitted viral variants, and the presence of immune memory responses in a growing number of vaccinated and/or virus-exposed individuals, has caused the pandemic to slowly wane in virulence. However, there are still patients with risk factors or comorbidities that put them at risk of poor outcomes in the event of having the coronavirus infectious disease 2019 (COVID-19). Among the different treatment options for patients with COVID-19, virus-targeted measures include antiviral drugs or monoclonal antibodies that may be provided in the early days of infection. The present expert consensus is based on a review of all the literature published between 1 July 2021 and 15 February 2022 that was carried out to establish the characteristics of patients, in terms of presence of risk factors or comorbidities, that may make them candidates for receiving any of the virus-targeted measures available in order to prevent a fatal outcome, such as severe disease or death. A total of 119 studies were included from the review of the literature and 159 were from the additional independent review carried out by the panelists a posteriori. Conditions found related to strong recommendation of the use of virus-targeted measures in the first days of COVID-19 were age above 80 years, or above 65 years with another risk factor; antineoplastic chemotherapy or active malignancy; HIV infection with CD4+ cell counts < 200/mm3; and treatment with anti-CD20 immunosuppressive drugs. There is also a strong recommendation against using the studied interventions in HIV-infected patients with a CD4+ nadir <200/mm3 or treatment with other immunosuppressants. Indications of therapies against SARS-CoV-2, regardless of vaccination status or history of infection, may still exist for some populations, even after COVID-19 has been declared to no longer be a global health emergency by the WHO.
1. Introduction
The Coronavirus Infectious Disease starting in 2019 (COVID-19) has already affected more than 10% of the global population and has caused around 1% of deaths among cases, according to recorded data that have probably underestimated the real figures [1,2,3].
The clinical manifestations of infection by the Severe Acute Respiratory Coronavirus type 2 (SARS-CoV-2) range from asymptomatic infection—in around 15% of infections—to multiple organ failure and death. Most symptomatic infections are limited to catarrhal symptoms, including mild pulmonary involvement, in around 80% of cases; severe pneumonia with hypoxia in around 15% of cases; and respiratory distress, shock or multiple organ failure in around 5% of cases [4,5]; overall fatality rate is today below 0.5%. The likelihood of severe disease has changed over the course of the pandemic, mainly for four reasons: the protective effect of vaccination [6], the selection for less lethal SARS-CoV-2 virus variants [7], the lower severity of reinfections and the availability of treatments to reduce the risk of progression of COVID-19 [8]. From the end of 2020, vaccination against SARS-CoV-2 has been the primary epidemiological determinant of change in hospitalization and death, and of transmission, to a lesser extent [9].According to the epidemiological studies carried out in the pre-vaccination era, the main risk factors for poor clinical outcome in patients with COVID-19 are being aged above 65 years, certain comorbidities [10,11,12,13] and different types of immunosuppression [14,15,16,17,18,19,20,21,22,23]. In vaccinated persons of all ages, a history of SARS-CoV-2 infection has been identified as one of the main protective factors against severe COVID-19 [18]. While greater body mass index (BMI) does appear to play an independent role, so do other factors associated with obesity, such as carbohydrate intolerance, diabetes, hyperlipidemia, or a sedentary lifestyle [24,25,26]. Evidence shows that a poorer prognosis may be expected in case of pulmonary [27,28], cardiovascular [29], kidney, liver and certain degenerative neurological diseases [30]. The degree of frailty and the need for care associated with these pathologies, together with age and various debilitating chronic diseases, may be the common reason for this poorer prognosis [31]. Immunosuppression is also a recognized risk factor for severe COVID-19 or death [32,33,34,35]. The analysis of the post-vaccination period continues to show that the elements that weigh most heavily in the stratification of the individual risk of a poor post-SARS-CoV-2 infection clinical outcome are vaccination status, history of infection, age, burden of comorbidities and immunosuppression [9,36,37,38,39].
It is not easy to establish the individual weight of many of the above-mentioned factors in the severity of COVID-19. Firstly, it would be necessary to ascertain whether each factor affects only the severity of the infection or whether it also contributes to a higher risk of death. Comorbidities are often analyzed in a general way, using definitions that include diseases with very different prognoses and at different stages of severity. Many factors identified as risk factors are associated with other comorbidities, producing statistical associations that do not always indicate causality. It is also necessary to analyze whether the effect of a particular disease is due to the actual disease process, to associated pathogenic factors or to the effect of certain drugs commonly used for its treatment. Chronic diseases that lead to frailty and dependency make patients more infection-prone. In addition, most analyses of prognostic factors come from retrospective studies, with the limitations that this entails in drawing conclusions [9]. Finally, despite the existence of current predictive scales with a well-adjusted number-needed-to-treat (NNT) to select patients with SARS-CoV-2 infection who would potentially benefit from a therapeutic measure in addition to the standard treatment [39,40,41,42,43], we still do not know what the weight of each one of these factors is in the current post-vaccination era.
2. Therapeutic Virus-Targeted Measures against SARS-CoV-2
SARS-CoV-2 disease is characterized by a defined pathochrony that conditions the clinical presentation of its different manifestations in the patient [4,44]. The early stages are dominated by viral replication, during which the patient presents general and respiratory tract symptoms like any other viral illness, such as fever, cough, dyspnea, odynophagia, asthenia and myalgia, headache, chills, vomiting and diarrhea. In some patients (15%), this initial phase leads to an inflammatory process because of an aberrant host immune response, causing diffuse alveolar damage and leading to the development of adult acute respiratory distress syndrome (ARDS). Typically, the first phase of the disease lasts for 7 days, although both phases may present simultaneously, intertwined or successively, and viral replication can be maintained in more severe and immunosuppressed patients for weeks [4,44].
From a therapeutic standpoint, it is important to discern whether the patient has viral replication for the use of additional virus-targeted measures (AVTMs), or has a state of proinflammation indicating the use of immunomodulators. The AVTMs currently available reduce the relative risk of progression by between 50% and 90% [45,46,47,48].
Neutralizing monoclonal antibody (mAb) technology, as a therapeutic alternative to previous emerging pathologies, has developed rapidly in recent years thanks to a combination of breakthroughs in single-cell genomic analysis techniques. These techniques make it possible to obtain the sequences of variable regions of B lymphocytes isolated from individuals exposed to different infectious agents, together with the availability of electron cryo-microscopy, which facilitates the precise characterization of the epitopes recognized by the antibodies [49,50,51,52,53,54].
The classification of the mAbs used against SARS-CoV-2 depends on the antibody binding site and mechanism. Depending on epitope recognition and binding mode, RBD-specific neutralizing mAbs are classified into four main classes (I, II, III and IV) [55]: (i) Class I and II neutralizing mAbs bind to the RBM region of the RBD. The mAbs that block this RBM-ACE2 interaction are “ACE2 blockers”. Class I mAbs bind to RBD only in the upward conformation, while class II mAbs block ACE2 binding and recognize both up and down RBD; (ii) Class III neutralizing mAbs block the ACE2 binding site outside the RBM and recognize both up and down RBD; and (iii) Class IV mAbs do not overlap with the ACE2 binding site and bind to a conserved region in RBD in the “up” conformation.
Therapeutic mAbs for the treatment of COVID-19 have been developed at an accelerated pace unprecedented for any other disease. Currently, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have a number of mAbs approved for clinical use, primarily in the early stages of infection and even as post-exposure prophylaxis, where antiviral treatment has been shown to be most effective in COVID-19. This list is changing and currently includes bamlanivimab (LY-CoV555) plus etesevimab (LY-CoV016), casirivimab (REGN10933) plus imdevimab (REGN10987), sotrovimab (VIR-7831) and bebtelovimab (LY-CoV1404) [56]. The combination of two human antibodies, tixagevimab and cilgavimab, has been cleared for use as pre-exposure prophylaxis in people at risk of severe COVID-19, in case of absence or poor response to vaccination [57]. A subsequent clinical trial [58] has demonstrated this combination’s efficacy at preventing fatal COVID-19 in high-risk patients with early symptoms; the drug may be ideal in ambulatory settings as administered in intramuscular injections. However, currently circulating viral variants present less susceptibility to this drug than to other mAbs.
Although the main function sought in these mAbs is the ability to neutralize infection, there is growing evidence that the antibodies’ effector functions may play an important role in protection against the more severe forms of COVID-19. This protection would be related to the functions of cytotoxicity, phagocytosis and stimulation of the antibody-mediated cellular response [59]. For instance, the administration of sotrovimab in the COMET-ICE clinical trial on susceptible variants showed that a favorable outcome was associated with a decrease in SARS-CoV-2 viral load in the respiratory tract and the normalization of the expression of 10 of the genes significantly associated with the inflammatory response predictive of severe outcome in COVID-19 [60].
Drugs able to block the life cycle of SARS-CoV-2 have proved efficacious in controlling the progression of COVID-19 into more severe forms, particularly in individuals with risk factors. The spike protein on the surface of the SARS-CoV-2 membrane binds to cellular angiotensin-converting enzyme 2 (ACE2) in the host. For the fusion of viral and cellular membranes, and the entrance of the viral ribonucleoprotein complex into the cell, an interaction between the SARS-CoV-2 spike protein and the cellular transmembrane serine protease 2 (TMPRSS2) is needed [61]. In cases of viral infection via endocytosis, the cathepsin-L is responsible for releasing the viral RNA into the host cell cytoplasm. Cellular furins are also needed in the process of viral maturation [62]. Drugs that interfere with a list of host enzymes to block SARS-CoV-2 replication are still under study, and none of them are ready for clinical use. The main mechanism of action of these compounds under development is: (i) interference with ACE receptor; (ii) inhibition of TMPRSS2; (iii) inhibition of furins; (iv) inhibition of cathepsin-L; and (v) interference with viral endocytosis.
Antiviral drugs developed for the treatment of COVID-19 target viral proteins needed to complete SARS-CoV-2 replication in infected cells. According to the mechanism of action of the drug, these compounds may be classified as: (i) RNA-dependent RNA polymerase (RdRP) inhibitors; (ii) viral protease inhibitors and (iii) maturation inhibitors.
Three direct acting antivirals have gained approval for the treatment of COVID-19:
Remdesivir: This drug is an analog of adenosine, which inhibits viral replication by acting as an RdRP inhibitor when incorporated into the nascent RNA strands, causing the termination of RNA synthesis. The benefit of remdesivir among hospitalized patients taken together is not completely clear; however, in subjects with severe disease but not under ventilation, the use of this drug is associated with (i) accelerated recovery; (ii) lower need of mechanical ventilation and (iii) reduced mortality [63,64,65,66,67,68,69,70,71,72]. Although the benefit of remdesivir among ventilated patients is not fully clear, an observational study also proved a reduction in mortality with remdesivir in this subset of patients [73]. In hospitalized patients, remdesivir is given for 5 days, as longer treatment has not demonstrated additional benefit; longer durations may be considered in immunosuppressed patients as prolonged replication and selection of viral resistance is possible [74].
Remdesivir may be considered as a second-choice option in non-hospitalized patients with non-severe COVID-19 to prevent disease worsening, particularly in subjects that have not been vaccinated, or if other comorbidities are present. Antiviral treatment for three days is associated with a reduced need for visits to the hospital or hospitalization and lower mortality [46]. The intravenous route of administration of remdesivir may be viewed as a limitation for the use of this drug in the ambulatory setting; the oral formulation of the drug is currently under study.
Nirmatrelvir/ritonavir: This drug is an inhibitor of the viral 3CL protease, which breaks down the viral polyproteins into nonstructural proteins that are needed for the assembly of new viral particles. Nirmatrelvir/ritonavir has shown efficacy in ambulatory patients at risk of adverse outcome, including unvaccinated subjects or SARS-CoV-2-naïve subjects, in terms of providing shorter viral shedding and lower risk of hospitalization or death [48,75,76]. The efficacy of the drug is more pronounced in subjects with risk factors such as older age, immunosuppression and cardiovascular or neurological disease. The benefit of this antiviral drug in vaccinated subjects or in those with a history of COVID-19 without risk factors is doubtful. Nirmatrelvir/ritonavir has been authorized for treating mild-to-moderate COVID-19 in adults and children. The active drug is taken orally in combination with ritonavir, a potent cytochrome P450-3A4 inhibitor that improves the pharmacokinetic profile of the drug. The reappearance of some symptoms of COVID-19 within 10 days after the end of treatment with nirmatrelvir/ritonavir has been described in a small proportion of patients [75,77]. This clinical relapse is associated with viral rebound; that is, with positive SARS-CoV-2 antigen or RT-PCR tests. COVID-19 recurrence is, in general, self-limited, but in patients with a risk of severe immunosuppression, retreatment with antivirals may be considered.
Molnupinavir: Once the RdRp incorporates this ribonucleoside analog into nascent viral RNA, the replication process is altered by the accumulation of mutations, driving the virus to error catastrophe and making replication impossible. In ambulatory patients with mild-to-moderate COVID-19 with additional comorbidities, the drug has shown reductions in the risk of progression, but is not so clear in the need for hospitalization and mechanical ventilation, or in mortality [47,51,78,79,80,81]. According to comparative analysis, molnupiravir is considered less effective than remdesivir or nirmatrelvir/ritonavir, so it is only considered a marginal option for non-hospitalized patients at risk of progression. Furthermore, a recent evaluation by the EMA has recommended against the marketing and use of molnupiravir and called for the withdrawal of the drug, which has been recalled by the marketing company. A re-evaluation by the EMA with novel evidence is currently being undertaken [82].
Antiviral drugs are active only in the presence of viral replication, which is estimated to happen within 5 to 7 days from the onset of symptoms; the benefit of these drugs in patients with non-severe or severe COVID-19, as in those with risk factors for poorer outcomes including unvaccinated or virus-naïve subjects, may be extended to a longer period. Beyond 7 days from symptom onset, the indication of antivirals should not be based on real-time PCR results, as viral RNA may be detected days after viral replication has subsided. The cycle threshold (Ct) may be a surrogate marker of SARS-CoV-2 RNA concentration; higher mortality from COVID-19 has been shown in hospitalized patients with Ct values below 25 [83]. Subgenomic RNA detection and antigen tests also have a good correlation with active viral replication [84,85]. The presence of viral replication, rather than the need for oxygen supply, is for many the guide to indicate antivirals in hospitalized patients with COVID-19 [86].
3. Main Objective
The aim of this expert consensus (MODUS Project) is to generate recommendations to establish the patient profiles that can benefit most from the use of AVTM against SARS-CoV-2, so that with these interventions the prognosis of the infection is expected to improve. Consequently, these recommendations may also help avoid indiscriminate and futile prescribing of these drugs.
This review addresses the characteristics and risk factors among patients with the diagnosis of COVID-19 that indicate progression to severe forms with poor prognosis. These patients become candidates for AVTM to halt progression to severe COVID-19. This project does not simply aim to generate a list of risk factors, but rather to produce recommendations intended to promote therapeutic actions.
4. Methods
A review of the literature published between 1 July 2021 and 15 February 2022 was conducted to identify scientific publications (original articles, cohort and case–control studies, meta-analyses or systematic reviews) describing which risk factors are indicators of poor prognosis in patients with SARS-CoV-2 infection, making them candidates for the use of AVTM, understood as antivirals or monoclonal antibodies, to halt progression to severe forms of COVID-19. Ex-post, when deemed relevant by the experts, published evidence after 15 February 2022 was accepted for inclusion in the analysis to provide additional data (Figure 1).
Figure 1.
Review process followed in the MODUS project.
The search was conducted in the following main sources of electronic reference libraries to access the available data: PubMed and The Cochrane Library. Related keywords, medical subject headings and free-text terms were used to search for the following concepts: COVID-19, SARS-CoV-2, poor prognosis, risk factors and mortality. The final search query was as follows: (((covid[Title/Abstract]) OR (sars-cov-2[Title/Abstract])) AND ((hospital admission[Title/Abstract]) OR (death[Title/Abstract]) or (mor*[title/abstract]) OR (bad outcomes[Title/Abstract]) OR (poor outcomes[Title/Abstract])) AND ((risk[Title/Abstract]) OR (predic*[Title/Abstract]) OR (prog*[Title/abstract]) OR (factor[Title/abstract])) AND (vacci*[Title/Abstract]))) AND ((“2021/6/1”[Date—Publication]: “3000”[Date—Publication])).
A total of 521 articles meeting the inclusion criteria were identified through the literature search. These records were analyzed according to title and abstract by 2 independent reviewers; if necessary, a third reviewer was involved in the decision to select or discard evidence. A total of 337 articles were discarded and 184 articles were selected. Once the evidence had been classified by headings, the articles were downloaded and forwarded to the editor responsible for each heading. The person responsible for each heading was tasked with distributing the bibliography among the team of assigned reviewers and with coordinating the review of the information and analysis of the evidence (GRADE). As mentioned above, in addition to the initial search provided to each writer, the authors were subsequently allowed, through a scoping review, to add additional references of high scientific value which, according to their expert judgement, provided new data that affected the quality and strength of the recommendations. A total of 159 articles outside the time range of the literature search were added.
A total of 278 articles were included in the final quantitative synthesis for the drafting of the list of risk factors and recommendations (Figure 2): 119 from the review of the literature and 159 from the additional independent review carried out by the actual editors a posteriori.
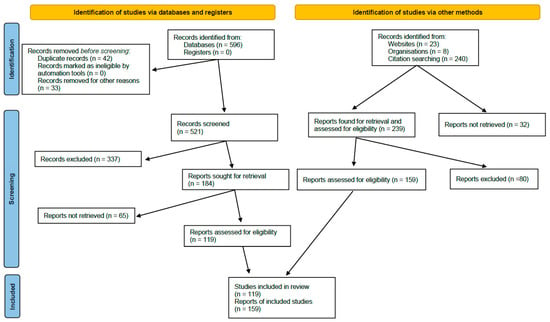
Figure 2.
PRISMA diagram of the literature review followed in the MODUS project.
A total of 37 experts, selected by the coordinators of the project (FJC, PB and MS), participated in the development of this document; all work at Spanish medical centers and all have recognized experience in treating COVID-19, according to publications, communications or participation in scientific panels. The areas of knowledge that were covered by this group of experts were primary care, emergencies, internal medicine, geriatrics, pulmonology, hematology, oncology, rheumatology, infectious diseases, microbiology, transplant medicine, intensive care and public health. A total of 20 drafters read the scientific evidence and established the preliminary list of risk factors under each heading. A three-round consensus validation was carried out with a larger group of 37 experts to ratify what had been analyzed after the systematic review of the literature. The objective was to reach an expert consensus on recommendations to establish the patient profiles that may benefit most from the use of ATVM.
The three-round consensus was conducted in two distinct phases: a face-to face meeting with 26 experts divided into three groups (25 October 2022) and an online questionnaire answered by 34 experts to validate the recommendations. Consensus was regarded as having been reached when 80% of the experts ticked the highest degree of agreement on a scale of 1 to 9.
The final recommendations for the treatment of SARS-CoV-2 infection derived from this review should be taken into account while considering several limitations. The temporal frame of the studies included has left the most recent evidence without, particularly those studies that refer to vaccinated or immune populations or to infections caused by currently circulating viral variants. It may be that these factors, better immunity in the population and novel viral variants, have modified the specific influence of certain factors on the prognosis of COVID-19. To overcome these limitations, panelists have been able to consider studies carried out before and after vaccination was implemented. Additionally, in the review process, panelists were able to include updated relevant publications after February 2022 to assess the impact of the Omicron variant, the last to cause many infections worldwide.
Given that the evidence about the influence of risk factors on COVID-19 has been shifting along the pandemic, a Bayesian statistical analysis would have been the ideal approach for this review. The large amount of information gathered for consideration made this comparison impossible.
5. Results
Considering that the aim of the MODUS Project is to determine the clinical factors of poor prognosis and/or poor outcome and/or death in patients with SARS-CoV-2 infection, what follows is the final list of risk factors extracted following the review of the available evidence and the expert meetings. In Table 1, there is a summary of the risk factors that were considered by the panel, and the level of evidence and recommendation for this consideration. The wording of these profiles has been simplified as far as possible with the aim of generating a useful list of “patient at risk” profiles for clinical practice.

Table 1.
List of risk factors.
There are several considerations to be addressed: (i) these are the factors to be considered as of the diagnosis of SARS-CoV-2 infection and at any level of care; (ii) these factors, unless otherwise specified, are considered to be applicable to the entire population meeting the criteria, regardless of whether the patient has received any dose(s) of vaccine, the time since the last dose and/or whether the patient has had COVID-19 and the time elapsed since then; and (iii) it also details the factors which, based on the available evidence, cannot be considered as risk factors, and nor can actions different from those that would be applied in the general population be recommended.
It is important to understand that the purpose of identifying several risk factors is to promote therapeutic action to prevent progression to more severe stages of the disease; in this case, to indicate specific treatment against SARS-CoV-2 with monoclonal antibodies (mAb) and/or antivirals within the first few days after diagnosis and for anyone presenting any of the factors listed (provided there is no other medical contraindication).
For each risk factor, the quality of the evidence supporting its consideration as a key factor is indicated, as is the strength of the recommendation for therapeutic action according to the criteria of the multidisciplinary group of experts participating in the MODUS Project.
- Elderly, frail and institutionalized patients
Rationale. Age is one of the main prognostic factors of a poor clinical outcome (hospitalization, need for intensive care or death) in patients infected with SARS-CoV-2, with a strong association with the age of 65 years and a maximum from the age of 70 years onwards, both in unvaccinated and vaccinated populations [87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102]. There is also a correlation between comorbidity and poorer clinical outcome, including mortality, at all ages, although particularly after 60 years of age. The strength of this association increases with the number of comorbidities affecting the patient, with the association being highest for three or more pathological processes, both in unvaccinated and vaccinated subjects, including pediatric patients [9,40,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106]. In nursing homes for older adults (NHOA), the factors associated with poorer clinical outcomes are age, health status and frailty [31,88,90,92,95,107,108,109,110,111,112,113]. There are different scales for determining frailty (CFS, Rockwood, FRAIL, ISAR, Green) [114], although in all of them the worst prognosis is defined by a score of 4 or higher. Table 2 summarizes the final recommendations of the panel.

Table 2.
Therapeutic recommendations for patients with COVID-19 according to greater age, frailty and institutionalization.
- Body weight
Rationale. In addition to the negative effect that obesity has on its own, particularly when BMI is around 40 kg/m2, obesity is often associated with other comorbidities such as diabetes or cardiovascular disease, and has an impact on the prognosis of patients with SARS-CoV-2 infection, both in the unvaccinated and vaccinated population, including the pediatric setting [97,98,103,107]. Table 3 summarizes the final recommendations of the panel.

Table 3.
Therapeutic recommendations for patients with COVID-19 according to elevated body weight.
- Kidney function
Rationale. Renal failure is an independent risk factor for mortality in any clinical process, both infectious and non-infectious. The impact of the association of functional renal failure (CrCl < 50 mL/min) with or without plasma exchange on the prognosis of patients with SARS-CoV-2 infection has been reviewed in both unvaccinated and vaccinated populations, including the pediatric population [9,38,87,107,115]. The prognosis in these patients, in terms of ICU admission and death, increases significantly with age (over 70 years), with comorbidity (CCI of 2 or more) and with the intensity of renal function deterioration (CrCl < 40 mL/min and hemodialyzed) [9,38,87,107,115]. Table 4 summarizes the final recommendations of the panel.

Table 4.
Therapeutic recommendations for patients with COVID-19 according to impairment in kidney function.
- Liver function
Rationale. The prognostic impact of liver failure has only been demonstrated in the most advanced stages. The impact of liver failure on the prognosis of patients with SARS-CoV-2 infection has been reviewed in both unvaccinated and vaccinated populations, including pediatrics [88,89,107,116]. Table 5 summarizes the final recommendations of the panel.

Table 5.
Therapeutic recommendations for patients with COVID-19 according to presence of liver failure.
- Solid organ transplantation
Rationale. The impact of solid organ transplantation (SOT) on the clinical course of SARS-CoV-2 infection has been analyzed [115,116,117,118,119]. A review based on 15 articles published up until March 2021 (with 265,839 participants, including 1485 recipients) showed that SOT was associated with an increased risk of ICU admission compared to non-transplant recipients (OR: 1.57; p = 0.02). The SOT population also had a higher adjusted mortality (HR: 1.54; p = 0.037) [117]. The data also show that the presence of comorbidities is more frequent in SOT recipients with SARS-CoV-2 infection compared to recipients without infection. In turn, SARS-CoV-2 infection increases the risk of developing post-transplant complications, such as major cardiovascular events, acute renal failure or graft rejection [115].
In a large multicenter cohort of SOT recipients during the first wave of the pandemic, the need for hospitalization and invasive mechanical ventilation (78% and 31%, respectively) was high, as was 28-day mortality (20.5%). Age over 65 years and the presence of congestive heart failure, chronic lung disease and obesity have been identified as independent risk factors for mortality or a poor outcome [118,119]. It is also worth noting that mortality in SOT recipients requiring hospitalization did not decrease significantly during the second wave of the pandemic [116].
SARS-CoV-2 vaccines, based either on non-replicating viral vectors or messenger RNA technology, show a lower efficacy in SOT recipients compared to the general population in terms of seroconversion rate or neutralizing antibody titers [120,121,122]. As a consequence, the development of severe SARS-CoV-2 infection requiring hospitalization has been reported in SOT recipients who had received a complete vaccination regimen [123,124]. Mortality in these cases of breakthrough infection can be considerable (9.3%) [124].
The administration of monoclonal antibodies directed against the spike glycoprotein in SOT recipients with SARS-CoV-2 infection has been shown to be safe and effective in reducing the risk of progression to severe disease, although the available evidence comes from observational studies [125,126,127]. More specifically, the use of sotrovimab in kidney transplant recipients over 55 years or with other risk factors for severe infection (diabetes, obesity, graft dysfunction, coronary heart disease or chronic lung disease) infected with the SARS-CoV-2 Omicron variant reduced both mortality and the need for ICU admission compared to a historical control group not treated with sotrovimab [127].
In the light of the data analyzed, it may be concluded that SOT is a prognostic factor for a poor clinical outcome (need for hospitalization, ICU admission or death) in SARS-CoV-2 infection, with the risk being higher in older recipients with graft dysfunction (particularly renal) or comorbidities. The association applies to both vaccinated and unvaccinated populations, although the risk of a poor outcome in the latter is lower [107]. Table 6 summarizes the final recommendations of the panel.

Table 6.
Therapeutic recommendations for patients with COVID-19 in solid organ transplant recipients.
- SARS-CoV-2 PCR-positive graft donors or recipients
Rationale. The potential feasibility of deceased COVID-19 patients with a diagnostic test for active SARS-CoV-2 infection (usually PCR) being able to act as solid organ or hematopoietic progenitor donors was considered from the outset of the pandemic. There are theoretical arguments for the risk of transmission of infection via solid organs and, to a lesser extent, hematopoietic progenitors; however, the available evidence for viable virus isolation in non-pulmonary organs and tissues is inconsistent [128].
Several case series, both multi- [129,130] and single-center [131,132,133] and individual cases [134], have shown favorable results with the use of organs (other than the lung) from deceased donors with a positive PCR for SARS-CoV-2 at the time of donation or in the days immediately preceding donation [135]. None of these studies demonstrated organ-mediated transmission or a worse-than-expected graft outcome considering donor and recipient characteristics [129,130,131,132,133,134].
In some centers, and in a pre-vaccination context, priority was given to selecting recipients with a positive serology for SARS-CoV-2 on the waiting list in order to ensure the presence of natural immunity [133]. It should be noted that none of these articles considered the preventive administration of antiviral drugs or monoclonal antibodies in the donor with the specific aim of reducing the theoretical risk of transmission, although it is true that there is no evidence to suggest that the administration of any type of preventive treatment could have a discernible impact on this theoretical risk of transmission.
In fact, the only documented cases of via-graft SARS-CoV-2 transmission occurred in lung transplant recipients and shared the common feature that no pre-donation molecular testing (PCR) had been performed on a donor lower respiratory tract sample [136,137]. Therefore, according to the evidence reviewed, the use of solid organs (other than lung and intestine) and hematopoietic progenitors from donors diagnosed with an active SARS-CoV-2 infection (PCR-positive) has been shown to be safe. Table 7 summarizes the final recommendations of the panel.

Table 7.
Therapeutic recommendations for patients with COVID-19 as graft donors or recipients.
- Hematopoietic stem cell transplantation
Rationale. Several observational studies, both retrospective [9,138,139,140] and prospective [141], have analyzed the clinical course of SARS-CoV-2 infection in hematopoietic stem cell transplantation (HSCT) recipients.
In a large multicenter cohort (n = 318) published in the pre-vaccine period, an ICU admission rate of 14% was observed, with an overall 30-day survival of 68% [15]. Being older [15,141], male gender [15], a period of less than one year since autologous hematopoietic stem cell transplantation (AHSCT), the presence of comorbidities and the presence of a high risk of death [15,140], the presence of comorbidities [138], the degree of immunosuppression [140,141], neutropenia [138] and certain clinical features at the time of diagnosis, such as the presence of pneumonia [138], were identified as predictors of death or unfavorable outcome. In addition, no significant prognostic differences were observed between autologous and allogeneic procedures [15,141].
On the other hand, none of the studies reviewed, including a systematic review and meta-analysis [32], reported the administration of monoclonal antibodies or antiviral drugs against SARS-CoV-2 and neither were specific recommendations made in two clinical practice guidelines [142,143].
In conclusion, and according to the evidence analyzed, HSCT is a prognostic factor for a poor clinical outcome (need for hospitalization, ICU admission or death) in patients infected with SARS-CoV-2. Table 8 summarizes the final recommendations of the panel.

Table 8.
Therapeutic recommendations for patients with COVID-19 with hematopoietic stem cell transplant.
- Oncologic patients
Rationale. Oncology and oncohematology patients have higher morbidity and mortality due to COVID-19. The data published to date corroborate this, while also shedding some light on the possibility of identifying subgroups of oncology patients who have a particularly poorer prognosis.
The existing evidence in this regard is scant and comes mostly from observational studies with a very heterogeneous data collection, obvious limitations considering the complexity of the moment and the imperative need for real-life data.
Moreover, the analysis of the evidence suggests that patients with active cancer have a higher risk of mortality from SARS-CoV-2 than the general population [36,90,106,107,108,110,144,145,146,147,148,149]. There are certain factors associated with these patients that may help to identify subgroups with a higher risk of increased mortality, including: (i) factors associated with neoplastic pathology (the presence of hematological malignancies) [110,146,147,148,150]; (ii) the presence of active disease and/or being on treatment [36,108,110,144,146,147,151,152,153,154,155,156]; (iii) refractory disease [156]; (iv) some subtypes of hematological malignancies (chronic lymphoid leukemia) [150] and solid tumors (lung cancer) [108,144]; (v) treatment-dependent factors (treatment with antiCD20) [156]; (vi) patient-dependent factors such as advanced age [88,90,144,146,150,151,156], presence of comorbidities [36,151,152] and high BMI [144]; and (vii) lower serological response to vaccination [153,154,155]. The evidence reviewed leads to the conclusion that oncology/oncohematology patients are particularly vulnerable to severe forms of SARS-CoV-2.
On the other hand, in the analysis of the evidence for these subgroups, patients with cancer in remission and/or without active treatment appear to have a lower mortality than those on active treatment [36,108,110,144,146,147,151]. Furthermore, these patients do not present a higher risk of COVID-19 mortality than the general population.
Patients with lymphoid hematological diseases—acute lymphoid leukemia (ALL), chronic lymphocytic leukemia (CLL) and/or non-Hodgkin’s lymphoma (NHL)—due to their prolonged immunosuppressed state, are more susceptible to SARS-CoV-2 infection compared to patients in remission and/or without active treatment, although there is no difference in mortality between them according to the underlying disease status [148,150,152].
However, the data do show that patients over 12 years of age weighing at least 40 kg and with stable-phase cancer and one or more comorbidities are, like the general population, at increased risk of severe forms of SARS-CoV-2 infection over and above their cancer patient status. These comorbidities include [88,145,157] (i) stable structural or functional airway disease, without oxygen dependency at the time of diagnosis; (ii) COPD on oxygen therapy with no change in oxygen dependence; (iii) functional renal insufficiency (CrCl < 50 mL/min) with or without plasma exchange; (iv) obesity with BMI > 40 as a sole disease, or BMI >30 and any other comorbidity; (v) insulin-dependent diabetes mellitus with poor control (glycated hemoglobin (Hb-A1c) > 6.5%); (vi) functional liver failure (Child–Pugh ≥ 10 points); (vii) established ischemic heart disease, with myocardial dysfunction or with normal myocardial function and two or more cardiovascular risk factors (hypertension (HT), hyperlipidemia, diabetes, obesity); (viii) congestive heart failure with a New York Heart Association (NYHA) classification ≥II and (ix) hematopoiesis disorders.
In summary, oncology/oncohematology patients with stable-phase cancer are not at increased risk of developing more severe forms of COVID-19 due to the nature of their pathology as long as they do not have associated comorbidities—which are already a risk factor for the general population—or are in a state of prolonged immunosuppression due to treatment. Table 9 summarizes the final recommendations of the panel.

Table 9.
Therapeutic recommendations for oncologic and oncohematologic patients with COVID-19.
- HIV infection
Rationale. Studies comparing the clinical features and prognosis of COVID-19 in people with and without human immunodeficiency virus (HIV) infection have yielded conflicting results. Some have found no significant differences in these aspects between the two populations [158,159,160,161,162,163,164,165,166,167,168,169], whereas others have found a higher risk of hospitalization, ICU admission or death from COVID-19 in people with HIV than in the general population [170,171,172,173,174,175]. These results must be viewed in the light of the different levels of antiretroviral treatment (ART) coverage and differences in socio-economic status and living conditions between people with and without HIV across the geographic regions in which these studies have been conducted.
In any case, many observational studies indicate that the prognosis of COVID-19 in people with HIV is largely determined by the same factors that influence the general population, such as gender, age and the presence of comorbidities [33,165,172,174,175,176,177,178,179,180,181,182,183,184,185,186,187], and by socio-economic status, living conditions and ethnicity [166,174,175,179,182,188]. The importance of demographic characteristics and comorbidity in this population is reinforced by the absence of prognostic differences between people with and without HIV in cohort studies matched for these variables [170,180,189,190].
The effect of the factors directly related to HIV on the prognosis of COVID-19 is still unknown, especially since many of the studies that have examined these aspects have been conducted in countries with good ART coverage and a low proportion of patients with immunosuppression or detectable viraemia. Nevertheless, an association between low CD4+ T-cell counts and increased risk of hospitalization, ICU admission and mortality from COVID-19 has been observed [33,107,170,187,188,191,192], with a cut-off point for defining a low CD4+ T-cell count of <200 cells/mm3 having been established in some studies [33,167,189] and of <350 cells/mm3 in others [187,191,193]. A poorer prognosis for COVID-19 has also been observed in HIV patients with a low CD4+ T-cell count nadir [178,187,194], with a threshold CD4+ T-cell count of <200 cells/mm3 established in one of them [187].
Detectable viral load has also been identified as a poor prognostic factor in several studies [163,183,191,192,195]. Among the available evidence, a large observational study in the US found no such association, although most HIV patients were on ART and had suppressed viral load [187]. Only in one retrospective study with fewer than 100 hospitalized patients in New York City was the risk of intubation and death higher in patients with a detectable viral load [195].
Finally, the effect of antiretroviral drugs on the natural history of COVID-19 is a highly controversial area. Some studies have found an association between treatment with tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) with a lower risk of SARS-CoV-2 infection and a better course of COVID-19 [167,186]. However, other studies have found no association between any antiretroviral and severity of infection [172,176,178,179]. Table 10 summarizes the final recommendations of the panel.

Table 10.
Therapeutic recommendations for HIV-infected patients with COVID-19.
- Primary and other secondary immunodeficiencies
Rationale. The pathogenesis of severe COVID-19 may be related to the characteristics of the infection, the impairment of the immune response leading to viral persistence and the effects of an excessive inflammatory response. In the first two cases, antiviral therapies are warranted, and in the second case, the use of immunomodulators for the treatment of severe COVID-19 is warranted.
Inborn errors of immunity (IEIs) are rare genetic defects that result in reduced expression or reduced or increased activity of certain proteins. These genetic changes affect germ cells and predispose to usually severe, even fatal, immune dysfunctions. These dysfunctions can lead to allergic reactions, recurrent opportunistic infections, autoimmune phenomena or the development of tumors. More specifically, with regard to acute viral infections, increased susceptibility to enterovirus, herpes simplex virus type I or Epstein–Barr virus, respiratory infections and reaction to attenuated vaccines have been reported [196,197,198,199,200,201].
More than 450 different IEIs are known, varying widely in their presentations and clinical manifestations; a distinction may be made between IEIs with impaired innate (e.g., chronic granulomatous disease) or adaptive (e.g., X-linked agammaglobulinemia, common variable immunodeficiency, etc.) immunity. In other cases, immune dysfunction involves molecular pathways, such as the complement pathway, IFNϓ/IL-12, IL-17, etc.
There are data on the evolution of COVID-19 in individuals affected by defects of innate immunity, humoral immune dysfunction (including common variable immunodeficiency (CVID), Bruton’s tyrosine kinase, agammaglobulinemia and hypogammaglobulinemia), defects in phagocytosis or bone marrow failure (including chronic granulomatous disease), immune dysregulation (including polyglandular autoimmune syndrome type I (PAS-1)), autoinflammatory processes (including familial Mediterranean fever and Aicardi–Goutières Syndrome) or complement deficiency (including hereditary angioedema or C3 deficiency).
The studies conducted in cohorts of patients with IEIs have not found a significantly different frequency of severe COVID-19 to that of the general population [202,203], although it is true that there is a much higher increase in recurrent/persistent forms of COVID-19 in real-life data, necessitating repeated treatment of patients with antivirals in combination with monoclonal antibodies (previously with plasma from convalescent donors) [204].
An estimated 10% of patients with IEIs have asymptomatic COVID-19 and 50% have mild COVID-19. However, severe cases occur at younger ages than expected in the general population and the ICU admission rate is also higher [205]. Globally, for patients with IEIs, the case fatality rate is between 8.1% and 9.5% [203,205], which could be compared to the 5–18% case fatality rate for the general population in the period of the pandemic when these studies were conducted. Thus, in a worst-case scenario, the COVID-19 case fatality rate in patients with IEIs is twice that of the general population.
On the other hand, in general, no differences have been observed between the different types of IEIs and severity of COVID-19 [205]. The poorer prognosis of COVID-19 in people with IEIs seems rather to depend on the coexistence of other comorbidities. This is the case for CVID, the most frequent immune disorder, which involves defective humoral immune function and various alterations in the cellular compartment.
CVID causes recurrent and severe respiratory infections which, in about 30% of patients, leave non-infectious complications (bronchiectasis, asthma and COPD; it is also associated with autoimmune and inflammatory phenomena leading to interstitial lung disease (ILD)). Patients with lung disease have a moderate risk of severe disease due to COVID-19 with hospitalization and possibly ICU admission and a moderate risk of death due to COVID-19. Of these comorbidities, COPD and ILD aggravate the prognosis of COVID-19, while the presence of bronchiectasis or asthma does not seem to have such a negative effect [206,207,208].
Peculiarly, APS-1 causes a higher risk of complications due to COVID-19. In this case, the risk of hospitalization (73%), ICU admission (58%) and death (15%) due to COVID-19 is much higher than in other IEIs [203,209,210,211]. The immune dysfunction associated with APS-1 is the production of autoantibodies against IFN-1. This mechanism of immunosuppression is more frequent in males and older people [212,213]. It has been proposed that the detection of these autoantibodies is a COVID-19-independent predictor of poor prognosis [214]. In X-linked Toll-like receptor 7 (TLR7) deficiency, and thus exclusively affecting males, an increased risk of severe or fatal COVID-19 has also been found [215,216,217].
On the other hand, there is little evidence of the risk of a poor clinical outcome in patients with anatomical or functional asplenia. There is also a predisposition to complications [21,218].
Finally, it is important to mention that activation of the cytokine storm and complement cascade is associated with severe COVID-19 [217,219]. For this reason, people with C3 deficiency or hereditary angioedema with C1 inhibitor deficiency have often been seen to have asymptomatic or mild COVID-19 [220,221]. Table 11 summarizes the final recommendations of the panel.

Table 11.
Therapeutic recommendations for patients with COVID-19 and primary or other secondary immunodeficiencies.
- Immune-mediated inflammatory diseases
Rationale. Patients with immune-mediated inflammatory diseases (IMIDs) may be at increased risk of severe COVID-19 because of the immune dysfunction caused by the actual disease or because of the immunosuppressive effect of many of the drugs used to treat these chronic conditions [222].
The evidence available regarding the possible negative effects of IMID comorbidity on the evolution of COVID-19 is contradictory. Some studies find a higher risk of hospitalization for COVID-19 in patients with Rheumatic and Musculoskeletal Diseases (RMSDs), while others indicate that they have a similar risk to the general population [223,224,225,226,227,228,229,230,231,232,233,234,235,236] or even lower [222]. These differences may well occur because in many studies it is not possible to separate the effect of the disease from that of the immunosuppressive treatment. On the other hand, the likelihood of hospitalization for causes other than COVID-19 may also be increased in patients with IMIDs. According to some of these studies, it may be said that the risk of death from COVID-19 is not increased by having IMID [222,225,236,237]. It is not possible to distinguish between the different IMIDs and their effect on the prognosis of COVID-19.
With regard to the effect of the immunosuppressive drugs used to treat MRDs, there is moderate evidence that glucocorticoids increase the severity of COVID-19, particularly if the chronic doses used exceed 10 mg daily [237,238,239,240,241,242,243,244,245].
There is no evidence of an association of the use of methotrexate, leflunomide or biological agents (anti-TNF, anti-IL1, anti-IL6, anti-IL17, anti-IL 12/23, anti-IL23, abatacept or belimumab) with severity or mortality consistently across all published studies. On the other hand, the data on JAK kinase inhibitors are inconsistent, as some studies show an association with severity or mortality, whereas others do not detect this effect.
It should also be noted that, in the first waves of the COVID-19 pandemic, hyperinflammatory states of severe cases hospitalized with SARS-CoV-2 infection were treated with IL-1 blocking agents (anakinra) and JAK inhibitors (e.g., baricitinib), and subsequent clinical trials demonstrated the protective and effective value of these drugs in counteracting the complications of cytokine release. Similarly, after some controversy, the same occurred with the indication of tocilizumab (IL-6 inhibitor agent) in severe cases of COVID-19.
Therefore, these drugs could play a certain protective role at the appropriate time in the evolution of patients with severe SARS-CoV-2 infection and, with regard to patients already receiving them as a base treatment for their IMID, it is possible that, while they cannot demonstrate a preventive effect by attenuating the baseline inflammatory state and secondary to COVID-19, at least they might not be associated with increased disease severity.
There are fewer studies of other immunosuppressants, and they are inconsistent. One study shows an association with mortality for mycophenolate, azathioprine, calcineurin inhibitors and cyclophosphamide together and another for mycophenolate individually. However, other studies do not detect this association with mycophenolate [233].
In contrast, there are consistent data on anti-CD20 drugs (rituximab and ocrelizumab), associating the latter with greater severity and mortality due to COVID-19 and lower vaccine protection on account of insufficient or no response to the vaccines administered, at least up to those comprising the 1st-, 2nd- and 3rd-dose strategy, although this probably extends to any vaccination strategy, including boosters [237,242,246,247,248,249,250].
After the consensus rounds, not included in recommendations, the expert group found new evidence on one of the points of lower consensus due to controversies in the published data. This new evidence supports a higher risk of progression to severe forms of COVID-19 disease in patients with RMSDs compared to the general population, and could support the need to promote therapeutic actions in these patients to prevent progression [251]. Table 12 summarizes the recommendations of the panel.

Table 12.
Therapeutic recommendations for patients with COVID-19 and autoimmune diseases.
Author Contributions
Conceptualization, F.J.C., P.B., M.S. and J.S.R.; Methodology, F.J.C., P.B. and M.S.; Software, F.J.C.; Validation, A.C., M.F.-R., P.P.-S., J.S.R., J.B., R.C., R.D., P.P.E., I.A.G.-C., J.M.G.d.C., S.B.H., F.J.M.-P., R.M., S.M., J.L.P. (José Luís Pablos), J.P., J.L.P. (José Luis Piñana); Formal analysis, F.J.C., P.B. and M.S.; Investigation, F.J.C., P.B., M.S., A.C., M.F.-R., P.P.-S., J.S.R., J.B., R.C., R.D., P.P.E., I.A.G.-C., J.M.G.d.C., S.B.H., F.J.M.-P., R.M., S.M., J.L.P. (José Luís Pablos), J.P., J.L.P. (José Luis Piñana); Resources, F.J.C.; Data curation, F.J.C., P.B., M.S., J.S.R., J.B., R.D.; Writing—original draft preparation, P.B.; Writing—review and editing, F.J.C., M.S.; Visualization, P.B.; Supervision, F.J.C., P.B., M.S.; Project administration, F.J.C.; Funding acquisition, F.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This expert consensus was technically supported with a grant provided by GSK Laboratories, Spain. Publication fees were paid by the Sociedad Española de Quimioterapia.
Institutional Review Board Statement
No approval was needed.
Informed Consent Statement
Not applicable.
Data Availability Statement
All published materials reviewed and all documents generated during the elaboration of this expert consensus are available upon request.
Acknowledgments
The MODUS group would like to thank the company ADELPHI Targis for their professional support of the realization of the project, from the design to the meetings. We would also like to thank GSK Laboratories for providing logistical support to the reviewers and panelists for the consensus meetings. Addenda MODUS Investigators: José María Aguado, (Infectious Diseases. Hospital General Universitario 12 de Octubre, Madrid), Bernardino Alcázar-Navarrete, (Pneumology. Hospital Universitario Virgen de las Nieves, Granada), Jesús Francisco Bermejo, (Institute of Biomedical Research of Salamanca, IBSAL, University of Salamanca, Salamanca), Ricard Ferrer, (Intensive Care Medicine. Hospital Universitario Vall d’Hebrón, Barcelona), Javier de la Fuente, (Internal Medicine. Hospital Ribera Povisa de Vigo, Pontevedra), Manuel Linares, (Clinical Microbiology. Hospital Universitario Príncipe de Asturias, Madrid), Pere Llorens, (Emergency Department. Hospital General Universitario de Alicante, Alicante), Juan Emilio Losa, (Infectious Diseases. Hospital Universitario Fundación Alcorcón, Madrid), Esperanza Merino, (Infectious Diseases. Hospital General Universitario Dr. Balmis de Alicante, Alicante), Óscar Miró, (Emergency Department. Hospital Clínic de Barcelona, Barcelona), José María Molero, (San Andrés Health Centre, Madrid), Julián Olalla, (Infectious Diseases. Hospital Costa del Sol, Málaga), Roger Paredes, (Infectious Diseases. Hospital Germans Trias i Pujol, Barcelona), Antonio Ramos, (Infectious Diseases. Internal Medicine. Hospital Universitario Puerta de Hierro Majadahonda, Madrid), Francisco del Rio, (Intensive Care Medicine. Transplant Coordination. Hospital Clínico Universitario San Carlos, Madrid), Pilar Rodríguez-Ledo, (Primary Care Medicine. Deputy Medical Director. Gerencia de Gestión Integrada de Lugo, Cervo e Monforte, Galicia), Alex Soriano, (Infectious Diseases. Hospital Clínic de Barcelona, Barcelona).
Conflicts of Interest
Although reviews, opinions and consensus decisions have been free and independent on the part of the authors, this project has been logistically funded by GSK. All coordinators, reviewers and panelists have given lectures sponsored by major pharmaceutical industry laboratories dedicated to the study of infectious pathology, including those dedicated to the study and treatment of SARS-CoV-2 infection (GSK, ASTRA-ZENECA, Gilead, MSD, Pfizer).
References
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center. 2022. Available online: https://coronavirus.jhu.edu/map.html (accessed on 5 September 2020).
- COVID-19 Situation Update for the EU/EEA, as of 31 August 2022. European Centre for Disease Prevention and Control. 2022. Available online: https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea (accessed on 5 September 2020).
- Centro de Coodinación de Alertas y Emergencias Sanitarias, Ministerio de Sanidad de España. Actualización No 589. Enfermedad por el Coronavirus (COVID-19). 2022. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_589_COVID-19.pdf (accessed on 15 October 2022).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, A.C.; Buchan, S.A.; Daneman, N.; Brown, K.A. Estimates of SARS-CoV-2 Omicron Variant Severity in Ontario, Canada. JAMA 2022, 327, 1286–1288. [Google Scholar] [CrossRef]
- Arribas, J.R.; Garcia-Vidal, C.; Paño, J.R.; Baño, J.R. Recomendaciones SEIMC Para El Manejo Clínico de Pacientes Con COVID-19. 2020, Volume 2. Available online: https://seimc.org/contenidos/documentoscientificos/recomendaciones/seimc-rc-2020-COVID19-manejoclinico.pdf (accessed on 15 October 2022).
- Yek, C.; Warner, S.; Wiltz, J.L.; Sun, J.; Adjei, S.; Mancera, A.; Silk, B.J.; Gundlapalli, A.V.; Harris, A.M.; Boehmer, T.K.; et al. Risk Factors for Severe COVID-19 Outcomes Among Persons Aged ≥ 18 Years Who Completed a Primary COVID-19 Vaccination Series—465 Health Care Facilities, United States, December 2020–October 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 19–25. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- Bonanad, C.; García-Blas, S.; Tarazona-Santabalbina, F.; Sanchis, J.; Bertomeu-González, V.; Fácila, L.; Ariza, A.; Núñez, J.; Cordero, A. The Effect of Age on Mortality in Patients with COVID-19: A Meta-Analysis with 611,583 Subjects. J. Am. Med. Dir. Assoc. 2020, 21, 915–918. [Google Scholar] [CrossRef]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S.; et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar] [CrossRef]
- Nandy, K.; Salunke, A.; Pathak, S.K.; Pandey, A.; Doctor, C.; Puj, K.; Sharma, M.; Jain, A.; Warikoo, V. Coronavirus disease (COVID-19): A systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1017–1025. [Google Scholar] [CrossRef]
- Westblade, L.F.; Brar, G.; Pinheiro, L.C.; Paidoussis, D.; Rajan, M.; Martin, P.; Goyal, P.; Sepulveda, J.L.; Zhang, L.; George, G.; et al. SARS-CoV-2 Viral Load Predicts Mortality in Patients with and without Cancer Who Are Hospitalized with COVID-19. Cancer Cell 2020, 38, 661–671.e2. [Google Scholar] [CrossRef]
- Sharma, A.; Bhatt, N.S.; Martin, A.S.; Abid, M.B.; Bloomquist, J.; Chemaly, R.F.; Dandoy, C.; Gauthier, J.; Gowda, L.; Perales, M.-A.; et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: An observational cohort study. Lancet Haematol. 2021, 8, e185–e193. [Google Scholar] [CrossRef] [PubMed]
- Daoussis, D.; Leonidou, L.; Kalogeropoulou, C.; Paliogianni, F.; Tzouvelekis, A. Protracted severe COVID-19 pneumonia following rituximab treatment: Caution needed. Rheumatol. Int. 2021, 41, 1839–1843. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sawalha, A.H.; Lu, Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2020, 33, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Gil, B.M.; Fernández-Ruiz, M.M.; Hernández, D.M.; Crespo, M.M.; Colmenero, J.M.; Coll, E.M.; Rubio, J.J. Organ Donation and Transplantation During the COVID-19 Pandemic: A Summary of the Spanish Experience. Transplantation 2021, 105, 29–36. [Google Scholar] [CrossRef]
- Garg, S.; Kim, L.; Whitaker, M.; O’halloran, A.; Cummings, C.; Holstein, R.; Prill, M.; Chai, S.J.; Kirley, P.D.; Alden, N.B.; et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2022, 69, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Bialek, S.; Boundy, E.; Bowen, V.; Chow, N.; Cohn, A.; Dowling, N.; Ellington, S.; Gierke, R.; Hall, A.; MacNeil, J.; et al. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 343–346. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Liguoro, I.; Pilotto, C.; Bonanni, M.; Ferrari, M.E.; Pusiol, A.; Nocerino, A.; Vidal, E.; Cogo, P. SARS-CoV-2 infection in children and newborns: A systematic review. Eur. J. Pediatr. 2020, 179, 1029–1046. [Google Scholar] [CrossRef]
- Bhopal, S.S.; Bagaria, J.; Olabi, B.; Bhopal, R. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolesc. Health 2021, 5, e12–e13. [Google Scholar] [CrossRef]
- Hamer, M.; Gale, C.R.; Kivimäki, M.; Batty, G.D. Overweight, obesity, and risk of hospitalization for COVID-19: A community-based cohort study of adults in the United Kingdom. Proc. Natl. Acad. Sci. USA 2020, 117, 21011–21013. [Google Scholar] [CrossRef]
- Fadini, G.P.; Morieri, M.L.; Boscari, F.; Fioretto, P.; Maran, A.; Busetto, L.; Bonora, B.M.; Selmin, E.; Arcidiacono, G.; Pinelli, S.; et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res. Clin. Pract. 2020, 168, 108374. [Google Scholar] [CrossRef]
- Sardu, C.; D’onofrio, N.; Balestrieri, M.L.; Barbieri, M.; Rizzo, M.R.; Messina, V.; Maggi, P.; Coppola, N.; Paolisso, G.; Marfella, R. Outcomes in Patients with Hyperglycemia Affected by COVID-19: Can We Do More on Glycemic Control? Diabetes Care 2020, 43, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Aveyard, P.; Gao, M.; Lindson, N.; Hartmann-Boyce, J.; Watkinson, P.; Young, D.; Coupland, C.A.C.; Tan, P.S.; Clift, A.K.; Harrison, D.; et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: A population cohort study. Lancet Respir. Med. 2021, 9, 909–923. [Google Scholar] [CrossRef]
- Beltramo, G.; Cottenet, J.; Mariet, A.-S.; Georges, M.; Piroth, L.; Tubert-Bitter, P.; Bonniaud, P.; Quantin, C. Chronic respiratory diseases are predictors of severe outcome in COVID-19 hospitalised patients: A nationwide study. Eur. Respir. J. 2021, 58, 2004474. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Heilbrunn, E.S.; Ba, D.M.; Chinchilli, V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238215. [Google Scholar] [CrossRef]
- Herman, C.; Mayer, K.; Sarwal, A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology 2020, 95, 77–84. [Google Scholar] [CrossRef]
- Candel, F.J.; Barreiro, P.; Román, J.S.; Carretero, M.D.M.; Sanz, J.C.; Pérez-Abeledo, M.; Ramos, B.; Viñuela-Prieto, J.M.; Canora, J.; Martínez-Peromingo, F.J.; et al. The demography and characteristics of SARS-CoV-2 seropositive residents and staff of nursing homes for older adults in the Community of Madrid: The SeroSOS study. Age Ageing 2021, 50, 1038–1047. [Google Scholar] [CrossRef]
- Belsky, J.A.; Tullius, B.P.; Lamb, M.G.; Sayegh, R.; Stanek, J.R.; Auletta, J.J. COVID-19 in immunocompromised patients: A systematic review of cancer, hematopoietic cell and solid organ transplant patients. J. Infect. 2021, 82, 329–338. [Google Scholar] [CrossRef]
- Dandachi, D.; Geiger, G.; Montgomery, M.W.; Karmen-Tuohy, S.; Golzy, M.; Antar, A.A.; Llibre, J.M.; Camazine, M.; Santiago, D.-D.; Carlucci, P.M.; et al. Characteristics, Comorbidities, and Outcomes in a Multicenter Registry of Patients with Human Immunodeficiency Virus and Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 73, e1964–e1972. [Google Scholar] [CrossRef] [PubMed]
- Zarifkar, P.; Kamath, A.; Robinson, C.; Morgulchik, N.; Shah, S.; Cheng, T.; Dominic, C.; Fehintola, A.; Bhalla, G.; Ahillan, T.; et al. Clinical Characteristics and Outcomes in Patients with COVID-19 and Cancer: A Systematic Review and Meta-analysis. Clin. Oncol. (R Coll Radiol) 2021, 33, e180–e191. [Google Scholar] [CrossRef] [PubMed]
- Vijenthira, A.; Gong, I.Y.; Fox, T.A.; Booth, S.; Cook, G.; Fattizzo, B.; Martín-Moro, F.; Razanamahery, J.; Riches, J.C.; Zwicker, J.; et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood 2020, 136, 2881–2892. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.A.C.; Mehta, N.; Keogh, R.H.; Diaz-Ordaz, K.; Khunti, K.; Lyons, R.A.; Kee, F.; Sheikh, A.; Rahman, S.; et al. Risk prediction of COVID-19 related death and hospital admission in adults after COVID-19 vaccination: National prospective cohort study. BMJ 2021, 374, n2244. [Google Scholar] [CrossRef] [PubMed]
- Centres for Disease Control and Prevention (CDC). Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html (accessed on 6 September 2022).
- Kompaniyets, L.; Pennington, A.F.; Goodman, A.B.; Rosenblum, H.G.; Belay, B.; Ko, J.Y.; Chevinsky, J.R.; Schieber, L.Z.; Summers, A.D.; Lavery, A.M.; et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized with COVID-19, March 2020–March 2021. Prev. Chronic Dis. 2021, 18, E66. [Google Scholar] [CrossRef] [PubMed]
- González Del Castillo, J.; Salmerón, P.P.; Jiménez, S.; Losa, J.E.; Berenguer, J.; Moreno, S.; Vázquez-Lima, M.; Burillo-Putze, G.; Grupo de Infecciones de la Sociedad Española de Medicina de Urgencias y Emergencias (INFURG-SEMES). Manejo de la Infección por SARS-CoV-2 en Urgencias. Rev. Española Urgenc. Emerg. 2022, 1, 23–27. Available online: www.cdc.gov/mmwr/volumes/71/wr/mm7101a4.htm (accessed on 6 September 2022).
- Bierle, D.M.; Ganesh, R.; Tulledge-Scheitel, S.; Hanson, S.N.; Arndt, L.L.; Wilker, C.G.; Razonable, R.R. Monoclonal Antibody Treatment of Breakthrough COVID-19 in Fully Vaccinated Individuals with High-Risk Comorbidities. J. Infect. Dis. 2022, 225, 598–602. [Google Scholar] [CrossRef]
- SEIMC Score Mortality—SEIMC—COVID-19. 2021. Available online: https://covid19.seimc.org/index.php/seimc-score-mortalidad/ (accessed on 6 September 2022).
- QCovidTM Risk Calculator. 2021. Available online: https://qcovid.org/ (accessed on 6 September 2022).
- Shinyapps. PrediCOVID-ED. Available online: https://predicovid.shinyapps.io/RISK_MODEL_COVID/ (accessed on 6 September 2022).
- Cevik, M.; Kuppalli, K.; Kindrachuk, J.; Peiris, M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ 2020, 371, m3862. [Google Scholar] [CrossRef]
- Gupta, A.; González-Rojas, Y.; Juárez, E.; Casal, M.C.; Sarkis, E.; Solis, J.; Falci, D.R.; Moya, H.Z.J.; Scott, N.; Cathcart, A.L.; et al. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Case, J.B.; Mackin, S.; Errico, J.; Chong, Z.; Madden, E.A.; Guarino, B.; Schmid, M.A.; Rosenthal, K.; Ren, K.; Jung, A.; et al. Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-CoV-2 Omicron lineage strains. Nat. Commun. 2022, 13, 3824. [Google Scholar] [CrossRef]
- Wu, M.Y.; Carr, E.J.; Harvey, R.; Mears, H.V.; Kjaer, S.; Townsley, H.; Hobbs, A.; Ragno, M.; Herman, L.S.; Adams, L.; et al. WHO’s Therapeutics and COVID-19 Living Guideline on mAbs needs to be reassessed. Lancet 2022, 400, 2193–2196. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Green, A.C.; Tazare, J.; Curtis, H.; Fisher, L.; Nab, L.; Schultze, A.; Mahalingasivam, V.; Parker, E.P.; Hulme, W.; et al. Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in non-hospitalised patients: An observational cohort study using the OpenSAFELY platform. BMJ 2022, 379, e071932. [Google Scholar] [CrossRef] [PubMed]
- Martin-Blondel, G.; Marcelin, A.-G.; Soulié, C.; Kaisaridi, S.; Lusivika-Nzinga, C.; Dorival, C.; Nailler, L.; Boston, A.; Melenotte, C.; Cabié, A.; et al. Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2. J. Infect. 2022, 85, e104–e108. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. SARS-CoV-2 Therapeutics Technical Briefing 3. Genomic Surveillance; 2022. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1074186/therapeutics-programme-technical-briefing-3.pdf (accessed on 18 October 2022).
- Bruel, T.; Stéfic, K.; Nguyen, Y.; Toniutti, D.; Staropoli, I.; Porrot, F.; Guivel-Benhassine, F.; Bolland, W.-H.; Planas, D.; Hadjadj, J.; et al. Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4 and BA.5 in patients receiving monoclonal antibodies. Cell. Rep. Med. 2022, 3, 100850. [Google Scholar] [CrossRef]
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.-M.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020, 588, 682–687. [Google Scholar] [CrossRef]
- Stanford University. Stanford Coronavirus Antiviral & Resistance Database (CoVDB). 2022. Available online: https://covdb.stanford.edu/ (accessed on 6 September 2022).
- Nguyen, Y.; Flahault, A.; Chavarot, N.; Melenotte, C.; Cheminant, M.; Deschamps, P.; Carlier, N.; Lafont, E.; Thomas, M.; Flamarion, E.; et al. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin. Microbiol. Infect. 2022, 28, 1654.e1–1654.e4. [Google Scholar] [CrossRef]
- Montgomery, H.; Hobbs, F.D.R.; Padilla, F.; Arbetter, D.; Templeton, A.; Seegobin, S.; Kim, K.; Campos, J.A.S.; Arends, R.H.; Brodek, B.H.; et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2022, 10, 985–996. [Google Scholar] [CrossRef]
- Bartsch, Y.C.; Tong, X.; Kang, J.; Avendaño, M.J.; Serrano, E.F.; García-Salum, T.; Pardo-Roa, C.; Riquelme, A.; Cai, Y.; Renzi, I.; et al. Omicron variant Spike-specific antibody binding and Fc activity are preserved in recipients of mRNA or inactivated COVID-19 vaccines. Sci. Transl. Med. 2022, 14, eabn9243. [Google Scholar] [CrossRef]
- Maher, M.C.; Soriaga, L.B.; Gupta, A.; Chen, Y.-P.; di Iulio, J.; Ledoux, S.; Smithey, M.J.; Cathcart, A.L.; McKusick, K.; Sun, D.; et al. Antibody therapy reverses biological signatures of COVID-19 progression. Cell. Rep. Med. 2022, 3, 100721. [Google Scholar] [CrossRef]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Hongchao, P.; Peto, R.; Ana-Maria, H.-R.; Marie-Pierre, P.; Vasee, S.; Quarraisha, A.K.; Marissa, M.A.; César, H.G.; Marie-Paule, K.; Reza, M.; et al. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497. [Google Scholar]
- Siemieniuk, R.A.; Bartoszko, J.J.; Zeraatkar, D.; Kum, E.; Qasim, A.; Martinez, J.P.D.; Izcovich, A.; Lamontagne, F.; Han, M.A.; Agarwal, A.; et al. Drug treatments for COVID-19: Living systematic review and network meta-analysis. BMJ 2020, 370, m2980.49. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.T.; Malani, P.N.; del Rio, C. COVID-19 Therapeutics for Nonhospitalized Patients. JAMA 2022, 327, 617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Rochwerg, B.; Agarwal, A.; Zeng, L.; Leo, Y.-S.; Appiah, J.A.; Agoritsas, T.; Bartoszko, J.; Brignardello-Petersen, R.; Ergan, B.; Ge, L.; et al. Remdesivir for severe COVID-19: A clinical practice guideline. BMJ 2020, 370, m2924. [Google Scholar] [CrossRef]
- Grundeis, F.; Ansems, K.; Dahms, K.; Thieme, V.; Metzendorf, M.I.; Skoetz, N.; Benstoem, C.; Mikolajewska, A.; Griesel, M.; Fichtner, F.; et al. Remdesivir for the treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 8, CD014962. [Google Scholar]
- Ader, F.; Bouscambert-Duchamp, M.; Hites, M.; Peiffer-Smadja, N.; Poissy, J.; Belhadi, D.; Diallo, A.; Lê, M.-P.; Peytavin, G.; Staub, T.; et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2022, 22, 209–221. [Google Scholar] [CrossRef]
- Kaka, A.S.; MacDonald, R.; Linskens, B.E.J.; Langsetmo, L.; Vela, M.K.; Duan-Porter, W.; Wilt, T.J. Major Update 2: Remdesivir for Adults with COVID-19: A Living Systematic Review and Meta-analysis for the American College of Physicians Practice Points. Ann. Intern. Med. 2022, 175, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, A.; Speich, B.; Mentré, F.; Rueegg, C.S.; Belhadi, D.; Assoumou, L.; Burdet, C.; Murthy, S.; Dodd, L.E.; Wang, Y.; et al. Effects of remdesivir in patients hospitalised with COVID-19: A systematic review and individual patient data meta-analysis of randomised controlled trials. Lancet Respir. Med. 2023, 11, 453–464. [Google Scholar] [CrossRef]
- Ali, K.; Azher, T.; Baqi, M.; Binnie, A.; Borgia, S.; Carrier, F.M.; Cavayas, Y.A.; Chagnon, N.; Cheng, M.P.; Conly, J.; et al. Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: A randomized controlled trial. Can. Med. Assoc. J. 2022, 194, E242–E251. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, E.; Chandak, A.; Zhang, Z.; Liang, S.; Thrun, M.; Gottlieb, R.L.; Kuritzkes, D.R.; Sax, P.E.; Wohl, D.A.; Casciano, R.; et al. Remdesivir Treatment in Hospitalized Patients with Coronavirus Disease 2019 (COVID-19): A Comparative Analysis of In-hospital All-cause Mortality in a Large Multicenter Observational Cohort. Clin. Infect. Dis. 2022, 75, e450–e458. [Google Scholar] [CrossRef]
- Hogan, J.I.; Duerr, R.; Dimartino, D.; Marier, C.; Hochman, S.E.; Mehta, S.; Wang, G.; Heguy, A. Remdesivir Resistance in Transplant Recipients with Persistent Coronavirus Disease 2019. Clin. Infect. Dis. 2023, 76, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients. Clin. Infect. Dis. 2023, 76, e342–e349. [Google Scholar] [CrossRef]
- Ganatra, S.; Dani, S.S.; Ahmad, J.; Kumar, A.; Shah, J.; Abraham, G.M.; McQuillen, D.P.; Wachter, R.M.; Sax, P.E. Oral Nirmatrelvir and Ritonavir in Nonhospitalized Vaccinated Patients with Coronavirus Disease 2019. Clin. Infect. Dis. 2023, 76, 563–572. [Google Scholar] [CrossRef]
- Rubin, R. From Positive to Negative to Positive Again—The Mystery of Why COVID-19 Rebounds in Some Patients Who Take Paxlovid. JAMA 2022, 327, 2380–2382. [Google Scholar] [CrossRef]
- Butler, C.C.; Hobbs, F.D.R.; Gbinigie, O.A.; Rahman, N.M.; Hayward, G.; Richards, D.B.; Dorward, J.; Lowe, D.M.; Standing, J.F.; Breuer, J.; et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): An open-label, platform-adaptive randomised controlled trial. Lancet 2023, 401, 281–293. [Google Scholar] [CrossRef]
- Wong, C.K.H.; Au, I.C.H.; Lau, K.T.K.; Lau, E.H.Y.; Cowling, B.J.; Leung, G.M. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: An observational study. Lancet 2022, 400, 1213–1222. [Google Scholar]
- Khoo, S.H.; FitzGerald, R.; Saunders, G.; Middleton, C.; Ahmad, S.; Edwards, C.J.; Hadjiyiannakis, D.; Walker, L.; Lyon, R.; Shaw, V.; et al. Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): A randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Infect. Dis. 2023, 23, 183. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Zarębska-Michaluk, D.; Rogalska, M.; Kryńska, J.A.; Kowalska, J.; Dutkiewicz, E.; Dobrowolska, K.; Jaroszewicz, J.; Moniuszko-Malinowska, A.; Rorat, M.; et al. Real-world experience with molnupiravir during the period of SARS-CoV-2 Omicron variant dominance. Pharmacol. Rep. 2022, 74, 1279–1285. [Google Scholar] [CrossRef]
- EMA. Refusal of the Marketing Authorisation for Lagevrio (Molnupiravir). 2023. Available online: www.ema.europa.eu/en/documents/smop-initial/questions-answers-refusal-marketing-authorisation-lagevrio-molnupiravir_en.pdf (accessed on 21 April 2023).
- Shah, V.P.; Farah, W.H.; Hill, J.C.; Hassett, L.C.; Binnicker, M.J.; Yao, J.D.; Murad, M.H. Association between SARS-CoV-2 cycle threshold values and clinical outcomes in patients with COVID-19: A systematic review and meta-analysis. Open Forum. Infect. Dis. 2021, 8, ofab453. [Google Scholar] [CrossRef]
- Alonso-Navarro, R.; Cuesta, G.; Santos, M.; Cardozo, C.; Rico, V.; Garcia-Pouton, N.; Tuset, M.; Bodro, M.; Morata, L.; Puerta-Alcalde, P.; et al. Qualitative subgenomic RNA to monitor the response to remdesivir in hos-pitalized patients with COVID-19: Impact on the length of hospital stay and mortality. Clin. Infect. Dis. 2022, 76, 32–38. [Google Scholar] [CrossRef]
- Kim, J.Y.; Bae, J.Y.; Bae, S.; Cha, H.H.; Kwon, J.S.; Suh, M.H.; Lee, H.J.; Jung, J.; Kim, M.J.; Cui, C.; et al. Diagnostic usefulness of subgenomic RNA detection of viable SARS-CoV-2 in patients with COVID-19. Clin. Microbiol. Infect. 2022, 28, 101–106. [Google Scholar] [CrossRef]
- Dai, C.L.; Kornilov, S.A.; Roper, R.T.; Cohen-Cline, H.; Jade, K.; Smith, B.; Heath, J.R.; Diaz, G.; Goldman, J.D.; Magis, A.T.; et al. Characteristics and Factors Associated with Coronavirus Disease 2019 Infection, Hospitalization, and Mortality Across Race and Ethnicity. Clin. Infect. Dis. 2021, 73, 2193–2204. [Google Scholar] [CrossRef]
- Gray, W.K.; Navaratnam, A.V.; Day, J.; Wendon, J.; Briggs, T.W.R. Changes in COVID-19 in-hospital mortality in hospitalised adults in England over the first seven months of the pandemic: An observational study using administrative data. Lancet Reg. Health Eur. 2021, 5, 100104. [Google Scholar] [CrossRef] [PubMed]
- Khamis, F.; Memish, Z.; Al Bahrani, M.; Al Dowaiki, S.; Pandak, N.; Al Bolushi, Z.; Al Salmi, I.; Al-Zakwani, I. Prevalence and predictors of in-hospital mortality of patients hospitalized with COVID-19 infection. J. Infect. Public Health 2021, 14, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Castilla, J.; Guevara, M.; Miqueleiz, A.; Baigorria, F.; Ibero-Esparza, C.; Navascués, A.; Trobajo-Sanmartín, C.; Martínez-Baz, I.; Casado, I.; Burgui, C.; et al. Risk Factors of Infection, Hospitalization and Death from SARS-CoV-2: A Population-Based Cohort Study. J. Clin. Med. 2021, 10, 2608. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, E.; Asgary, A.; Tavakoli, N.; Zali, A.; Dastan, F.; Daaee, A.; Badakhshan, M.; Esmaily, H.; Jamaldini, S.H.; Safari, S.; et al. Symptom Prediction and Mortality Risk Calculation for COVID-19 Using Machine Learning. Front. Artif. Intell. 2021, 4, 673527. [Google Scholar] [CrossRef]
- Agrawal, U.; Katikireddi, S.V.; McCowan, C.; Mulholland, R.H.; Azcoaga-Lorenzo, A.; Amele, S.; Fagbamigbe, A.F.; Vasileiou, E.; Grange, Z.; Shi, T.; et al. COVID-19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV-19 vaccinations in 2·57 million people in Scotland (EAVE II): A prospective cohort study. Lancet Respir. Med. 2021, 9, 1439–1449. [Google Scholar] [CrossRef]
- Lana, R.M.; Freitas, L.P.; Codeço, C.T.; Pacheco, A.G.; de Carvalho, L.M.F.; Villela, D.A.M.; Coelho, F.C.; Cruz, O.G.; Niquini, R.P.; Porto, V.B.G.; et al. Identification of priority groups for COVID-19 vaccination in Brazil. Cad Saude Publica 2021, 37, e00049821. [Google Scholar] [CrossRef]
- Agrawal, U.; Azcoaga-Lorenzo, A.; Fagbamigbe, A.F.; Vasileiou, E.; Henery, P.; Simpson, C.R.; Stock, S.J.; Shah, S.A.; Robertson, C.; Woolhouse, M.; et al. Association between multimorbidity and mortality in a cohort of patients admitted to hospital with COVID-19 in Scotland. J. R. Soc. Med. 2022, 115, 22–30. [Google Scholar] [CrossRef]
- Ouattara, E.; Bruandet, A.; Borde, A.; Lenne, X.; Binder-Foucard, F.; Le-Bourhis-Zaimi, M.; Muller, J.; Loc, P.T.B.; Séguret, F.; du Montcel, S.T.; et al. Risk factors of mortality among patients hospitalised with COVID-19 in a critical care or hospital care unit: Analysis of the French national medico administrative database. BMJ Open Respir. Res. 2021, 8, e001002. [Google Scholar] [CrossRef]
- Lapi, F.; Domnich, A.; Marconi, E.; Rossi, A.; Grattagliano, I.; Lagolio, E.; Medea, G.; Sessa, A.; Cricelli, I.; Icardi, G.; et al. Predicting the risk of severe COVID-19 outcomes in primary care: Development and validation of a vulnerability index for equitable allocation of effective vaccines. Expert Rev. Vaccines 2022, 21, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Discacciati, M.G.; Siani, S.; Campa, A.; Nakaya, H.I. Why should obese youth be prioritized in COVID-19 vaccination programs? A nationwide retrospective study. Lancet Reg. Health Am. 2022, 7, 100167. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Schäffer, A.A.; Merzon, E.; Green, I.; Magen, E.; Golan-Cohen, A.; Vinker, S.; Ruppin, E. A calculator for COVID-19 severity prediction based on patient risk factors and number of vaccines received. Microorganisms 2022, 10, 1238. [Google Scholar] [CrossRef]
- Davies, M.A.; Kassanjee, R.; Rousseau, P.; Morden, E.; Johnson, L.; Solomon, W.; Hsiao, N.Y.; Hussey, H.; Meintjes, G.; Paleker, M.; et al. Western Cape and South African National Departments of Health in collaboration with the National Institute for Communicable Diseases in South Africa Affiliations. Outcomes of laboratory-confirmed SARS-CoV-2 infection in the Omicron-driven fourth wave compared with previous waves in the Western Cape Province, South Africa. Trop. Med. Int. Health 2022, 27, 564–573. [Google Scholar] [CrossRef]
- Esteban, C.; Villanueva, A.; García-Gutierrez, S.; Aramburu, A.; Gorordo, I.; Quintana, J.M. Working Group TC. COPD in SARS-CoV-2 pandemic. baseline characteristics related to hospital admissions. Expert Rev. Respir. Med. 2022, 16, 477–484. [Google Scholar] [PubMed]
- Portuondo-Jimenez, J.; Bilbao-González, A.; Tíscar-González, V.; Garitano-Gutiérrez, I.; García-Gutiérrez, S.; Martínez-Mejuto, A.; Santiago-Garin, J.; Arribas-García, S.; García-Asensio, J.; Chart-Pascual, J.; et al. Modelling the risk of hospital admission of lab confirmed SARS-CoV-2-infected patients in primary care: A population-based study. Intern. Emerg. Med. 2022, 17, 1211–1221. [Google Scholar] [CrossRef]
- Moreno-Perez, O.; Ribes, I.; Boix, V.; Martinez-García, M.; Otero-Rodriguez, S.; Reus, S.; Sánchez-Martínez, R.; Ramos, J.M.; Chico-Sánchez, P.; Merino, E. Hospitalized patients with breakthrough COVID-19: Clinical features and poor outcome predictors. Int. J. Infect. Dis. 2022, 118, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Antoon, J.W.; Grijalva, C.G.; Thurm, C.; Richardson, T.; Spaulding, A.B.; Ii, R.J.T.; Reyes, M.A.; Shah, S.S.; Burns, J.E.; Kenyon, C.C.; et al. Factors Associated with COVID-19 Disease Severity in US Children and Adolescents. J. Hosp. Med. 2021, 16, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Harwood, R.; Yan, H.T.; Da Camara, N.T.; Smith, C.; Ward, J.; Tudur-Smith, C.; Linney, M.; Clark, M.; Whittaker, E.; Saatci, D.; et al. Which children and young people are at higher risk of severe disease and death after hospitalisation with SARS-CoV-2 infection in children and young people: A systematic review and individual patient meta-analysis. Eclinicalmedicine 2022, 44, 101287. [Google Scholar] [CrossRef]
- Boudou, M.; Óhaiseadha, C.; Garvey, P.; O’dwyer, J.; Hynds, P. Modelling COVID-19 severity in the Republic of Ireland using patient co-morbidities, socioeconomic profile and geographic location, February to November 2020. Sci. Rep. 2021, 11, 18474. [Google Scholar] [CrossRef]
- Ge, E.; Li, Y.; Wu, S.; Candido, E.; Wei, X. Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: A population-based cohort study. PLoS ONE 2021, 16, e0258154. [Google Scholar] [CrossRef]
- Agrawal, U.; Bedston, S.; McCowan, C.; Oke, J.; Patterson, L.; Robertson, C.; Akbari, A.; Azcoaga-Lorenzo, A.; Bradley, D.T.; Fagbamigbe, A.F.; et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: Pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. Lancet 2022, 400, 1305–1320. [Google Scholar] [CrossRef]
- Semenzato, L.; Botton, J.; Drouin, J.; Cuenot, F.; Dray-Spira, R.; Weill, A.; Zureik, M. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: A cohort study of 66 million people. Lancet Reg. Health Eur. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Yates, T.; Zaccardi, F.; Islam, N.; Razieh, C.; Gillies, C.L.; Lawson, C.A.; Chudasama, Y.; Rowlands, A.; Davies, M.J.; Docherty, A.B.; et al. Obesity, Ethnicity, and Risk of Critical Care, Mechanical Ventilation, and Mortality in Patients Admitted to Hospital with COVID-19: Analysis of the ISARIC CCP-UK Cohort. Obesity (Silver Spring) 2021, 29, 1223–1230. [Google Scholar] [CrossRef]
- Treskova-Schwarzbach, M.; Haas, L.; Reda, S.; Pilic, A.; Borodova, A.; Karimi, K.; Koch, J.; Nygren, T.; Scholz, S.; Schönfeld, V.; et al. Pre-existing health conditions and severe COVID-19 outcomes: An umbrella review approach and meta-analysis of global evidence. BMC Med. 2021, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Antos, A.; Kwong, M.L.; Balmorez, T.; Villanueva, A.; Murakami, S. Unusually High Risks of COVID-19 Mortality with Age-Related Comorbidities: An Adjusted Meta-Analysis Method to Improve the Risk Assessment of Mortality Using the Comorbid Mortality Data. Infect. Dis. Rep. 2021, 13, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Román, J.S.; Candel, F.J.; Carretero, M.D.M.; Sanz, J.C.; Pérez-Abeledo, M.; Barreiro, P.; Viñuela-Prieto, J.M.; Ramos, B.; Canora, J.; Barba, R.; et al. Cross-Sectional Analysis of Risk Factors for Outbreak of COVID-19 in Nursing Homes for Older Adults in the Community of Madrid. Gerontology 2023, 69, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Henrina, J.; Lim, M.A.; Lawrensia, S.; Yonas, E.; Vania, R.; Huang, I.; Lukito, A.A.; Suastika, K.; Kuswardhani, R.T.; et al. Clinical frailty scale and mortality in COVID-19: A systematic review and dose-response meta-analysis. Arch. Gerontol. Geriatr. 2021, 93, 104324. [Google Scholar] [CrossRef]
- Díez-Villanueva, P.; Arizá-Solé, A.; Vidán, M.T.; Bonanad, C.; Formiga, F.; Sanchis, J.; Sánchez, F.J.M.; Ros, V.R.; Fernández, M.S.; Bueno, H.; et al. Recomendaciones de la Sección de Cardiología Geriátrica de la Sociedad Española de Cardiología para la valoración de la fragilidad en el anciano con cardiopatía. Rev. Esp. Cardiol. 2019, 72, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Vinson, A.J.M.; Agarwal, G.; Dai, R.; Anzalone, A.J.M.; Lee, S.B.; French, E.B.; Olex, A.M.; Madhira, V.M.; Mannon, R.B. COVID-19 in Solid Organ Transplantation: Results of the National COVID Cohort Collaborative. Transplant. Direct 2021, 7, e775. [Google Scholar] [CrossRef]
- Coll, E.; Fernández-Ruiz, M.; Padilla, M.; Moreso, F.; Hernández-Vicente, A.; Yañez, I.; Molina, M.; Vázquez-Sánchez, T.; Crespo, M.; Facundo, C.; et al. COVID-19 in Solid Organ Transplant Recipients in Spain Throughout 2020: Catching the Wave? Transplantation 2021, 105, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Ao, G.; Wang, Y.; Qi, X.; Nasr, B.; Bao, M.; Gao, M.; Sun, Y.; Xie, D. The association between severe or death COVID-19 and solid organ transplantation: A systematic review and meta-analysis. Transplant. Rev. (Orlando) 2021, 35, 100628. [Google Scholar] [CrossRef] [PubMed]
- Linares, L.; Cofan, F.; Diekmann, F.; Herrera, S.; Marcos, M.A.; Castel, M.A.; Farrero, M.; Colmenero, J.; Ruiz, P.; Crespo, G.; et al. A propensity score-matched analysis of mortality in solid organ transplant patients with COVID-19 compared to non-solid organ transplant patients. PLoS ONE 2021, 16, e0247251. [Google Scholar] [CrossRef]
- Kates, O.S.; Haydel, B.M.; Florman, S.S.; Rana, M.M.; Chaudhry, Z.S.; Ramesh, M.S.; Safa, K.; Kotton, C.N.; Blumberg, E.A.; Besharatian, B.D.; et al. Coronavirus Disease 2019 in Solid Organ Transplant: A Multicenter Cohort Study. Clin. Infect. Dis. 2021, 73, e4090–e4099. [Google Scholar] [CrossRef]
- Swai, J.; Gui, M.; Long, M.; Wei, Z.; Hu, Z.; Liu, S. Humoral and cellular immune response to severe acute respiratory syndrome coronavirus-2 vaccination in haemodialysis and kidney transplant patients. Nephrology (Carlton) 2022, 27, 7–24. [Google Scholar] [CrossRef]
- Hamm, S.R.; Møller, D.L.; Pérez-Alós, L.; Hansen, C.B.; Pries-Heje, M.M.; Heftdal, L.D.; Hasselbalch, R.B.; Fogh, K.; Madsen, J.R.; Armenteros, J.J.A.; et al. Decline in Antibody Concentration 6 Months After Two Doses of SARS-CoV-2 BNT162b2 Vaccine in Solid Organ Transplant Recipients and Healthy Controls. Front. Immunol. 2022, 13, 832501. [Google Scholar] [CrossRef]
- Callaghan, C.J.; Mumford, L.; Curtis, R.M.K.; Williams, S.V.; Whitaker, H.; Andrews, N.; Bernal, J.L.; Ushiro-Lumb, I.; Pettigrew, G.J.; Thorburn, D.; et al. Real-world Effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S Vaccines Against SARS-CoV-2 in Solid Organ and Islet Transplant Recipients. Transplantation 2022, 106, 436–446. [Google Scholar] [CrossRef]
- Yetmar, Z.A.; Bhaimia, E.; Bierle, D.M.; Ganesh, R.; Razonable, R.R. Breakthrough COVID-19 after SARS-CoV-2 vaccination in solid organ transplant recipients: An analysis of symptomatic cases and monoclonal antibody therapy. Transpl. Infect. Dis. 2022, 24, e13779. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.X.; Moore, L.W.; Anjan, S.; Rahamimov, R.; Sifri, C.D.; Ali, N.M.; Morales, M.K.; Tsapepas, D.S.; Basic-Jukic, N.; Miller, R.A.; et al. Risk of Breakthrough SARS-CoV-2 Infections in Adult Transplant Recipients. Transplantation 2021, 105, E265–E266. [Google Scholar] [CrossRef]
- Pinchera, B.; Buonomo, A.R.; Scotto, R.; Carrano, R.; Salemi, F.; Galluccio, F.; Guarino, M.; Viceconte, G.; Moriello, N.S.; Giaccone, A.; et al. Sotrovimab in Solid Organ Transplant Patients with Early, Mild/Moderate SARS-CoV-2 Infection: A Single-center Experience. Transplantation 2022, 106, e343–e345. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.J.; Hardesty, A.; Vieira, K.; Farmakiotis, D. Use of anti-spike monoclonal antibodies in kidney transplant recipients with COVID-19: Efficacy, ethnic and racial disparities. Am. J. Transplant. 2022, 22, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Chavarot, N.; Melenotte, C.; Amrouche, L.; Rouzaud, C.; Sberro-Soussan, R.; Pavie, J.; Martinez, F.; Pouvaret, A.; Leruez-Ville, M.; Cantin, D.; et al. Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with Omicron infection. Kidney Int. 2022, 101, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Gaussen, A.; Hornby, L.M.; Rockl, G.M.; O’brien, S.; Delage, G.; Sapir-Pichhadze, R.M.; Drews, S.J.; Weiss, M.J.; Lewin, A. Evidence of SARS-CoV-2 Infection in Cells, Tissues, and Organs and the Risk of Transmission Through Transplantation. Transplantation 2021, 105, 1405–1422. [Google Scholar] [CrossRef] [PubMed]
- Neidlinger, N.A.; Smith, J.A.; D’alessandro, A.M.; Roe, D.; Taber, T.E.; Pereira, M.R.; Friedman, A.L. Organ recovery from deceased donors with prior COVID-19: A case series. Transpl. Infect. Dis. 2021, 23, e13503. [Google Scholar] [CrossRef]
- Romagnoli, R.; Gruttadauria, S.; Tisone, G.; Ettorre, G.M.; De Carlis, L.; Martini, S.; Tandoi, F.; Trapani, S.; Saracco, M.; Luca, A.; et al. Liver transplantation from active COVID-19 donors: A lifesaving opportunity worth grasping? Am. J. Transplant. 2021, 21, 3919–3925. [Google Scholar] [CrossRef]
- Sigler, R.; Shah, M.; Schnickel, G.; Pretorius, V.; Dan, J.; Taremi, M.; Aslam, S. Successful heart and kidney transplantation from a deceased donor with PCR positive COVID-19. Transpl. Infect. Dis. 2021, 23, e13707. [Google Scholar] [CrossRef]
- Koval, C.E.; Poggio, E.D.; Lin, Y.C.; Kerr, H.; Eltemamy, M.; Wee, A. Early success transplanting kidneys from donors with new SARS-CoV-2 RNA positivity: A report of 10 cases. Am. J. Transplant. 2021, 21, 3743–3749. [Google Scholar] [CrossRef] [PubMed]
- Saracco, M.; Romagnoli, R.; Martini, S. Solid non-lung organs from COVID-19 donors in seropositive or naive recipients: Where do we stand? Transpl. Infect. Dis. 2022, 24, e13761. [Google Scholar] [CrossRef] [PubMed]
- Dhand, A.; Gass, A.; Nishida, S.; Kai, M.; Berger, K.; Wolf, D.; Ohira, S.; Sogawa, H.; Lee, L.; Lebovics, E.; et al. Successful transplantation of organs from a deceased donor with early SARS-CoV-2 infection. Am. J. Transplant. 2021, 21, 3804–3805. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, E.M.; Kaul, D.R.; Wolfe, C.R. The pandemic provides a pathway: What we know and what we need to know about using COVID positive donors. Transpl. Infect. Dis. 2021, 23, e13727. [Google Scholar] [CrossRef]
- Kaul, D.R.; Valesano, A.L.; Petrie, J.G.; Sagana, R.; Lyu, D.; Lin, J.; Stoneman, E.; Smith, L.M.; Lephart, P.; Lauring, A.S. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am. J. Transplant. 2021, 21, 2885–2889. [Google Scholar] [CrossRef]
- Kumar, D.; Humar, A.; Keshavjee, S.; Cypel, M. A call to routinely test lower respiratory tract samples for SARS-CoV-2 in lung donors. Am. J. Transplant. 2021, 21, 2623–2624. [Google Scholar] [CrossRef]
- Shah, G.L.; DeWolf, S.; Lee, Y.J.; Tamari, R.; Dahi, P.B.; Lavery, J.A.; Ruiz, J.D.; Devlin, S.M.; Cho, C.; Peled, J.U.; et al. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J. Clin. Investig. 2020, 130, 6656–6667. [Google Scholar] [CrossRef]
- Lupo-Stanghellini, M.T.; Xue, E.; Mastaglio, S.; Oltolini, C.; Angelillo, P.; Messina, C.; Piemontese, S.; Girlanda, S.; Farina, F.; Lazzari, L.; et al. COVID-19 in recipients of allogeneic stem cell transplantation: Favorable outcome. Bone Marrow Transplant. 2021, 56, 2312–2315. [Google Scholar] [CrossRef]
- Camargo, J.F.; Mendoza, M.A.; Lin, R.; Moroz, I.V.; Anderson, A.D.; Morris, M.I.; Natori, Y.; Natori, A.; Raja, M.; Lekakis, L.; et al. Clinical presentation and outcomes of COVID-19 following hematopoietic cell transplantation and cellular therapy. Transpl. Infect. Dis. 2021, 23, e13625. [Google Scholar] [CrossRef]
- Ljungman, P.; de la Camara, R.; Mikulska, M.; Tridello, G.; Aguado, B.; Al Zahrani, M.; Apperley, J.; Berceanu, A.; Bofarull, R.M.; Calbacho, M.; et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia 2021, 35, 2885–2894. [Google Scholar] [CrossRef]
- Orchard, K.; Dignan, F.L.; Lee, J.; Pearce, R.; Desai, M.; McFarlane, E.; Parkin, A.; Shearn, P.; Snowden, J.A. The NICE COVID-19 rapid guideline on haematopoietic stem cell transplantation: Development, implementation and impact. Br. J. Haematol. 2021, 192, 467–473. [Google Scholar] [CrossRef]
- Ljungman, P.; Transplantation, F.T.E.S.F.B.A.M.; Mikulska, M.; De La Camara, R.; Basak, G.W.; Chabannon, C.; Corbacioglu, S.; Duarte, R.; Dolstra, H.; Lankester, A.C.; et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020, 55, 2071–2076. [Google Scholar] [CrossRef]
- Tehrani, D.M.; Wang, X.; Rafique, A.M.; Hayek, S.S.; Herrmann, J.; Neilan, T.G.; Desai, P.; Morgans, A.; Lopez-Mattei, J.; Parikh, R.V.; et al. Impact of cancer and cardiovascular disease on in-hospital outcomes of COVID-19 patients: Results from the american heart association COVID-19 cardiovascular disease registry. Cardio-Oncology 2021, 7, 28. [Google Scholar] [CrossRef]
- Bushman, D.; Davidson, A.; Pathela, P.; Greene, S.K.; Weiss, D.; Reddy, V.; New York City Fatal Case-Control St New York City Fatal Case-Control Study Team; Latash, J. Risk Factors for Death Among Hospitalized Patients Aged 21–64 Years Diagnosed with COVID-19—New York City, March 13–April 9, 2020. J. Racial Ethn. Health Disparities 2022, 9, 1584–1599. [Google Scholar] [CrossRef]
- Roel, E.; Pistillo, A.; Recalde, M.; Fernández-Bertolín, S.; Aragón, M.; Soerjomataram, I.; Jenab, M.; Puente, D.; Prieto-Alhambra, D.; Burn, E.; et al. Cancer and the risk of coronavirus disease 2019 diagnosis, hospitalisation and death: A population-based multistate cohort study including 4 618 377 adults in Catalonia, Spain. Int. J. Cancer 2022, 150, 782–794. [Google Scholar] [CrossRef]
- Mittelman, M.; Magen, O.; Barda, N.; Dagan, N.; Oster, H.S.; Leader, A.; Balicer, R. Effectiveness of the BNT162b2mRNA COVID-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood 2022, 139, 1439–1451. [Google Scholar] [CrossRef]
- Martínez, J.C.; Sica, R.A.; Stockerl-Goldstein, K.; Rubinstein, S.M. COVID-19 in Patients with Hematologic Malignancies: Outcomes and Options for Treatments. Acta Haematol. 2022, 145, 244–256. [Google Scholar] [CrossRef]
- Elkrief, A.; Wu, J.T.; Jani, C.; Enriquez, K.T.; Glover, M.; Shah, M.R.; Shaikh, H.G.; Beeghly-Fadiel, A.; French, B.; Jhawar, S.R.; et al. Learning through a Pandemic: The Current State of Knowledge on COVID-19 and Cancer. Cancer Discov. 2022, 12, 303–330. [Google Scholar] [CrossRef]
- Arellano-Llamas, A.A.; Vela-Ojeda, J.; Hernandez-Caballero, A. Chronic Lymphocytic Leukemia in the SARS-CoV-2 Pandemic. Curr. Oncol. Rep. 2022, 24, 209–213. [Google Scholar] [CrossRef]
- Pinato, D.J.; Scotti, L.; Gennari, A.; Colomba-Blameble, E.; Dolly, S.; Loizidou, A.; Chester, J.; Mukherjee, U.; Zambelli, A.; Aguilar-Company, J.; et al. Determinants of enhanced vulnerability to coronavirus disease 2019 in UK patients with cancer: A European study. Eur. J. Cancer 2021, 150, 190–202. [Google Scholar] [CrossRef]
- Ribera, J.-M.; Morgades, M.; Coll, R.; Barba, P.; López-Lorenzo, J.-L.; Montesinos, P.; Foncillas, M.-A.; Cabrero, M.; Gómez-Centurión, I.; Morales, M.-D.; et al. Frequency, Clinical Characteristics and Outcome of Adults with Acute Lymphoblastic Leukemia and COVID 19 Infection in the First vs. Second Pandemic Wave in Spain. Clin. Lymphoma Myeloma Leuk. 2021, 21, e801–e809. [Google Scholar] [CrossRef]
- Shmueli, E.S.; Itay, A.; Margalit, O.; Berger, R.; Halperin, S.; Jurkowicz, M.; Levin, E.G.; Levy, I.; Olmer, L.; Regev-Yochay, G.; et al. Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy—A single center prospective study. Eur. J. Cancer 2021, 157, 124–131. [Google Scholar] [CrossRef]
- Brosh-Nissimov, T.; Orenbuch-Harroch, E.; Chowers, M.; Elbaz, M.; Nesher, L.; Stein, M.; Maor, Y.; Cohen, R.; Hussein, K.; Weinberger, M.; et al. BNT162b2 vaccine breakthrough: Clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin. Microbiol. Infect. 2021, 27, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Verbruggen, L.; Teuwen, L.; Vanhoutte, G.; Kerckhove, S.V.; Raats, S.; Van der Massen, I.; De Keersmaecker, S.; Debie, Y.; Huizing, M.; et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open 2021, 6, 100274. [Google Scholar] [CrossRef]
- Duléry, R.; Lamure, S.; Delord, M.; Di Blasi, R.; Chauchet, A.; Hueso, T.; Rossi, C.; Drenou, B.; Fischer, B.D.; Soussain, C.; et al. Prolonged in-hospital stay and higher mortality after COVID-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am. J. Hematol. 2021, 96, 934–944. [Google Scholar] [CrossRef]
- Safari, M.; Faradmal, J.; Bashirian, S.; Soltanian, A.R.; Khazaei, S.; Roshanaei, G. Identifying the Risk Factors for Mortality in Patients with Cancer and COVID-19 in Hamadan, the West of Iran. J. Gastrointest. Cancer 2022, 53, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Blanco, J.L.; Reyes-Urueña, J.M.; Davies, M.-A.; Sued, O.; Marcos, M.A.; Martínez, E.; Bertagnolio, S.; Alcamí, J.; Miro, J.M.; et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV 2021, 8, e294–e305. [Google Scholar] [CrossRef]
- Gervasoni, C.; Meraviglia, P.; Riva, A.; Giacomelli, A.; Oreni, L.; Minisci, D.; Atzori, C.; Ridolfo, A.; Cattaneo, D. Clinical Features and Outcomes of Patients with Human Immunodeficiency Virus with COVID-19. Clin. Infect. Dis. 2020, 71, 2276–2278. [Google Scholar] [CrossRef] [PubMed]
- Karmen-Tuohy, S.; Carlucci, P.M.; Zervou, F.N.; Zacharioudakis, I.M.; Rebick, G.; Klein, E.; Reich, J.; Jones, S.; Rahimian, J. Outcomes Among HIV-Positive Patients Hospitalized with COVID-19. JAIDS J. Acquir. Immune Defic. Syndr. 2020, 85, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Byrd, K.M.; Beckwith, C.G.; Garland, J.M.; Johnson, J.E.; Aung, S.; Cu-Uvin, S.; Farmakiotis, D.; Flanigan, T.; Gillani, F.S.; Macias-Gil, R.; et al. SARS-CoV-2 and HIV coinfection: Clinical experience from Rhode Island, United States. J. Int. AIDS Soc. 2020, 23, e25573. [Google Scholar] [CrossRef]
- Inciarte, A.; Gonzalez-Cordon, A.; Rojas, J.; Torres, B.; De Lazzari, E.; De La Mora, L.; Martinez-Rebollar, M.; Laguno, M.; Callau, P.; Gonzalez-Navarro, A.; et al. Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV: A single-center, prospective observational study. AIDS 2020, 34, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.J.; Woodward, B.L.; Alom, S.; Harky, A. Coronavirus disease 2019 (COVID-19) outcomes in HIV/AIDS patients: A systematic review. HIV Med. 2020, 21, 567–577. [Google Scholar] [CrossRef]
- Shalev, N.; Scherer, M.; Lasota, E.D.; Antoniou, P.; Yin, M.T.; Zucker, J.; Sobieszczyk, M.E. Clinical Characteristics and Outcomes in People Living with Human Immunodeficiency Virus Hospitalized for Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2294–2297. [Google Scholar] [CrossRef]
- Sigel, K.; Swartz, T.; Golden, E.; Paranjpe, I.; Somani, S.; Richter, F.; De Freitas, J.K.; Miotto, R.; Zhao, S.; Polak, P.; et al. Coronavirus 2019 and People Living with Human Immunodeficiency Virus: Outcomes for Hospitalized Patients in New York City. Clin. Infect. Dis. 2020, 71, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, D.; Mara, E.; Hsu, L.; Scheer, S.; Rutherford, G.; Enanoria, W.; Gandhi, M. COVID-19 Susceptibility and Outcomes Among People Living with HIV in San Francisco. J. Acquir. Immune. Defic. Syndr. 2021, 86, 19–21. [Google Scholar] [CrossRef]
- Berenguer, J.; Díez, C.; Martín-Vicente, M.; Micán, R.; Pérez-Elías, M.J.; García-Fraile, L.J.; Vidal, F.; Suárez-García, I.; Podzamczer, D.; Del Romero, J.; et al. Prevalence and factors associated with SARS-CoV-2 seropositivity in the Spanish HIV Research Network Cohort. Clin. Microbiol. Infect. 2021, 27, 1678–1684. [Google Scholar] [CrossRef]
- Martín-Vicente, M.; Berenguer, J.; Muñoz-Gómez, M.J.; Díez, C.; Micán, R.; Pérez-Elías, M.J.; García-Fraile, L.J.; Peraire, J.; Suárez-García, I.; Jiménez-Sousa, M.; et al. Similar humoral immune responses against the SARS-CoV-2 spike protein in HIV and non-HIV individuals after COVID-19. J. Infect. 2022, 84, 418–467. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.L.; Ambrosioni, J.; Garcia, F.; Martínez, E.; Soriano, A.; Mallolas, J.; Miro, J.M. COVID-19 in patients with HIV: Clinical case series. Lancet HIV 2020, 7, e314–e316. [Google Scholar] [CrossRef]
- Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases (South Africa). Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a Population Cohort Study from the Western Cape Province, South Africa. Clin Infect Dis. 2021, 73, e2005–e2015. [Google Scholar] [CrossRef]
- Mellor, M.M.; Bast, A.C.; Jones, N.R.; Roberts, N.W.; Ordóñez-Mena, J.M.; Reith, A.J.; Butler, C.C.; Matthews, P.C.; Dorward, J. Risk of adverse coronavirus disease 2019 outcomes for people living with HIV. AIDS 2021, 35, F1–F10. [Google Scholar] [CrossRef]
- Braunstein, S.L.; Lazar, R.; Wahnich, A.; Daskalakis, D.C.; Blackstock, O.J. Coronavirus Disease 2019 (COVID-19) Infection Among People with Human Immunodeficiency Virus in New York City: A Population-Level Analysis of Linked Surveillance Data. Clin. Infect. Dis. 2021, 72, E1021–E1029. [Google Scholar] [CrossRef] [PubMed]
- Triant, V.A.; Gandhi, R.T. When Epidemics Collide: Why People with Human Immunodeficiency Virus May Have Worse Coronavirus Disease 2019 Outcomes and Implications for Vaccination. Clin. Infect. Dis. 2021, 72, e1030–e1034. [Google Scholar] [CrossRef] [PubMed]
- Varshney, K.; Ghosh, P.; Stiles, H.; Iriowen, R. Risk Factors for COVID-19 Mortality Among People Living with HIV: A Scoping Review. AIDS Behav. 2022, 26, 2256–2265. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Croxford, S.E.; Nash, S.; Khawam, J.; Kirwan, P.; Kall, M.; Bradshaw, D.; Sabin, C.; Miller, R.F.; Post, F.A.; et al. COVID-19 mortality among people with diagnosed HIV compared to those without during the first wave of the COVID-19 pandemic in England. HIV Med. 2022, 23, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Vizcarra, P.; Pérez-Elías, M.J.; Quereda, C.; Moreno, A.; Vivancos, M.J.; Dronda, F.; Casado, J.L.; Moreno, S.; Fortún, J.; Navas, E.; et al. Description of COVID-19 in HIV-infected individuals: A single-center, prospective cohort. Lancet HIV 2020, 7, e554–e564. [Google Scholar] [CrossRef]
- Meyerowitz, E.A.; Kim, A.Y.; Ard, K.L.; Basgoz, N.; Chu, J.T.; Hurtado, R.M.; Lee, C.K.; He, W.; Minukas, T.; Nelson, S.; et al. Disproportionate burden of coronavirus disease 2019 among racial minorities and those in congregate settings among a large cohort of people with HIV. AIDS 2020, 34, 1781–1787. [Google Scholar] [CrossRef]
- Di Biagio, A.; Ricci, E.; Calza, L.; Squillace, N.; Menzaghi, B.; Rusconi, S.; Orofino, G.; Bargiacchi, O.; Molteni, C.; Valsecchi, L.; et al. Factors associated with hospital admission for COVID-19 in HIV patients. AIDS 2020, 34, 1983–1985. [Google Scholar] [CrossRef]
- Etienne, N.; Karmochkine, M.; Slama, L.; Pavie, J.; Batisse, D.; Usubillaga, R.; Letembet, V.-A.; Brazille, P.; Canouï, E.; Slama, D.; et al. HIV infection and COVID-19: Risk factors for severe disease. AIDS 2020, 34, 1771–1774. [Google Scholar] [CrossRef]
- Hadi, Y.B.; Naqvi, S.F.Z.; Kupec, J.T.; Sarwari, A.R. Characteristics and outcomes of COVID-19 in patients with HIV: A multicentre research network study. AIDS 2020, 34, F3–F8. [Google Scholar] [CrossRef]
- Mirzaei, H.; McFarland, W.; Karamouzian, M.; Sharifi, H. COVID-19 Among People Living with HIV: A Systematic Review. AIDS Behav. 2021, 25, 85–92. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Rentsch, C.T.; MacKenna, B.; Schultze, A.; Mehrkar, A.; Bates, C.J.; Eggo, R.M.; Morton, C.E.; Bacon, S.C.J.; Inglesby, P.; et al. HIV infection and COVID-19 death: A population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021, 8, e24–e32. [Google Scholar] [CrossRef] [PubMed]
- Tesoriero, J.M.; Swain, C.A.E.; Pierce, J.L.; Zamboni, L.; Wu, M.; Holtgrave, D.R.; Gonzalez, C.J.; Udo, T.; Morne, J.E.; Hart-Malloy, R.; et al. COVID-19 Outcomes Among Persons Living with or without Diagnosed HIV Infection in New York State. JAMA Netw. Open 2021, 4, e2037069. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.E.; Peluso, M.J.; Margus, C.; Lopes, J.P.M.; He, C.; Gaisa, M.M.; Osorio, G.; Aberg, J.A.; Mullen, M.P. Clinical Outcomes and Immunologic Characteristics of Coronavirus Disease 2019 in People with Human Immunodeficiency Virus. J. Infect. Dis. 2021, 223, 403–408. [Google Scholar] [CrossRef] [PubMed]
- BHIVA DEGPSAS and PA for the Clinical Study of A (APECS). BHIVA, DAIG, EACS, GESIDA, Polish Scientific AIDS Society and Portuguese Association for the clinical study of AIDS (APECS). Available online: https://www.bhiva.org/joint-statement-on-risk-of-COVID-19-for-PLWH-and-SARS-CoV-2-vaccine-advice (accessed on 6 September 2022).
- del Amo, J.; Polo, R.; Moreno, S.; Jarrín, I.; Hernán, M.A. SARS-CoV-2 infection and coronavirus disease 2019 severity in persons with HIV on antiretroviral treatment. AIDS 2022, 36, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.E.; Ignacio, R.A.B.; Whitney, B.M.; Delaney, J.A.; Nance, R.M.; Bamford, L.; Wooten, D.; Keruly, J.C.; Burkholder, G.; Napravnik, S.; et al. Factors Associated with Severity of COVID-19 Disease in a Multicenter Cohort of People with HIV in the United States, March–December 2020. JAIDS J. Acquir. Immune Defic. Syndr. 2022, 90, 369–376. [Google Scholar] [CrossRef]
- Childs, K.; Post, F.A.; Norcross, C.; Ottaway, Z.; Hamlyn, E.; Quinn, K.; Juniper, T.; Taylor, C. Hospitalized Patients with COVID-19 and Human Immunodeficiency Virus: A Case Series. Clin. Infect. Dis. 2020, 71, 2021–2022. [Google Scholar] [CrossRef]
- Díez, C.; Del Romero-Raposo, J.; Mican, R.; López, J.C.; Blanco, J.R.; Calzado, S.; Samperiz, G.; Portilla, J.; García-Fraile, L.J.; Gutiérrez, F.; et al. COVID-19 in hospitalized HIV-positive and HIV-negative patients: A matched study. HIV Med. 2021, 22, 867–876. [Google Scholar] [CrossRef]
- Lang, R.; Humes, E.; Coburn, S.B.; Horberg, M.A.; Fathi, L.F.; Watson, E.; Jefferson, C.R.; Park, L.S.; Gordon, K.S.; Akgün, K.M.; et al. Analysis of Severe Illness After Postvaccination COVID-19 Breakthrough Among Adults with and without HIV in the US. JAMA Netw. Open 2022, 5, e2236397. [Google Scholar] [CrossRef]
- Jassat, W.; Cohen, C.; Tempia, S.; Masha, M.; Goldstein, S.; Kufa, T.; Murangandi, P.; Savulescu, D.; Walaza, S.; Bam, J.-L.; et al. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: A cohort study. Lancet HIV 2021, 8, e554–e567. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.; Patel, R.C.; Zhang, J.; Guo, S.; Zheng, Q.; Olex, A.L.; Olatosi, B.; Weissman, S.B.; Islam, J.Y.; et al. Associations between HIV infection and clinical spectrum of COVID-19: A population level analysis based on US National COVID Cohort Collaborative (N3C) data. Lancet HIV 2021, 8, e690–e700. [Google Scholar] [CrossRef]
- Danwang, C.; Noubiap, J.J.; Robert, A.; Yombi, J.C. Outcomes of patients with HIV and COVID-19 co-infection: A systematic review and meta-analysis. AIDS Res. Ther. 2022, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Casado, J.L.; Härter, G.; Vizcarra, P.; Moreno, A.; Cattaneo, D.; Meraviglia, P.; Spinner, C.D.; Schabaz, F.; Grunwald, S.; et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med. 2021, 22, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.V.; Felsen, U.R.; Fisher, M.; Fazzari, M.J.; Ginsberg, M.S.; Beil, R.; Akiyama, M.J.; Anastos, K.; Hanna, D.B. Clinical Outcomes and Inflammatory Markers by HIV Serostatus and Viral Suppression in a Large Cohort of Patients Hospitalized with COVID-19. J. Acquir. Immune Defic. Syndr. 2021, 86, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Jouanguy, E.; Zhang, Q.; Abel, L.; Puel, A.; Casanova, J.L. Human inborn errors of immunity to infection affecting cells other than leukocytes: From the immune system to the whole organism. Curr. Opin. Immunol. 2019, 59, 88–100. [Google Scholar] [CrossRef]
- Moens, L.; Meyts, I. Recent human genetic errors of innate immunity leading to increased susceptibility to infection. Curr. Opin. Immunol. 2020, 62, 79–90. [Google Scholar] [CrossRef]
- Zhang, S.Y. Herpes simplex virus encephalitis of childhood: Inborn errors of central nervous system cell-intrinsic immunity. Hum. Genet. 2020, 139, 911–918. [Google Scholar] [CrossRef]
- Tangye, S.G.; Latour, S. Primary immunodeficiencies reveal the molecular requirements for effective host defense against EBV infection. Blood 2020, 135, 644–655. [Google Scholar] [CrossRef]
- Lamborn, I.T.; Su, H.C. Genetic determinants of host immunity against human rhinovirus infections. Hum. Genet. 2020, 139, 949–959. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, Z.A.; Abramova, I.; Aldave, J.C.; Al-Herz, W.; Bezrodnik, L.; Boukari, R.; Bousfiha, A.A.; Cancrini, C.; Condino-Neto, A.; Dbaibo, G.; et al. X-linked agammaglobulinemia (XLA): Phenotype, diagnosis, and therapeutic challenges around the world. World Allergy Organ. J. 2019, 12, 100018. [Google Scholar] [CrossRef]
- Marcus, N.; Frizinsky, S.; Hagin, D.; Ovadia, A.; Hanna, S.; Farkash, M.; Maoz-Segal, R.; Agmon-Levin, N.; Broides, A.; Nahum, A.; et al. Minor Clinical Impact of COVID-19 Pandemic on Patients with Primary Immunodeficiency in Israel. Front. Immunol. 2021, 11, 614086. [Google Scholar] [CrossRef]
- Meyts, I.; Bucciol, G.; Quinti, I.; Neven, B.; Fischer, A.; Seoane, E.; Lopez-Granados, E.; Gianelli, C.; Robles-Marhuenda, A.; Jeandel, P.-Y.; et al. Coronavirus disease 2019 in patients with inborn errors of immunity: An international study. J. Allergy Clin. Immunol. 2021, 147, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, T.L.; Rasmussen, L.D.; Drabe, C.H.; Larsen, C.S.; Hansen, A.E.; Stærkind, M.; Knudsen, L.S.; Hansen, C.H.; Obel, N. Outcome of SARS-CoV-2 infection among patients with common variable immunodeficiency and a matched control group: A Danish nationwide cohort study. Front Immunol. 2022, 13, 994253. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Bucciol, G.; Meyts, I. Mechanisms underlying host defense and disease pathology in response to severe acute respiratory syndrome (SARS)-CoV2 infection: Insights from inborn errors of immunity. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed]
- Pardhan, S.; Wood, S.; Vaughan, M.; Trott, M. The Risk of COVID-19 Related Hospitalsation, Intensive Care Unit Admission and Mortality in People with Underlying Asthma or COPD: A Systematic Review and Meta-Analysis. Front. Med. (Lausanne) 2021, 8, 668808. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.J.; Menon, A.A.; Ghosh, A.J.; Putman, R.K.; Fredenburgh, L.E.; El-Chemaly, S.Y.; Goldberg, H.J.; Baron, R.M.; Hunninghake, G.M.; Doyle, T.J. Increased Odds of Death for Patients with Interstitial Lung Disease and COVID-19: A Case–Control Study. Am. J. Respir. Crit. Care Med. 2020, 202, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Lemarquis, A.; Campbell, T.; Aranda-Guillén, M.; Hennings, V.; Brodin, P.; Kämpe, O.; Blennow, K.; Zetterberg, H.; Wennerås, C.; Eriksson, K.; et al. Severe COVID-19 in an APS1 patient with interferon autoantibodies treated with plasmapheresis. J. Allergy Clin. Immunol. 2021, 148, 96–98. [Google Scholar] [CrossRef]
- Bastard, P.; Orlova, E.; Sozaeva, L.; Lévy, R.; James, A.; Schmitt, M.M.; Ochoa, S.; Kareva, M.; Rodina, Y.; Gervais, A.; et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J. Exp. Med. 2021, 218, e20210554. [Google Scholar] [CrossRef]
- Meisel, C.; Akbil, B.; Meyer, T.; Lankes, E.; Corman, V.M.; Staudacher, O.; Unterwalder, N.; Kölsch, U.; Drosten, C.; Mall, M.A.; et al. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J. Clin. Investig. 2021, 131, e150867. [Google Scholar] [CrossRef]
- du Bois, H.; Heim, T.A.; Rahman, S.A.; Yagnik, B.; Bastard, P.; Gervais, A.; Le Voyer, T.; Rosain, J.; Philippot, Q.; Manry, J.; et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci. Immunol. 2021, 6, eabl4340. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Manry, J.; Bastard, P.; Gervais, A.; Le Voyer, T.; Rosain, J.; Philippot, Q.; Michailidis, E.; Hoffmann, H.-H.; Eto, S.; Garcia-Prat, M.; et al. The risk of COVID-19 death is much greater and age dependent with type I IFN autoantibodies. Proc. Natl. Acad. Sci. USA 2022, 119, e2200413119. [Google Scholar] [CrossRef] [PubMed]
- van der Made, C.I.; Simons, A.; Schuurs-Hoeijmakers, J.; Heuvel, G.V.D.; Mantere, T.; Kersten, S.; van Deuren, R.C.; Steehouwer, M.; van Reijmersdal, S.V.; Jaeger, M.; et al. Presence of Genetic Variants Among Young Men with Severe COVID-19. JAMA 2020, 324, 663–673. [Google Scholar] [CrossRef]
- Asano, T.; Boisson, B.; Onodi, F.; Matuozzo, D.; Moncada-Velez, M.; Renkilaraj, M.R.L.M.; Zhang, P.; Meertens, L.; Bolze, A.; Materna, M.; et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 2021, 6, eabl4348. [Google Scholar] [CrossRef] [PubMed]
- Holter, J.C.; Pischke, S.E.; de Boer, E.; Lind, A.; Jenum, S.; Holten, A.R.; Tonby, K.; Barratt-Due, A.; Sokolova, M.; Schjalm, C.; et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc. Natl. Acad. Sci. USA 2020, 117, 25018–25025. [Google Scholar] [CrossRef]
- Karimi, M.; De Sanctis, V. Implications of SARSr-CoV 2 infection in thalassemias: Do patients fall into the “high clinical risk” category? Acta Biomed. 2020, 91, 50–56. [Google Scholar] [CrossRef]
- Ramlall, V.; Thangaraj, P.M.; Meydan, C.; Foox, J.; Butler, D.; Kim, J.; May, B.; De Freitas, J.K.; Glicksberg, B.S.; Mason, C.E.; et al. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 2020, 26, 1609–1615. [Google Scholar] [CrossRef]
- Grumach, A.S.; Goudouris, E.; Junior, S.D.; Marcelino, F.C.; Alonso, M.L.O.; Martins, R.D.O.; Arpon, M.A.; Valle, S.O.R. COVID-19 affecting hereditary angioedema patients with and without C1 inhibitor deficiency. J. Allergy Clin. Immunol. Pract. 2021, 9, 508–510. [Google Scholar] [CrossRef]
- Goudouris, E.S.; Pinto-Mariz, F.; Mendonça, L.O.; Aranda, C.S.; Guimarães, R.R.; Kokron, C.; Barros, M.T.; Anísio, F.; Alonso, M.L.O.; Marcelino, F.; et al. Outcome of SARS-CoV-2 Infection in 121 Patients with Inborn Errors of Immunity: A Cross-Sectional Study. J. Clin. Immunol. 2021, 41, 1479–1489. [Google Scholar] [CrossRef]
- Pablos, J.L.; Galindo, M.; Carmona, L.; Lledó, A.; Retuerto, M.; Blanco, R.; Gonzalez, A.; Martinez-Lopez, D.; Castrejón, I.; Alvaro-Gracia, J.M.; et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: A multicentric matched cohort study. Ann. Rheum. Dis. 2020, 79, 1544–1549. [Google Scholar] [CrossRef]
- Bachiller-Corral, J.; Boteanu, A.; Garcia-Villanueva, M.J.; de la Puente, C.; Revenga, M.; Diaz-Miguel, M.C.; Rodriguez-Garcia, A.; Morell-Hita, J.L.; Valero, M.; Larena, C.; et al. Risk of Severe COVID-19 Infection in Patients with Inflammatory Rheumatic Diseases. J. Rheumatol. 2021, 48, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, A.H.; Grondal, G.; Kristjansson, M.; Jonsdottir, T.; Love, T.J.; Gudbjornsson, B. Prevalence, admission rates and hypoxia due to COVID-19 in patients with rheumatic disorders treated with targeted synthetic or biologic disease modifying antirheumatic drugs or methotrexate: A nationwide study from Iceland. Ann. Rheum. Dis. 2021, 80, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Bower, H.; Frisell, T.; Di Giuseppe, D.; Delcoigne, B.; Ahlenius, G.-M.; Baecklund, E.; Chatzidionysiou, K.; Feltelius, N.; Forsblad-D’Elia, H.; Kastbom, A.; et al. Impact of the COVID-19 pandemic on morbidity and mortality in patients with inflammatory joint diseases and in the general population: A nationwide Swedish cohort study. Ann. Rheum. Dis. 2021, 80, 1086–1093. [Google Scholar] [CrossRef]
- Cordtz, R.; Lindhardsen, J.; Soussi, B.G.; Vela, J.; Uhrenholt, L.; Westermann, R.; Kristensen, S.; Nielsen, H.; Torp-Pedersen, C.; Dreyer, L. Incidence and severeness of COVID-19 hospitalization in patients with inflammatory rheumatic disease: A nationwide cohort study from Denmark. Rheumatology (Oxford) 2021, 60, SI59–SI67. [Google Scholar] [CrossRef]
- Conway, R.; Grimshaw, A.A.; Konig, M.F.; Putman, M.; Duarte-García, A.; Tseng, L.Y.; Cabrera, D.M.; Chock, Y.P.E.; Degirmenci, H.B.; Duff, E. SARS–CoV-2 Infection and COVID-19 Outcomes in Rheumatic Diseases: A Systematic Literature Review and Meta-Analysis. Arthritis Rheumatol. 2022, 74, 766–775. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef]
- Fredi, M.; Cavazzana, I.; Moschetti, L.; Andreoli, L.; Franceschini, F.; Airò, P.; Bazzani, C.; Crisafulli, F.; Filippini, M.; Frassi, M.; et al. COVID-19 in patients with rheumatic diseases in northern Italy: A single-center observational and case–control study. Lancet Rheumatol. 2020, 2, e549–e556. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. COVID-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef] [PubMed]
- Emmi, G.; Bettiol, A.; Mattioli, I.; Silvestri, E.; Di Scala, G.; Urban, M.L.; Vaglio, A.; Prisco, D. SARS-CoV-2 infection among patients with systemic autoimmune diseases. Autoimmun. Rev. 2020, 19, 102575. [Google Scholar] [CrossRef]
- Lu, C.; Li, S.; Liu, Y. Role of immunosuppressive therapy in rheumatic diseases concurrent with COVID-19. Ann. Rheum. Dis. 2020, 79, S737–S739. [Google Scholar] [CrossRef]
- MacKenna, B.; Kennedy, N.A.; Mehrkar, A.; Rowan, A.; Galloway, J.; Matthewman, J.; Mansfield, K.E.; Bechman, K.; Yates, M.; Brown, J.; et al. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: A nationwide cohort study in the OpenSAFELY platform. Lancet Rheumatol. 2022, 4, e490–e506. [Google Scholar] [CrossRef]
- Jovani, V.; Calabuig, I.; Peral-Garrido, M.L.; Tovar-Sugrañes, E.; López-González, M.-D.; Bernabeu, P.; Martínez, A.; Esteve-Vives, J.; León-Ramírez, J.-M.; Moreno-Perez, O.; et al. Incidence of severe COVID-19 in a Spanish cohort of 1037 patients with rheumatic diseases treated with biologics and JAK-inhibitors. Ann. Rheum. Dis. 2022, 81, e131. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.A.; Gerosa, M.; Beretta, L.; Bellocchi, C.; Argolini, L.M.; Moroni, L.; Della Torre, E.; Artusi, C.; Nicolosi, S.; Caporali, R.; et al. COVID-19 in systemic lupus erythematosus: Data from a survey on 417 patients. Semin. Arthritis Rheum. 2020, 50, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Bajocchi, G.; Mancuso, P.; Galli, E.; Muratore, F.; Boiardi, L.; Catanoso, M.; Pipitone, N.; Cassone, G.; Girolimetto, N.; et al. Susceptibility and severity of COVID-19 in patients treated with bDMARDS and tsDMARDs: A population-based study. Ann. Rheum. Dis. 2020, 79, 986–988. [Google Scholar] [CrossRef] [PubMed]
- FAI2R /SFR/SNFMI/SOFREMIP/CRI/IMIDIATE Consortium and Contributors. Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: Data from the French RMD COVID-19 cohort of 694 patients. Ann. Rheum. Dis. 2021, 80, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Stastna, D.; Menkyova, I.; Drahota, J.; Mazouchova, A.; Adamkova, J.; Ampapa, R.; Grunermelova, M.; Peterka, M.; Recmanova, E.; Rockova, P.; et al. Multiple sclerosis, neuromyelitis optica spectrum disorder and COVID-19: A pandemic year in Czechia. Mult. Scler. Relat. Disord. 2021, 54, 103104. [Google Scholar] [CrossRef]
- Wetwittayakhlang, P.; Albader, F.; Golovics, P.A.; Hahn, G.D.; Bessissow, T.; Bitton, A.; Afif, W.; Wild, G.; Lakatos, P.L. Clinical Outcomes of COVID-19 and Impact on Disease Course in Patients with Inflammatory Bowel Disease. Can. J. Gastroenterol. Hepatol. 2021, 2021, 7591141. [Google Scholar] [CrossRef]
- Brenner, E.J.; Ungaro, R.C.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.-F.; Reinisch, W.; Ruemmele, F.M.; et al. Corticosteroids, But Not TNF Antagonists, Are Associated with Adverse COVID-19 Outcomes in Patients with Inflammatory Bowel Diseases: Results from an International Registry. Gastroenterology 2020, 159, 481–491.e3. [Google Scholar] [CrossRef]
- Gianfrancesco, M.; Hyrich, K.L.; Al-Adely, S.; Carmona, L.; Danila, M.I.; Gossec, L.; Izadi, Z.; Jacobsohn, L.; Katz, P.; Lawson-Tovey, S.; et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: Data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2020, 79, 859–866. [Google Scholar] [CrossRef]
- Strangfeld, A.; Schäfer, M.; Gianfrancesco, M.A.; Lawson-Tovey, S.; Liew, J.W.; Ljung, L.; Mateus, E.F.; Richez, C.; Santos, M.J.; Schmajuk, G.; et al. Factors associated with COVID-19-related death in people with rheumatic diseases: Results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2021, 80, 930–942. [Google Scholar] [CrossRef]
- Hasseli, R.; Mueller-Ladner, U.; Hoyer, B.F.; Krause, A.; Lorenz, H.-M.; Pfeil, A.; Richter, J.; Schäfer, M.; Schmeiser, T.; Strangfeld, A.; et al. Older age, comorbidity, glucocorticoid use and disease activity are risk factors for COVID-19 hospitalisation in patients with inflammatory rheumatic and musculoskeletal diseases. RMD Open 2021, 7, e001464. [Google Scholar] [CrossRef] [PubMed]
- Attauabi, M.; Seidelin, J.B.; Felding, O.K.; Wewer, M.D.; Arp, L.K.V.; Sarikaya, M.Z.; Egeberg, A.; Vladimirova, N.; Bendtsen, F.; Burisch, J. Coronavirus disease 2019, immune-mediated inflammatory diseases and immunosuppressive therapies—A Danish population-based cohort study. J. Autoimmun. 2021, 118, 102613. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Pan, J.; Vasileiou, E.; Robertson, C.; Sheikh, A. Risk of serious COVID-19 outcomes among adults with asthma in Scotland: A national incident cohort study. Lancet Respir. Med. 2022, 10, 347–354. [Google Scholar] [CrossRef]
- Spiera, R.; Jinich, S.; Jannat-Khah, D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann. Rheum. Dis. 2021, 80, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, J.A.; Connolly, C.M.; Boyarsky, B.J.; Werbel, W.A.; Christopher-Stine, L.; Garonzik-Wang, J.; Segev, D.L.; Paik, J.J. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021, 80, 1351–1352. [Google Scholar] [CrossRef]
- Raiker, R.; DeYoung, C.; Pakhchanian, H.; Ahmed, S.; Kavadichanda, C.; Gupta, L.; Kardeş, S. Outcomes of COVID-19 in patients with rheumatoid arthritis: A multicenter research network study in the United States. Semin. Arthritis Rheum. 2022, 51, 1057–1066. [Google Scholar] [CrossRef]
- Andersen, K.M.; Bates, B.A.; Rashidi, E.S.; Olex, A.L.; Mannon, R.B.; Patel, R.C.; Singh, J.; Sun, J.; Auwaerter, P.G.; Ng, D.K.; et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: A retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol. 2022, 4, e33–e41. [Google Scholar] [CrossRef]
- Garcillán, B.; Salavert, M.; Regueiro, J.R.; Díaz-Castroverde, S. Response to Vaccines in Patients with Immune-Mediated Inflammatory Diseases: A Narrative Review. Vaccines 2022, 10, 297. [Google Scholar] [CrossRef]
- Bournia, V.-K.; Fragoulis, G.E.; Mitrou, P.; Mathioudakis, K.; Tsolakidis, A.; Konstantonis, G.; Tseti, I.; Vourli, G.; Tektonidou, M.G.; Paraskevis, D.; et al. Different COVID-19 outcomes among systemic rheumatic diseases: A nation-wide cohort study. Rheumatology (Oxford) 2023, 62, 1047–1056. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).