Subjective Perception of Recovery and Measured Olfactory Function in COVID-19 Patients
Abstract
1. Introduction
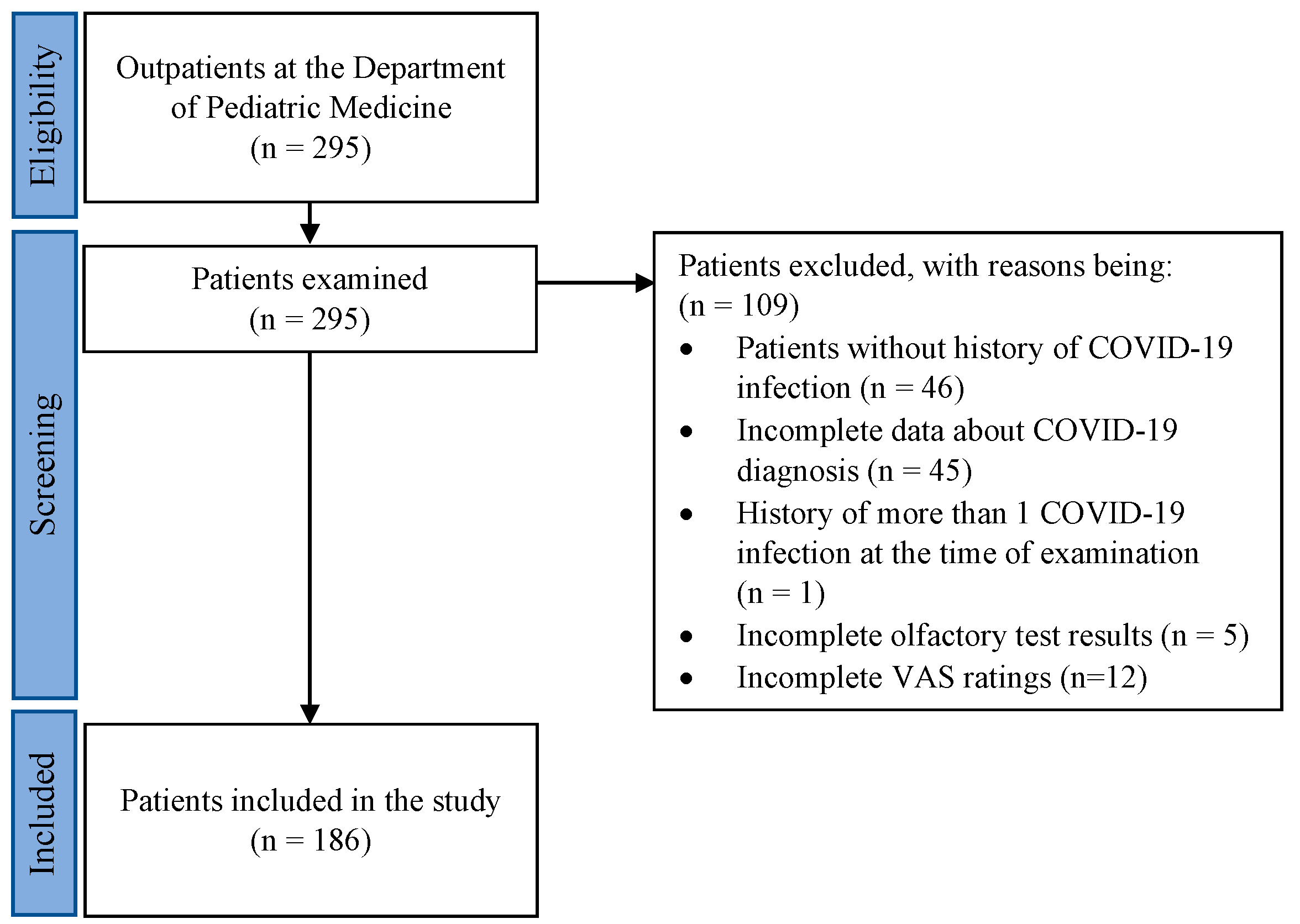
2. Materials and Methods
2.1. Participants
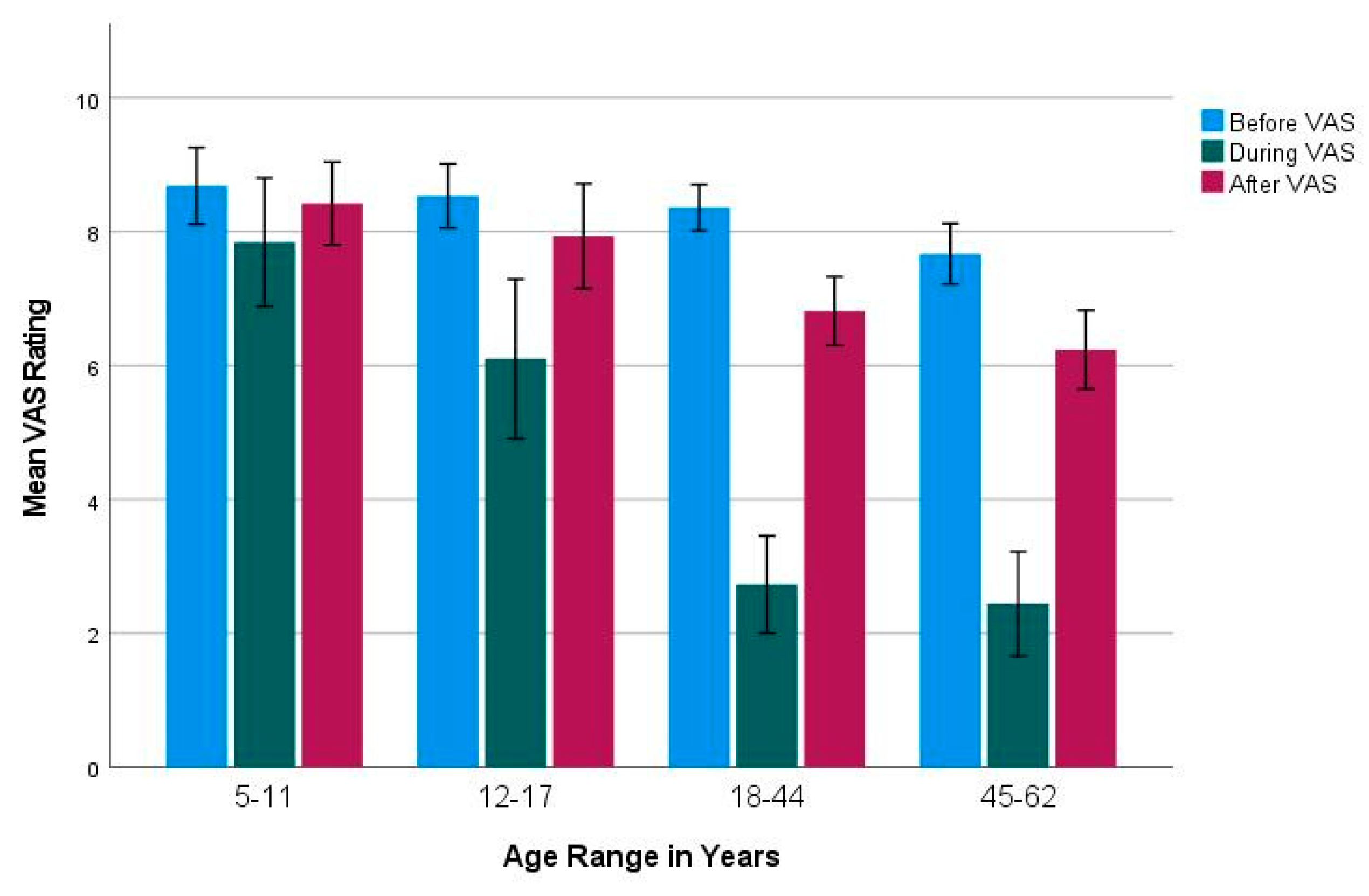
2.2. Visual Analogue Scale (before, during, after COVID-19 Infection)
2.3. Recovery Classes
2.4. Age Groups
2.5. Psychophysical Olfactory Tests
2.6. Cognitive Symptoms and Parosmia
2.7. Data Analysis
3. Results
3.1. Recovery Classes
3.2. Subjective Olfactory Dysfunction and Recovery
3.3. Cognitive Symptoms and Parosmia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, O.P.; Bhandari, P.; Raut, A.; Kacimi, S.E.O.; Huy, N.T. Coronavirus Disease (COVID-19): Comprehensive Review of Clinical Presentation. Front. Public Health 2021, 8, 582932. [Google Scholar] [CrossRef] [PubMed]
- Borsetto, D.; Hopkins, C.; Philips, V.; Obholzer, R.; Tirelli, G.; Polesel, J.; Calvanese, L.; Boscolo-Rizzo, P. Self-reported alteration of sense of smell or taste in patients with COVID-19: A systematic review and meta-analysis on 3563 patients. Rhinology 2020, 58, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Yang, X.; Liu, L.; Wu, T.; Cui, L.; Mou, Y.; Sun, Y. Prevalence and prognosis of otorhinolaryngological symptoms in patients with COVID-19: A systematic review and meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 49–60. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, A.; Meena, J.; Sankar, J.M. A systematic review and meta-analysis of otorhinolaryngological manifestations of coronavirus disease 2019 in paediatric patients. J. Laryngol. Otol. 2022, 136, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Marchese-Ragona, R.; Restivo, D.A.; De Corso, E.; Vianello, A.; Nicolai, P.; Ottaviano, G. Loss of smell in COVID-19 patients: A critical review with emphasis on the use of olfactory tests. Acta Otorhinolaryngol. Ital. 2020, 40, 241–247. [Google Scholar] [CrossRef]
- Han, P.; Su, T.; Qin, M.; Chen, H.; Hummel, T. A systematic review of olfactory related questionnaires and scales. Rhinology 2021, 59, 133–143. [Google Scholar] [CrossRef]
- Landis, B.; Hummel, T.; Hugentobler, M.; Giger, R.; Lacroix, J. Ratings of Overall Olfactory Function. Chem. Senses 2003, 28, 691–694. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Neri, G.; D’Ardes, D.; De Luca, C.; Marinari, S.; Porreca, E.; Cipollone, F.; Vecchiet, J.; Falcicchia, C.; Panichi, V.; et al. Smell and Taste in Severe COVID-19: Self-Reported vs. Testing. Front. Med. 2020, 7, 589409. [Google Scholar] [CrossRef]
- Lechien, J.R.; Cabaraux, P.; Chiesa-Estomba, C.M.; Khalife, M.; Hans, S.; Calvo-Henriquez, C.; Martiny, D.; Journe, F.; Sowerby, L.; Saussez, S. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck 2020, 42, 1583–1590. [Google Scholar] [CrossRef]
- Hannum, M.E.; Ramirez, V.A.; Lipson, S.J.; Herriman, R.D.; Toskala, A.K.; Lin, C.; Joseph, P.V.; Reed, D.R. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19–positive patients compared to subjective methods: A systematic review and meta-analysis. Chem. Senses 2020, 45, 865–874. [Google Scholar] [CrossRef]
- Prajapati, D.P.; Shahrvini, B.; Said, M.; Srinivas, S.; DeConde, A.S.; Yan, C.H. Assessment of patient recognition of coronavirus disease 2019 (COVID-19)-associated olfactory loss and recovery: A longitudinal study. Int. Forum Allergy Rhinol. 2021, 11, 1529–1537. [Google Scholar] [CrossRef]
- Nørgaard, H.J.; Fjaeldstad, A.W. Differences in Correlation between Subjective and Measured Olfactory and Gustatory Dysfunctions after Initial Ear, Nose and Throat Evaluation. Int. Arch. Otorhinolaryngol. 2021, 25, e563–e569. [Google Scholar] [CrossRef]
- Oleszkiewicz, A.; Schriever, V.A.; Croy, I.; Hähner, A.; Hummel, T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 719–728. [Google Scholar] [CrossRef]
- Reden, J.; Mueller, A.; Mueller, C.; Konstantinidis, I.; Frasnelli, J.; Landis, B.N.; Hummel, T. Recovery of Olfactory Function Following Closed Head Injury or Infections of the Upper Respiratory Tract. Arch. Otolaryngol.-Head Neck Surg. 2006, 132, 265–269. [Google Scholar] [CrossRef]
- Schriever, V.A.; Agosin, E.; Altundag, A.; Avni, H.; Van, H.C.; Cornejo, C.; Santos, G.D.L.; Fishman, G.; Fragola, C.; Guarneros, M.; et al. Development of an International Odor Identification Test for Children: The Universal Sniff Test. J. Pediatr. 2018, 198, 265–272.e3. [Google Scholar] [CrossRef]
- Zou, L.; Dworschak, A.; Alizadeh, R.; Kamrava, S.; Alwashahi, M.; Bock, M.; Boesveldt, S.; Singh, B.; Brusevold, I.; Voznessenskaya, V.; et al. “U-Sniff”—The international odor identification test for children: An extension of its normative database and study of global reliability. Rhinology 2020, 58, 471–476. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.; Pauli, E.; Kobal, G. ‘Sniffin’ Sticks’: Olfactory Performance Assessed by the Combined Testing of Odour Identification, Odor Discrimination and Olfactory Threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Vaira, L.A.; Deiana, G.; Fois, A.G.; Pirina, P.; Madeddu, G.; De Vito, A.; Babudieri, S.; Petrocelli, M.; Serra, A.; Bussu, F.; et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck 2020, 42, 1252–1258. [Google Scholar] [CrossRef]
- Gözen, E.D.; Aliyeva, C.; Tevetoğlu, F.; Karaali, R.; Balkan, I.I.; Yener, H.M.; Özdoğan, H.A. Evaluation of Olfactory Function with Objective Tests in COVID-19-Positive Patients: A Cross-Sectional Study. Ear Nose Throat J. 2021, 100 (Suppl. S2), 169S–173S. [Google Scholar] [CrossRef]
- Lechien, J.R.; Wajsblat, S.; Horoi, M.; Boscolo-Rizzo, P.; Le Bon, S.D.; Vaira, L.A.; Saussez, S. Comparison of prevalence and evolution of COVID-19 olfactory disorders in patients infected by D614 (wild) and B.1.1.7. Alpha variant: A brief report. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 3461–3467. [Google Scholar] [CrossRef]
- Ferreli, F.; Gaino, F.; Russo, E.; Di Bari, M.; Rossi, V.; De Virgilio, A.; Di Stadio, A.; Spriano, G.; Mercante, G. Long-term olfactory dysfunction in COVID-19 patients: 18-month follow-up study. Int. Forum Allergy Rhinol. 2022, 12, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, T.; Masala, C.; Pinna, I.; Orofino, G.; Solla, P.; Rocchi, L.; Defazio, G. Qualitative smell/taste disorders as sequelae of acute COVID-19. Neurol. Sci. 2021, 42, 4921–4926. [Google Scholar] [CrossRef] [PubMed]
- Vandersteen, C.; Payne, M.; Dumas, L.É.; Cancian, É.; Plonka, A.; D’Andréa, G.; Chirio, D.; Demonchy, É.; Risso, K.; Askenazy-Gittard, F.; et al. Olfactory Training in Post-COVID-19 Persistent Olfactory Disorders: Value Normalization for Threshold but Not Identification. J. Clin. Med. 2022, 11, 3275. [Google Scholar] [CrossRef] [PubMed]
- Somekh, I.; Hanna, H.Y.; Heller, E.; Bibi, H.; Somekh, E. Age-Dependent Sensory Impairment in COVID-19 Infection and its Correlation with ACE2 Expression. Pediatr. Infect. Dis. J. 2020, 39, e270–e272. [Google Scholar] [CrossRef]
- Malcangi, G.; Inchingolo, A.D.; Inchingolo, A.M.; Santacroce, L.; Marinelli, G.; Mancini, A.; Vimercati, L.; Maggiore, M.E.; D’oria, M.T.; Hazballa, D.; et al. COVID-19 Infection in Children, Infants and Pregnant Subjects: An Overview of Recent Insights and Therapies. Microorganisms 2021, 9, 1964. [Google Scholar] [CrossRef]
- Buonsenso, D.; Martino, L.; Morello, R.; De Rose, C.; Valentini, P. Chronic Olfactory Dysfunction in Children with Long COVID: A Retrospective Study. Children 2022, 9, 1251. [Google Scholar] [CrossRef]
- McWilliams, M.P.; Coelho, D.H.; Reiter, E.R.; Costanzo, R.M. Recovery from COVID-19 smell loss: Two-years of follow up. Am. J. Otolaryngol. 2022, 43, 103607. [Google Scholar] [CrossRef]

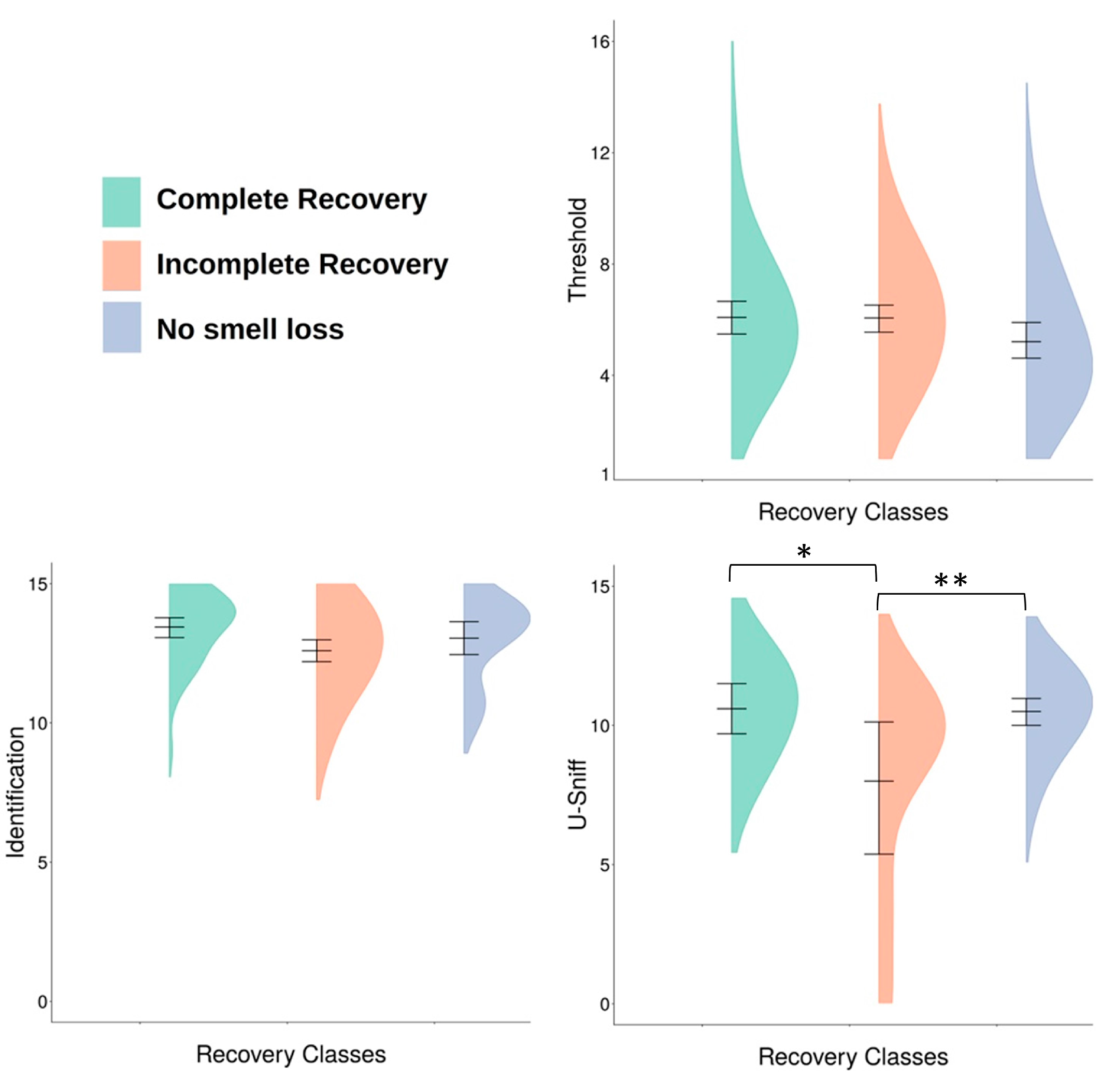
| Recovery Class | n | Mean Threshold (SD) | n | Mean U-Sniff (SD) | n | Mean Identification (SD) |
|---|---|---|---|---|---|---|
| Complete Recovery | 57 | 6.00 (2.36) | 11 | 10.64 a (1.43) | 46 | 13.43 (1.29) |
| Incomplete Recovery | 77 | 5.95 (2.28) | 9 | 8.11 ab (3.66) | 68 | 12.71 (1.77) |
| No Smell Loss | 52 | 5.21 (2.24) | 29 | 10.38 b (1.50) | 23 | 12.96 (1.52) |
| Total | 186 | 5.76 (2.31) | 49 | 10.02 (2.19) | 137 | 12.99 (1.61) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cancellieri, E.; Hernandez, A.K.; Degkwitz, H.; Kahre, E.; Blankenburg, J.; Horst, T.S.; Czyborra, P.; Boscolo-Rizzo, P.; Hummel, T. Subjective Perception of Recovery and Measured Olfactory Function in COVID-19 Patients. Viruses 2023, 15, 1418. https://doi.org/10.3390/v15071418
Cancellieri E, Hernandez AK, Degkwitz H, Kahre E, Blankenburg J, Horst TS, Czyborra P, Boscolo-Rizzo P, Hummel T. Subjective Perception of Recovery and Measured Olfactory Function in COVID-19 Patients. Viruses. 2023; 15(7):1418. https://doi.org/10.3390/v15071418
Chicago/Turabian StyleCancellieri, Emilia, Anna Kristina Hernandez, Helena Degkwitz, Elisabeth Kahre, Judith Blankenburg, Theresa S. Horst, Paula Czyborra, Paolo Boscolo-Rizzo, and Thomas Hummel. 2023. "Subjective Perception of Recovery and Measured Olfactory Function in COVID-19 Patients" Viruses 15, no. 7: 1418. https://doi.org/10.3390/v15071418
APA StyleCancellieri, E., Hernandez, A. K., Degkwitz, H., Kahre, E., Blankenburg, J., Horst, T. S., Czyborra, P., Boscolo-Rizzo, P., & Hummel, T. (2023). Subjective Perception of Recovery and Measured Olfactory Function in COVID-19 Patients. Viruses, 15(7), 1418. https://doi.org/10.3390/v15071418






