Dengue Virus Infection Alters Inter-Endothelial Junctions and Promotes Endothelial–Mesenchymal-Transition-like Changes in Human Microvascular Endothelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Virus and Cell Lines
2.3. Plasmid Transfection
2.4. Amplification and Virus Titration
2.5. Infection of Microvascular Endothelial Cells and Production of Conditioned Media
2.6. Early-Stage and Post-Infective Stage Inhibition Assays
2.7. Evaluation of Transfection Efficiency, c-ABL Expression Level, and CRKII Phosphorylation
2.8. Determination of Actin Reorganization and Expression of VE-Cadherin, ZO-1, N-Cadherin, and Vimentin
2.9. Cell Migration Assays
2.10. Statistical Analysis
3. Results
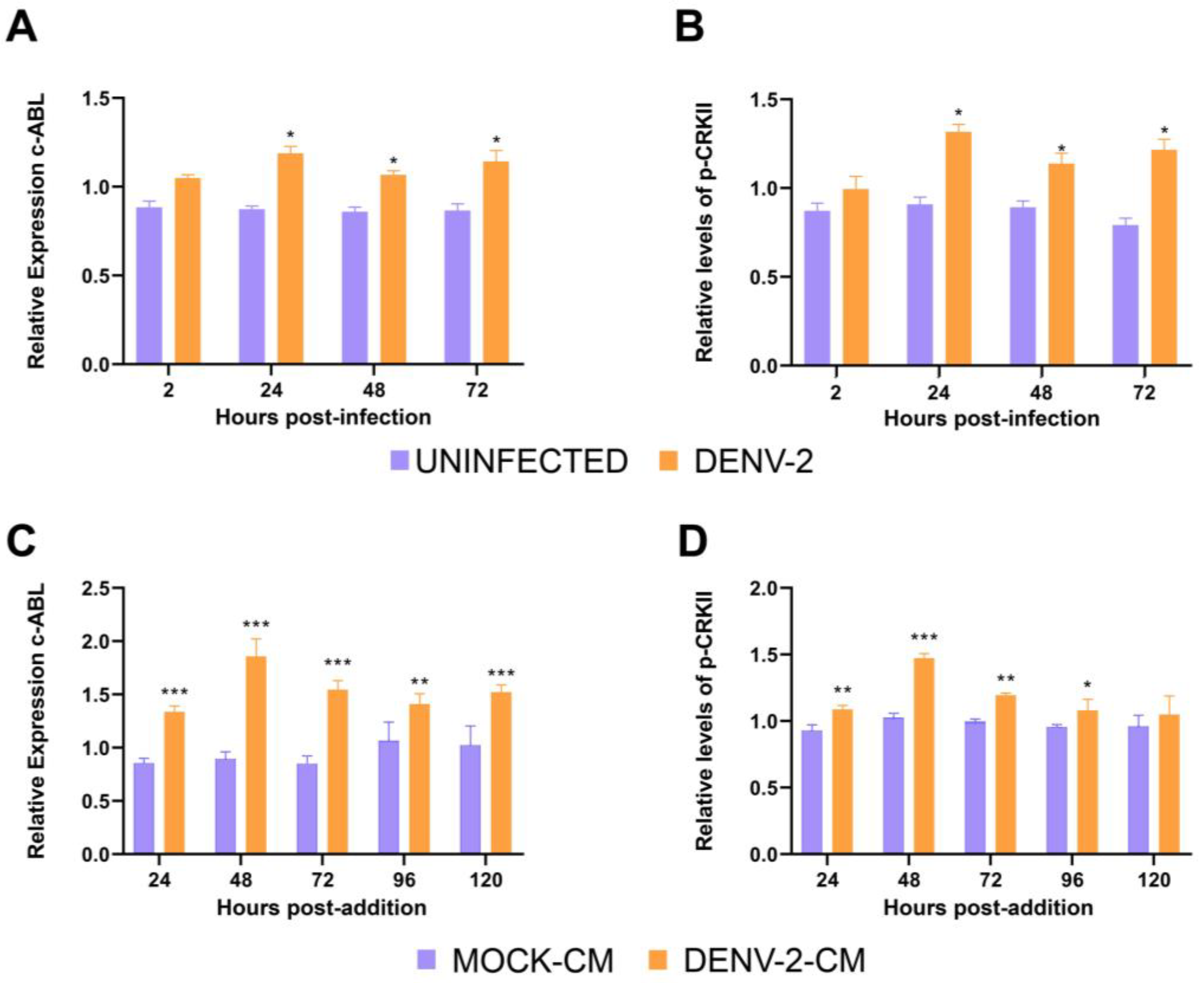
3.1. DENV-2 Infection Increases the Expression of c-ABL Kinase and Its Phosphorylated Cellular Target CRKII in HMEC-1 Cells
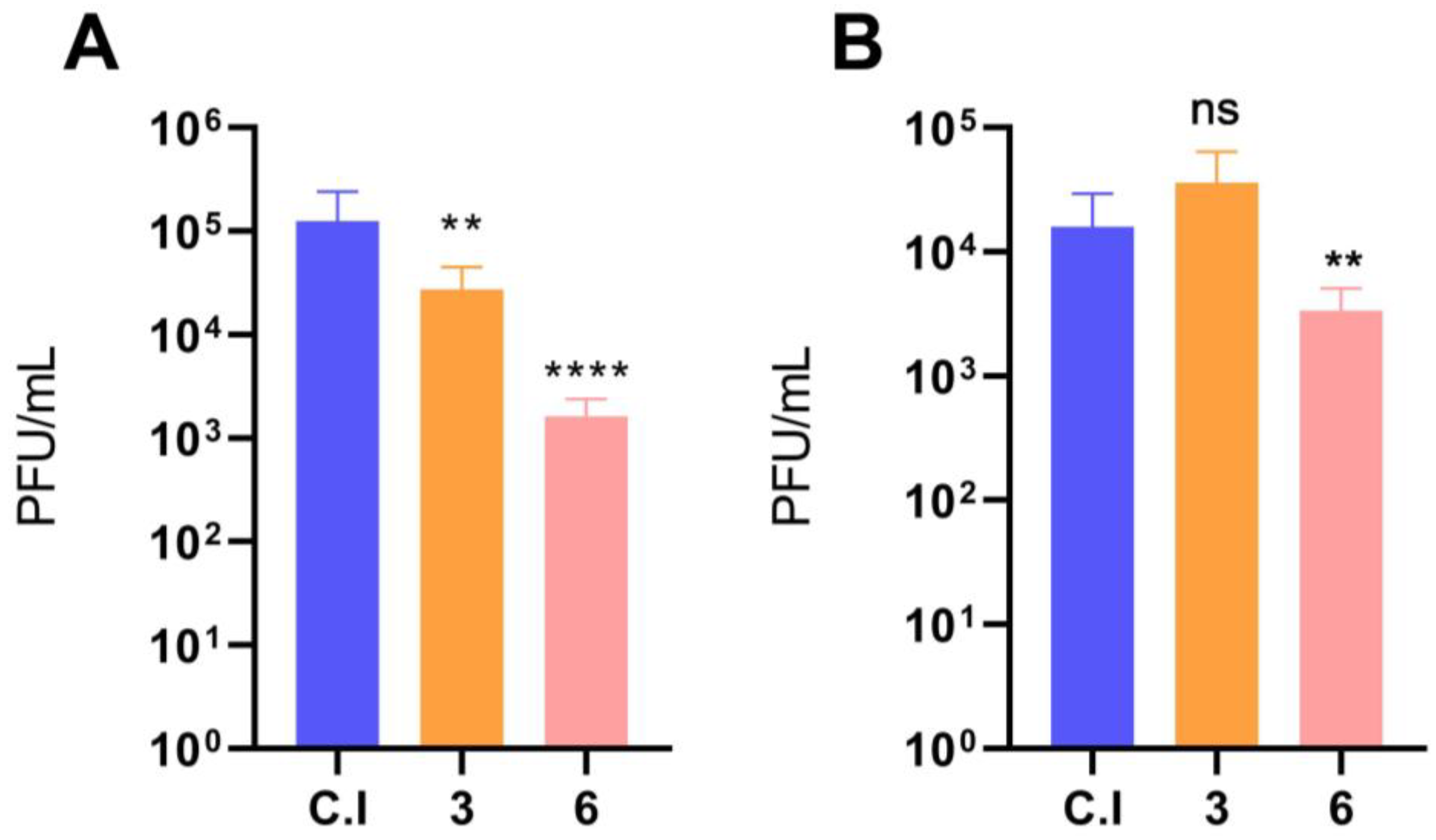
3.2. Imatinib Inhibits the Early Stages of DENV-2 Infection in HMEC-1 Cells
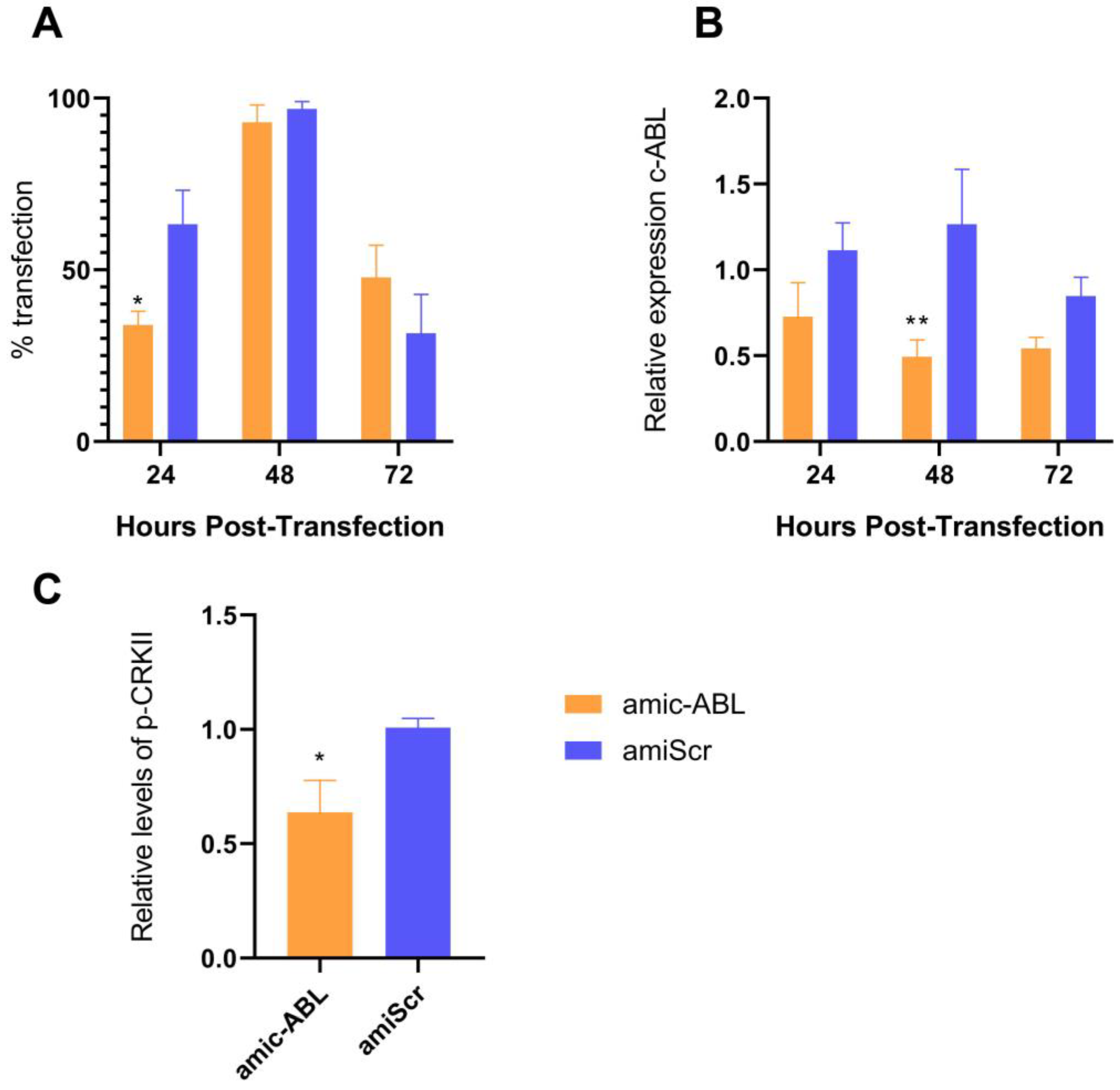
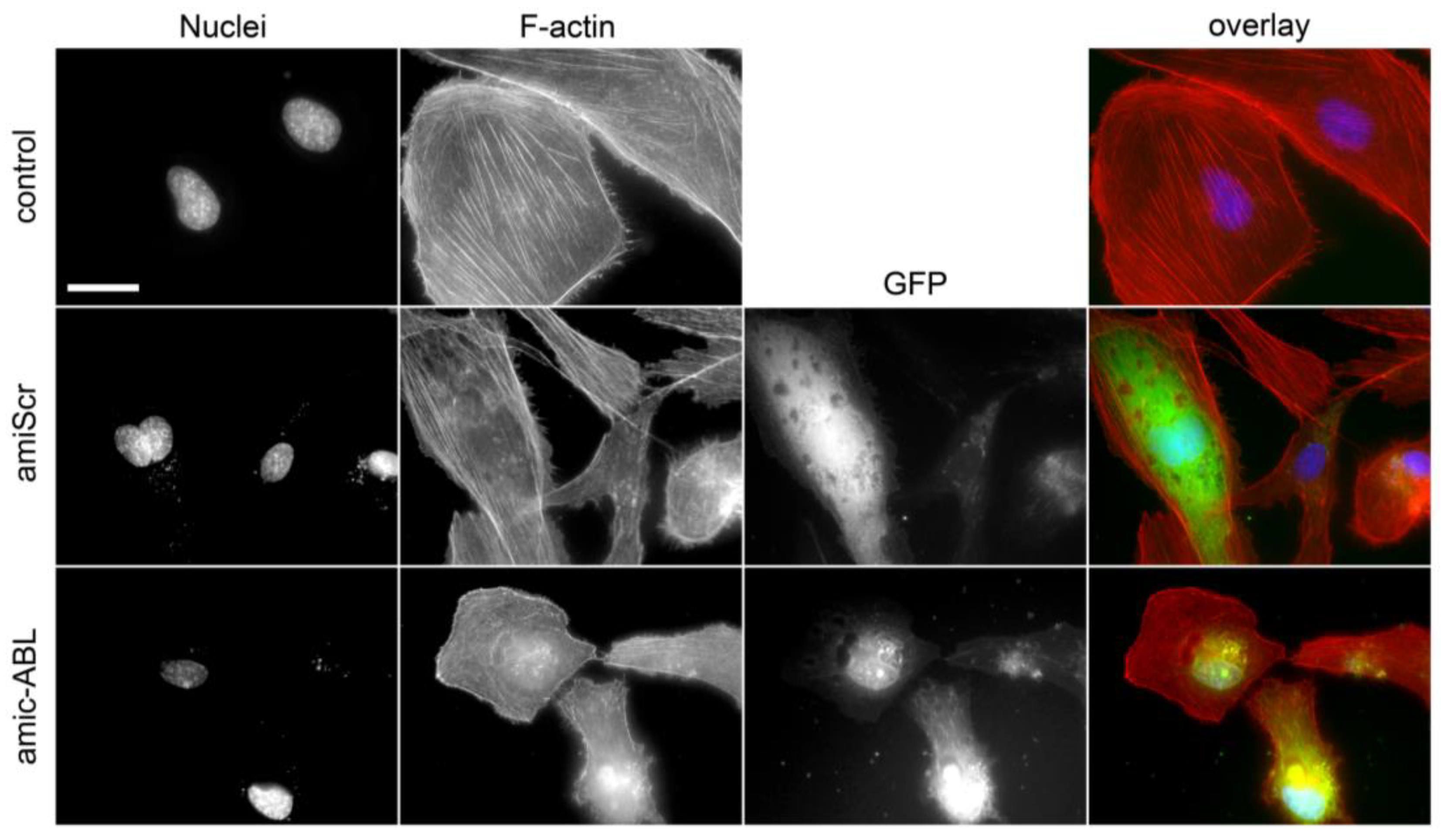
3.3. c-ABL Depletion Reduces CRKII Phosphorylation and Promotes Actin Cytoskeleton Reorganization
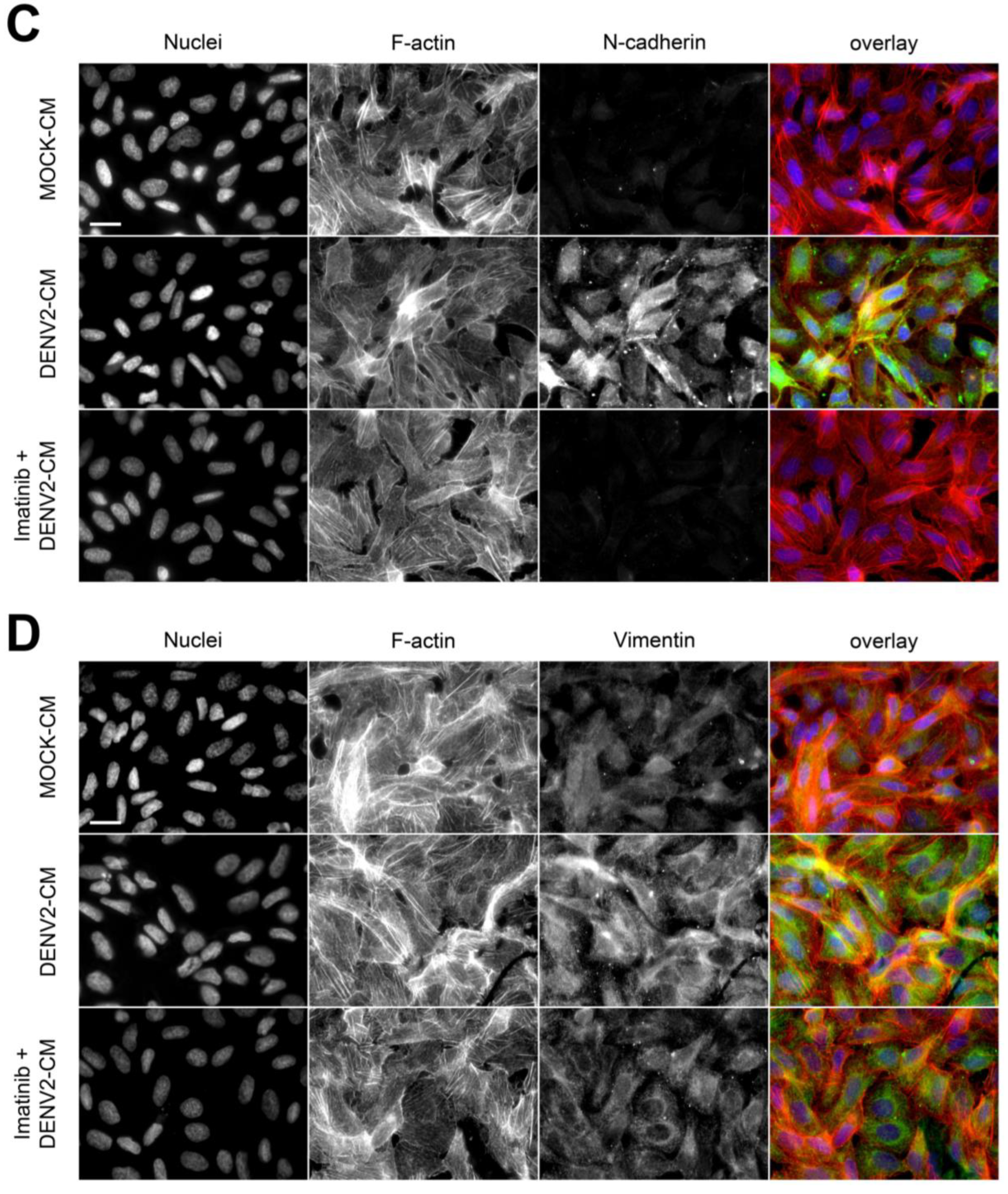
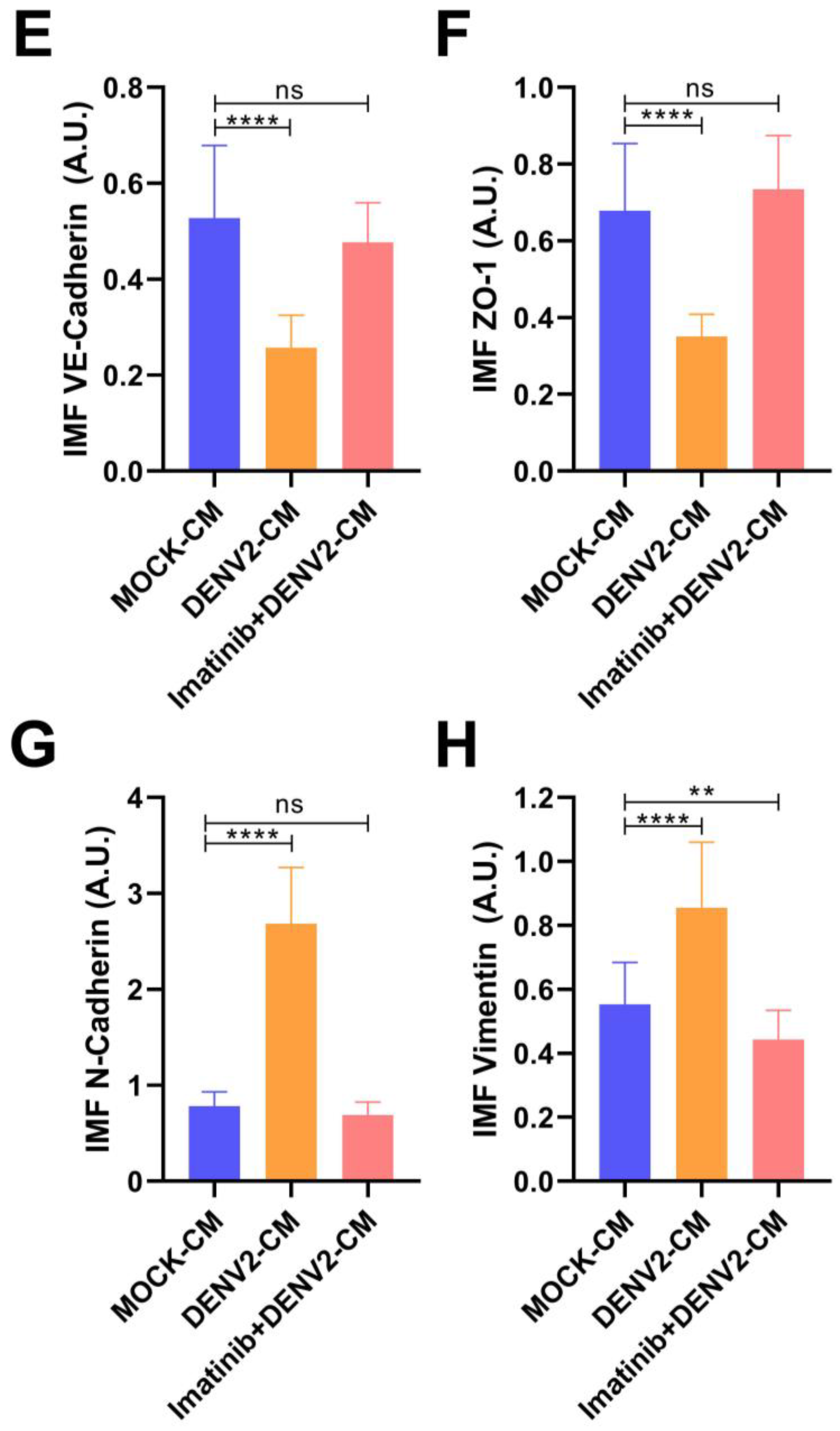
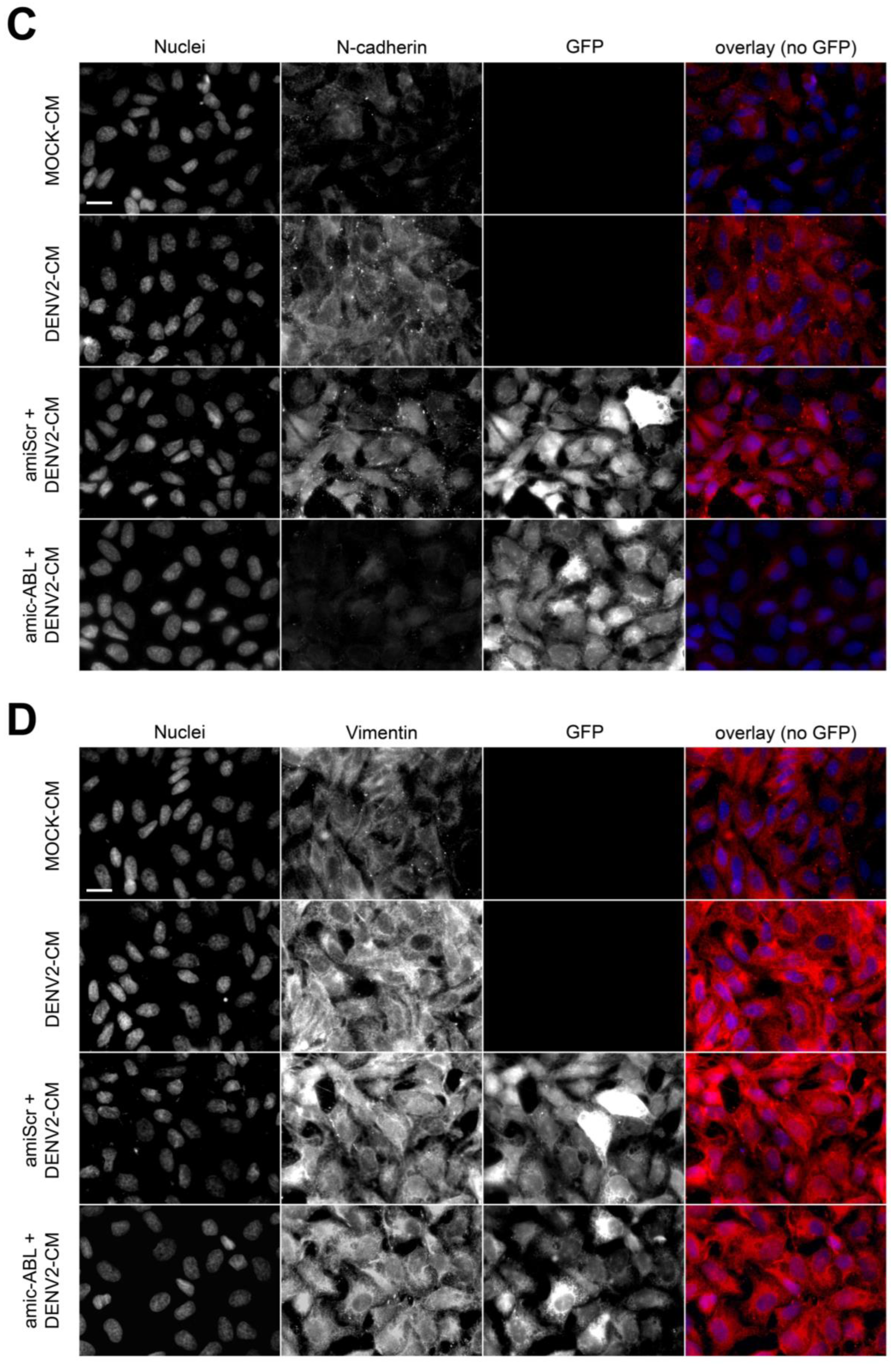
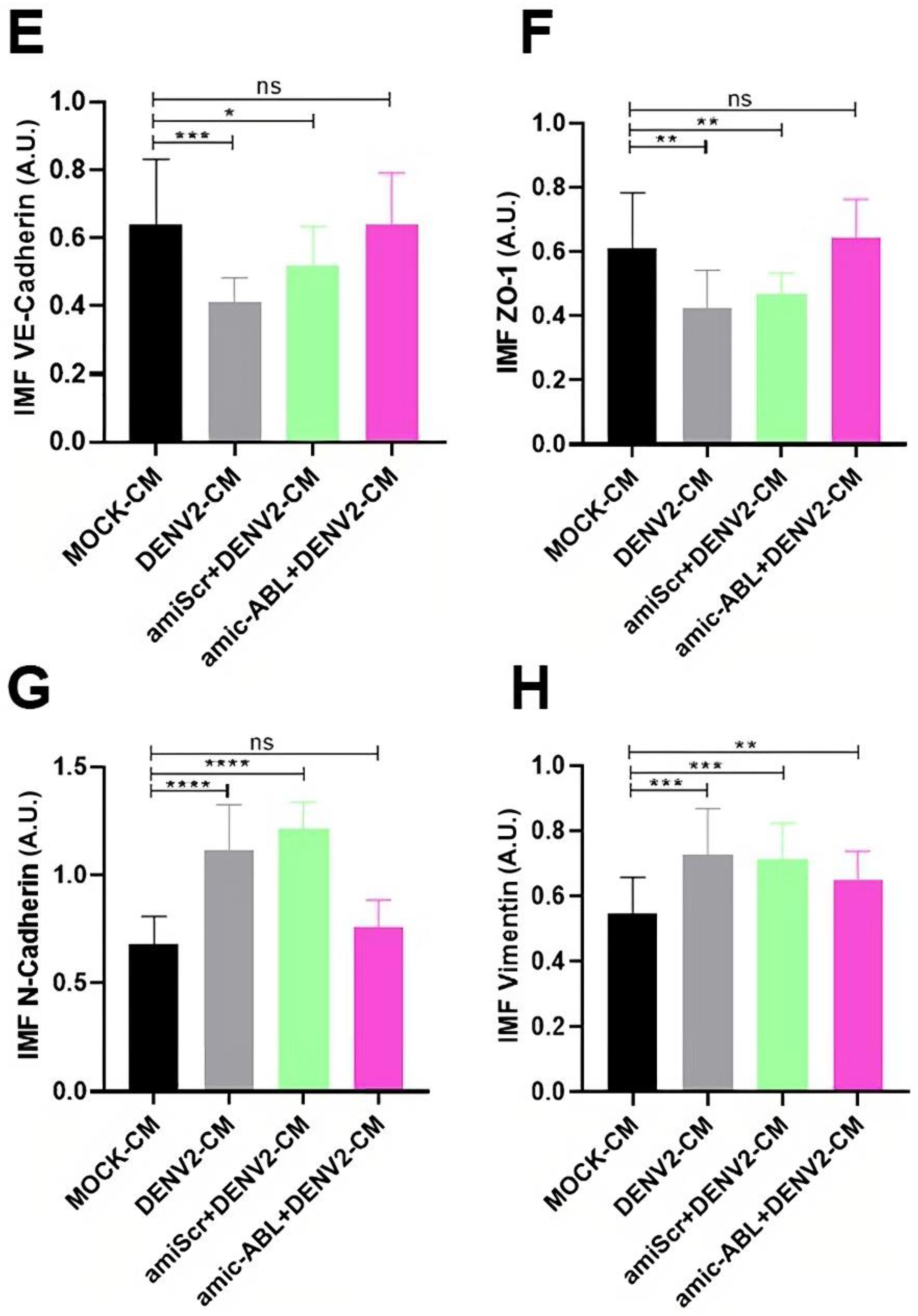
3.4. DENV2 Infection Decreases VE-Cadherin and ZO-1 and Increases N-Cadherin and Vimentin in a c-ABL-Dependent Manner
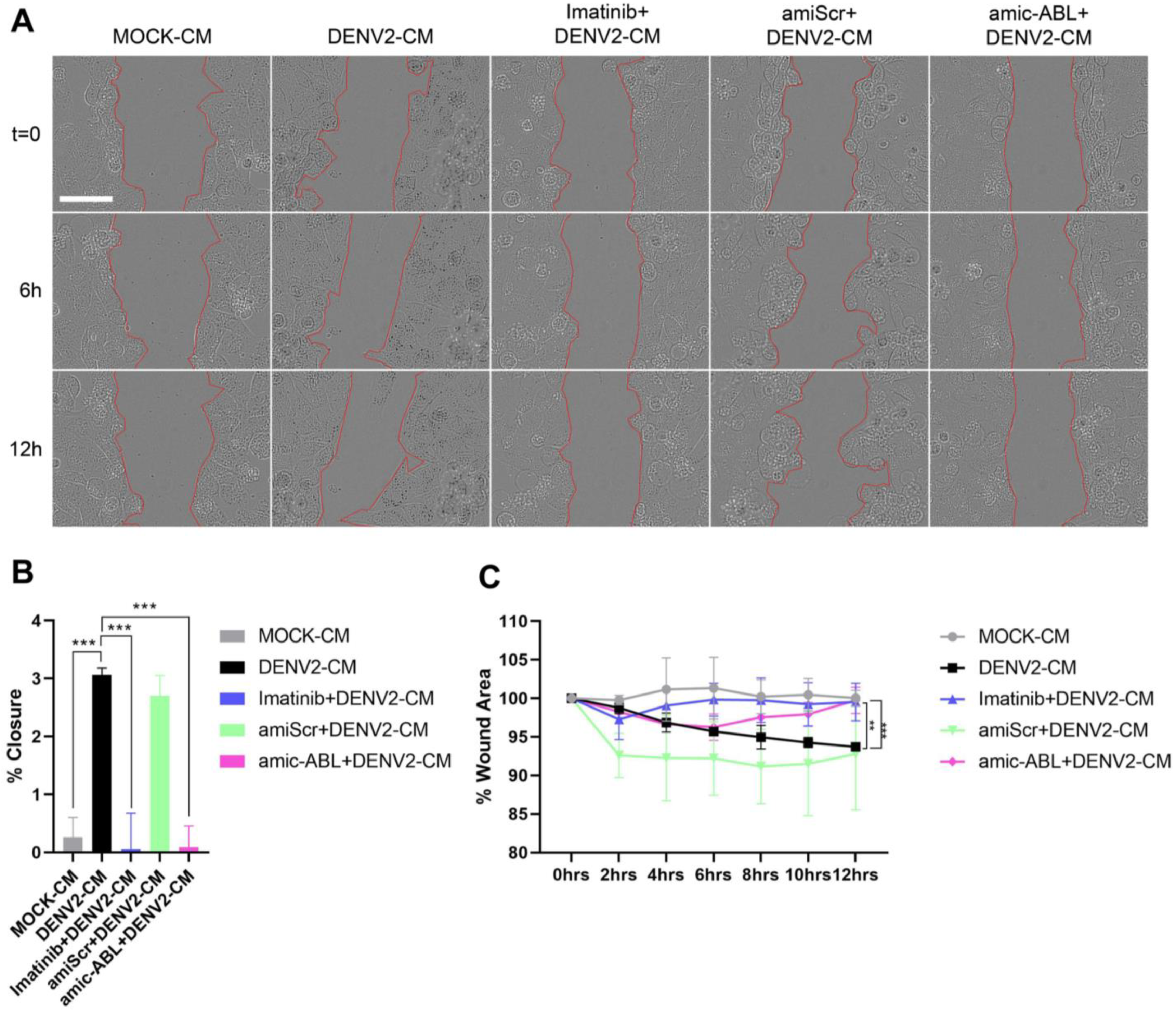
3.5. DENV-2 Infection Induces Cell Motility in a c-ABL-Dependent Manner
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Prim. 2016, 2, 16055. [Google Scholar] [CrossRef]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Yount, B.; Pantoja, P.; Henein, S.; Meganck, R.M.; McBride, J.; Munt, J.E.; Baric, T.J.; Zhu, D.; Scobey, T.; et al. A live dengue virus vaccine carrying a chimeric envelope glycoprotein elicits dual DENV2-DENV4 serotype-specific immunity. Nat. Commun. 2023, 14, 1371. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.; Valero, N.; Mosquera, J.; Montiel, M.; Reyes, E.; Larreal, Y.; Alvarez-Mon, M. Increased expression of cytokines, soluble cytokine receptors, soluble apoptosis ligand and apoptosis in dengue. Virology 2014, 452–453, 42–51. [Google Scholar] [CrossRef]
- Kreutzman, A.; Colom-Fernandez, B.; Jimenez, A.M.; Ilander, M.; Cuesta-Mateos, C.; Perez-Garcia, Y.; Arevalo, C.D.; Bruck, O.; Hakanen, H.; Saarela, J.; et al. Dasatinib Reversibly Disrupts Endothelial Vascular Integrity by Increasing Non-Muscle Myosin II Contractility in a ROCK-Dependent Manner. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6697–6707. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.N.; Vestweber, D. Mechanisms Ensuring Endothelial Junction Integrity Beyond VE-Cadherin. Front. Physiol. 2020, 11, 519. [Google Scholar] [CrossRef]
- Bazzoni, G.; Dejana, E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef]
- Srikiatkhachorn, A.; Kelley, J.F. Endothelial cells in dengue hemorrhagic fever. Antivir. Res. 2014, 109, 160–170. [Google Scholar] [CrossRef]
- Alvarez-Diaz, D.A.; Gutierrez-Diaz, A.A.; Orozco-Garcia, E.; Puerta-Gonzalez, A.; Bermudez-Santana, C.I.; Gallego-Gomez, J.C. Dengue virus potentially promotes migratory responses on endothelial cells by enhancing pro-migratory soluble factors and miRNAs. Virus Res. 2019, 259, 68–76. [Google Scholar] [CrossRef]
- Royall, J.A.; Berkow, R.L.; Beckman, J.S.; Cunningham, M.K.; Matalon, S.; Freeman, B.A. Tumor necrosis factor and interleukin 1 alpha increase vascular endothelial permeability. Am. J. Physiol. 1989, 257, L399–L410. [Google Scholar] [CrossRef]
- Beatty, P.R.; Puerta-Guardo, H.; Killingbeck, S.S.; Glasner, D.R.; Hopkins, K.; Harris, E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci. Transl. Med. 2015, 7, 304ra141. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anupriya, M.G.; Modak, A.; Sreekumar, E. Dengue virus or NS1 protein induces trans-endothelial cell permeability associated with VE-Cadherin and RhoA phosphorylation in HMEC-1 cells preventable by Angiopoietin-1. J. Gen. Virol. 2018, 99, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- van Nieuw Amerongen, G.P.; van Delft, S.; Vermeer, M.A.; Collard, J.G.; van Hinsbergh, V.W. Activation of RhoA by thrombin in endothelial hyperpermeability: Role of Rho kinase and protein tyrosine kinases. Circ. Res. 2000, 87, 335–340. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef]
- Cilloni, D.; Saglio, G. Molecular pathways: BCR-ABL. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 930–937. [Google Scholar] [CrossRef]
- Sirvent, A.; Benistant, C.; Roche, S. Cytoplasmic signalling by the c-Abl tyrosine kinase in normal and cancer cells. Biol. Cell. 2008, 100, 617–631. [Google Scholar] [CrossRef]
- Bradley, W.D.; Koleske, A.J. Regulation of cell migration and morphogenesis by Abl-family kinases: Emerging mechanisms and physiological contexts. J. Cell. Sci. 2009, 122, 3441–3454. [Google Scholar] [CrossRef] [PubMed]
- Zandy, N.L.; Playford, M.; Pendergast, A.M. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc. Natl. Acad. Sci. USA 2007, 104, 17686–17691. [Google Scholar] [CrossRef]
- Yang, L.; Lin, C.; Liu, Z.R. P68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing Axin from beta-catenin. Cell 2006, 127, 139–155. [Google Scholar] [CrossRef]
- Luttman, J.H.; Colemon, A.; Mayro, B.; Pendergast, A.M. Role of the ABL tyrosine kinases in the epithelial-mesenchymal transition and the metastatic cascade. Cell Commun. Signal. 2021, 19, 59. [Google Scholar] [CrossRef]
- Anselmi, F.; Orlandini, M.; Rocchigiani, M.; De Clemente, C.; Salameh, A.; Lentucci, C.; Oliviero, S.; Galvagni, F. c-ABL modulates MAP kinases activation downstream of VEGFR-2 signaling by direct phosphorylation of the adaptor proteins GRB2 and NCK1. Angiogenesis 2012, 15, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Chislock, E.M.; Pendergast, A.M. Abl family kinases regulate endothelial barrier function in vitro and in mice. PLoS ONE 2013, 8, e85231. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chang, A.; Chang, L.; Niessen, K.; Eapen, S.; Setiadi, A.; Karsan, A. Differential regulation of transforming growth factor beta signaling pathways by Notch in human endothelial cells. J. Biol. Chem. 2009, 284, 19452–19462. [Google Scholar] [CrossRef]
- Clark, M.J.; Miduturu, C.; Schmidt, A.G.; Zhu, X.; Pitts, J.D.; Wang, J.; Potisopon, S.; Zhang, J.; Wojciechowski, A.; Hann Chu, J.J.; et al. GNF-2 Inhibits Dengue Virus by Targeting Abl Kinases and the Viral E Protein. Cell Chem. Biol. 2016, 23, 443–452. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Prestwood, T.R.; Shresta, S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 2010, 7, 128–139. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, M.; Castellanos, J.E.; Gallego-Gomez, J.C. Statins reduce dengue virus production via decreased virion assembly. Intervirology 2011, 54, 202–216. [Google Scholar] [CrossRef]
- Mayer, B.J.; Hirai, H.; Sakai, R. Evidence that SH2 domains promote processive phosphorylation by protein-tyrosine kinases. Curr. Biol. 1995, 5, 296–305. [Google Scholar] [CrossRef]
- Tanaka, S.; Ouchi, T.; Hanafusa, H. Downstream of Crk adaptor signaling pathway: Activation of Jun kinase by v-Crk through the guanine nucleotide exchange protein C3G. Proc. Natl. Acad. Sci. USA 1997, 94, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, R.L.; Martins, I.; Davidson, B.L. Artificial microRNAs as siRNA shuttles: Improved safety as compared to shRNAs in vitro and in vivo. Mol. Ther. 2009, 17, 169–175. [Google Scholar] [CrossRef]
- Li, R.; Knight, J.F.; Park, M.; Pendergast, A.M. Abl Kinases Regulate HGF/Met Signaling Required for Epithelial Cell Scattering, Tubulogenesis and Motility. PLoS ONE 2015, 10, e0124960. [Google Scholar] [CrossRef]
- Kumar, S.; Das, A.; Sen, S. Extracellular matrix density promotes EMT by weakening cell-cell adhesions. Mol. Biosyst. 2014, 10, 838–850. [Google Scholar] [CrossRef]
- Swimm, A.I.; Bornmann, W.; Jiang, M.; Imperiale, M.J.; Lukacher, A.E.; Kalman, D. Abl family tyrosine kinases regulate sialylated ganglioside receptors for polyomavirus. J. Virol. 2010, 84, 4243–4251. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.B.; Bergelson, J.M. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 2006, 124, 119–131. [Google Scholar] [CrossRef]
- Harmon, B.; Campbell, N.; Ratner, L. Role of Abl kinase and the Wave2 signaling complex in HIV-1 entry at a post-hemifusion step. PLoS Pathog. 2010, 6, e1000956. [Google Scholar] [CrossRef]
- Reeves, P.M.; Bommarius, B.; Lebeis, S.; McNulty, S.; Christensen, J.; Swimm, A.; Chahroudi, A.; Chavan, R.; Feinberg, M.B.; Veach, D.; et al. Disabling poxvirus pathogenesis by inhibition of Abl-family tyrosine kinases. Nat. Med. 2005, 11, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Orozco-García, E.; Trujillo-Correa, A.; Gallego-Gómez, J.C. Cell Biology of Virus Infection. In Cell Biology-New Insights; Najman, S., Ed.; IntechOpen: London, UK, 2016; Volume 1. [Google Scholar]
- Kanlaya, R.; Pattanakitsakul, S.N.; Sinchaikul, S.; Chen, S.T.; Thongboonkerd, V. Alterations in actin cytoskeletal assembly and junctional protein complexes in human endothelial cells induced by dengue virus infection and mimicry of leukocyte transendothelial migration. J. Proteome Res. 2009, 8, 2551–2562. [Google Scholar] [CrossRef]
- Nie, Y.; Hui, L.; Guo, M.; Yang, W.; Huang, R.; Chen, J.; Wen, X.; Zhao, M.; Wu, Y. Rearrangement of Actin Cytoskeleton by Zika Virus Infection Facilitates Blood-Testis Barrier Hyperpermeability. Virol. Sin. 2021, 36, 692–705. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Li, J.; Wu, W.; Jiu, Y. The Role of Host Cytoskeleton in Flavivirus Infection. Virol. Sin. 2019, 34, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Meza, H.; Castillo-Alvarez, A.; Gonzalez-Bonilla, C.; Meza, I. Cross-talk between Rac1 and Cdc42 GTPases regulates formation of filopodia required for dengue virus type-2 entry into HMEC-1 cells. J. Gen. Virol. 2009, 90, 2902–2911. [Google Scholar] [CrossRef]
- Stuart, J.R.; Gonzalez, F.H.; Kawai, H.; Yuan, Z.M. c-Abl interacts with the WAVE2 signaling complex to induce membrane ruffling and cell spreading. J. Biol. Chem. 2006, 281, 31290–31297. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Katayama, H.; Kiyokawa, E.; Ota, S.; Kurata, T.; Gotoh, N.; Otsuka, N.; Shibata, M.; Matsuda, M. Phosphorylation of CrkII adaptor protein at tyrosine 221 by epidermal growth factor receptor. J. Biol. Chem. 1998, 273, 17186–17191. [Google Scholar] [CrossRef]
- Abassi, Y.A.; Vuori, K. Tyrosine 221 in Crk regulates adhesion-dependent membrane localization of Crk and Rac and activation of Rac signaling. EMBO J. 2002, 21, 4571–4582. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Ruco, L.; Dejana, E. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J. Cell Biol. 1998, 140, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Labernadie, A.; Kato, T.; Brugues, A.; Serra-Picamal, X.; Derzsi, S.; Arwert, E.; Weston, A.; Gonzalez-Tarrago, V.; Elosegui-Artola, A.; Albertazzi, L.; et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 2017, 19, 224–237. [Google Scholar] [CrossRef]
- Brasch, J.; Harrison, O.J.; Ahlsen, G.; Carnally, S.M.; Henderson, R.M.; Honig, B.; Shapiro, L. Structure and binding mechanism of vascular endothelial cadherin: A divergent classical cadherin. J. Mol. Biol. 2011, 408, 57–73. [Google Scholar] [CrossRef]
- Gerhardt, H.; Wolburg, H.; Redies, C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev. Dyn. 2000, 218, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Maitre, J.L.; Heisenberg, C.P. Three functions of cadherins in cell adhesion. Curr. Biol. 2013, 23, R626–R633. [Google Scholar] [CrossRef]
- Pan, P.; Li, G.; Shen, M.; Yu, Z.; Ge, W.; Lao, Z.; Fan, Y.; Chen, K.; Ding, Z.; Wang, W.; et al. DENV NS1 and MMP-9 cooperate to induce vascular leakage by altering endothelial cell adhesion and tight junction. PLoS Pathog. 2021, 17, e1008603. [Google Scholar] [CrossRef]
- Lin, J.C.; Lin, S.C.; Chen, W.Y.; Yen, Y.T.; Lai, C.W.; Tao, M.H.; Lin, Y.L.; Miaw, S.C.; Wu-Hsieh, B.A. Dengue viral protease interaction with NF-kappaB inhibitor alpha/beta results in endothelial cell apoptosis and hemorrhage development. J. Immunol. 2014, 193, 1258–1267. [Google Scholar] [CrossRef]
- Idris, F.; Muharram, S.H.; Zaini, Z.; Alonso, S.; Diah, S. Invasion of a murine in vitro blood-brain barrier co-culture model by dengue virus serotypes 1 to 4. Arch. Virol. 2019, 164, 1069–1083. [Google Scholar] [CrossRef]
- Inyoo, S.; Suttitheptumrong, A.; Pattanakitsakul, S.N. Synergistic Effect of TNF-alpha and Dengue Virus Infection on Adhesion Molecule Reorganization in Human Endothelial Cells. Jpn. J. Infect. Dis. 2017, 70, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, H.; Remmers, N.; Hollingsworth, M.A. Loss of E-cadherin and epithelial to mesenchymal transition is not required for cell motility in tissues or for metastasis. Tissue Barriers 2014, 2, e969112. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudero-Flórez, M.; Torres-Hoyos, D.; Miranda-Brand, Y.; Boudreau, R.L.; Gallego-Gómez, J.C.; Vicente-Manzanares, M. Dengue Virus Infection Alters Inter-Endothelial Junctions and Promotes Endothelial–Mesenchymal-Transition-like Changes in Human Microvascular Endothelial Cells. Viruses 2023, 15, 1437. https://doi.org/10.3390/v15071437
Escudero-Flórez M, Torres-Hoyos D, Miranda-Brand Y, Boudreau RL, Gallego-Gómez JC, Vicente-Manzanares M. Dengue Virus Infection Alters Inter-Endothelial Junctions and Promotes Endothelial–Mesenchymal-Transition-like Changes in Human Microvascular Endothelial Cells. Viruses. 2023; 15(7):1437. https://doi.org/10.3390/v15071437
Chicago/Turabian StyleEscudero-Flórez, Manuela, David Torres-Hoyos, Yaneth Miranda-Brand, Ryan L. Boudreau, Juan Carlos Gallego-Gómez, and Miguel Vicente-Manzanares. 2023. "Dengue Virus Infection Alters Inter-Endothelial Junctions and Promotes Endothelial–Mesenchymal-Transition-like Changes in Human Microvascular Endothelial Cells" Viruses 15, no. 7: 1437. https://doi.org/10.3390/v15071437
APA StyleEscudero-Flórez, M., Torres-Hoyos, D., Miranda-Brand, Y., Boudreau, R. L., Gallego-Gómez, J. C., & Vicente-Manzanares, M. (2023). Dengue Virus Infection Alters Inter-Endothelial Junctions and Promotes Endothelial–Mesenchymal-Transition-like Changes in Human Microvascular Endothelial Cells. Viruses, 15(7), 1437. https://doi.org/10.3390/v15071437





