Comparison of the Biological Basis for Non-HIV Transmission to HIV-Exposed Seronegative Individuals, Disease Non-Progression in HIV Long-Term Non-Progressors and Elite Controllers
Abstract
1. Introduction
2. Mechanisms of Non-HIV Transmission to HESN Individuals
2.1. Adaptive Immunity in HESN Individuals
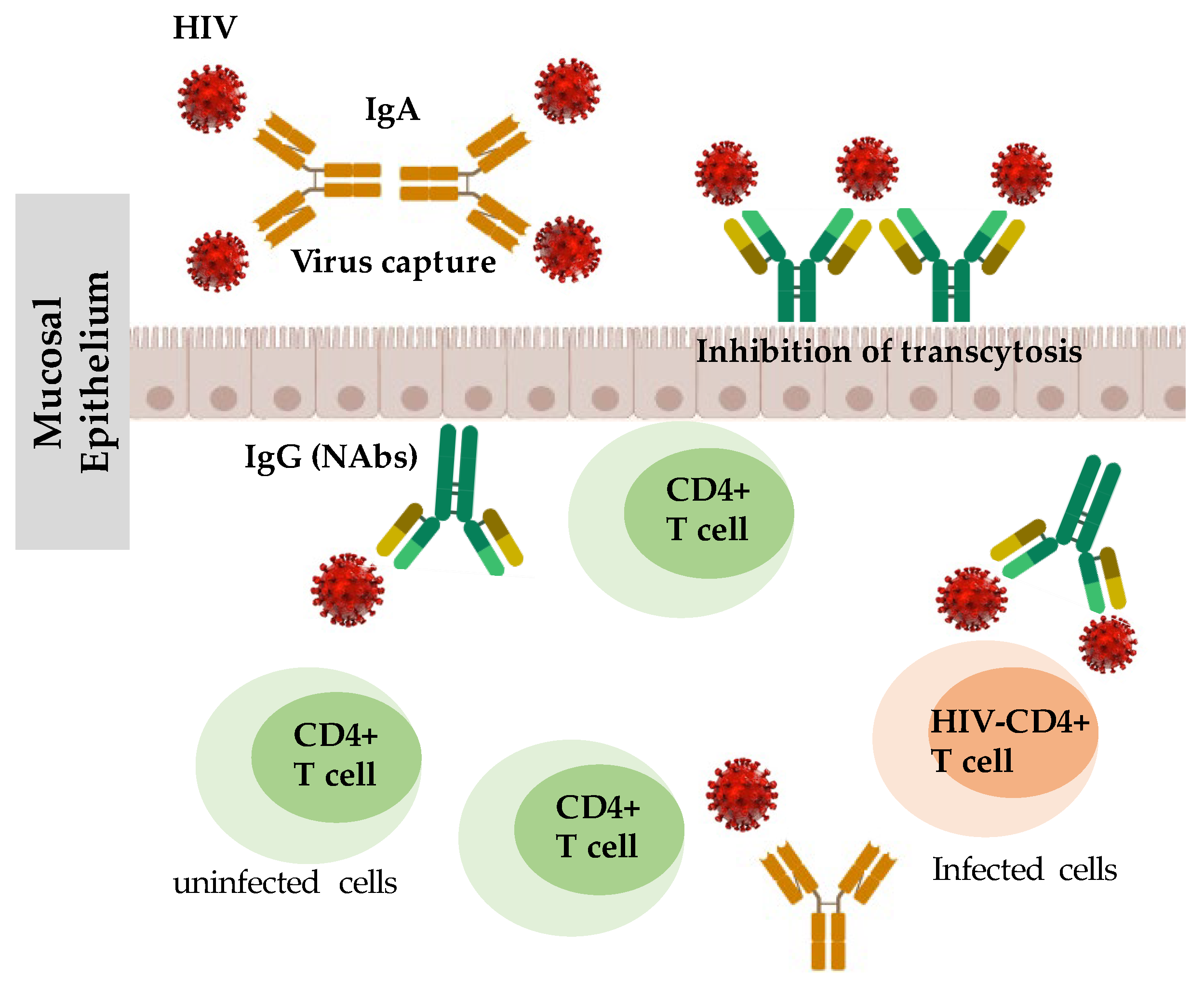
2.1.1. Humoral Immune Responses in HESN Individuals
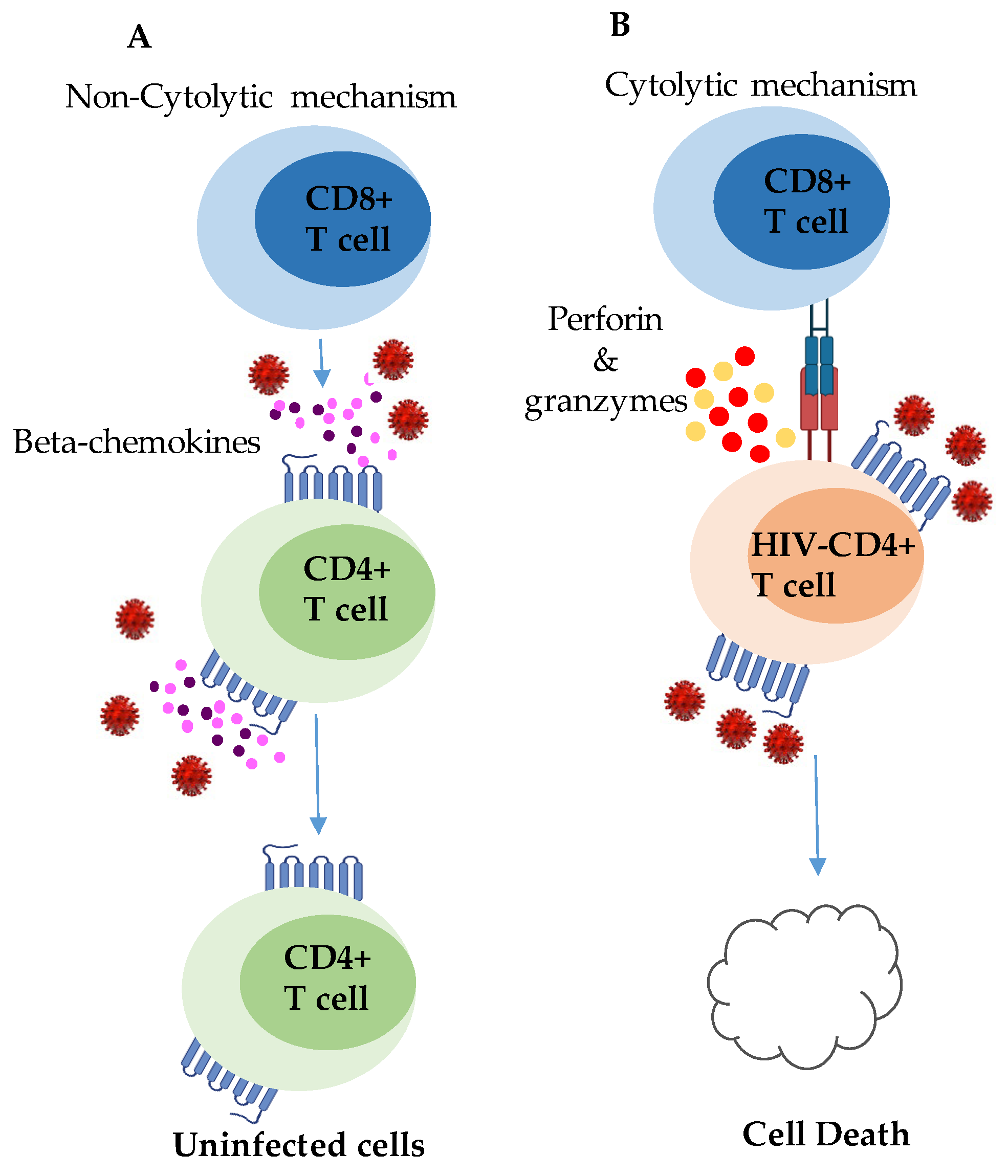
2.1.2. CD8+ T-Cell Responses in HESN Individuals
2.2. Innate Intracellular Antiviral Proteins in HESN Individuals
2.3. Innate Immunity in HESN Individuals
3. Mechanisms of Disease Non-Progression in HIV LTNPs
3.1. Attenuated Viruses in LTNPs
3.2. Innate Intracellular Antiviral Proteins in LTNPs
3.2.1. Endogenous Antiretroviral Protein APOBEC3G
3.2.2. The Trim5α
3.3. The Role of Genetic Factors in Disease Non-Progression in HIV LTNPs
3.4. The Role of Adaptive Immune Responses in LTNPs
3.4.1. Humoral Immunity
3.4.2. CD8+ T Cells
4. Mechanisms of Spontaneous and Durable HIV Control by Elite Controllers
4.1. Adaptive Immune Response in HIV Elite Controllers
4.1.1. Humoral Immune Responses
4.1.2. CD4+ T-Cell Responses
4.1.3. CD8+ T-Cell Responses in HIV Controllers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biraro, S.; Ruzagira, E.; Kamali, A.; Whitworth, J.; Grosskurth, H.; Weiss, H.A. HIV-1 transmission within marriage in rural Uganda: A longitudinal study. PLoS ONE 2013, 8, e55060. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, B.L.; de Bruyn, G.; Farquhar, C. HIV-1-discordant couples in sub-Saharan Africa: Explanations and implications for high rates of discordancy. Curr. HIV Res. 2007, 5, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Lopalco, L.; Mazzotta, F.; Lo Caputo, S.; Veas, F.; Clerici, M.; Group, E.S.N.S. The ‘immunologic advantage’ of HIV-exposed seronegative individuals. AIDS 2009, 23, 161–175. [Google Scholar] [CrossRef]
- Hokello, J.; Sharma, A.L.; Tyagi, M. An Update on the HIV DNA Vaccine Strategy. Vaccines 2021, 9, 605. [Google Scholar] [CrossRef]
- Hokello, J.; Sharma, A.L.; Dimri, M.; Tyagi, M. Insights into the HIV Latency and the Role of Cytokines. Pathogens 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Zicari, S.; Sharma, A.L.; Sahu, G.; Dubrovsky, L.; Sun, L.; Yue, H.; Jada, T.; Ochem, A.; Simon, G.; Bukrinsky, M.; et al. DNA dependent protein kinase (DNA-PK) enhances HIV transcription by promoting RNA polymerase II activity and recruitment of transcription machinery at HIV LTR. Oncotarget 2020, 11, 699–726. [Google Scholar] [CrossRef]
- Alqatawni, A.; Sharma, A.L.; Attilus, B.; Tyagi, M.; Daniel, R. Shedding Light on the Role of Extracellular Vesicles in HIV Infection and Wound Healing. Viruses 2020, 12, 584. [Google Scholar] [CrossRef]
- Sharma, A.L.; Hokello, J.; Sonti, S.; Zicari, S.; Sun, L.; Alqatawni, A.; Bukrinsky, M.; Simon, G.; Chauhan, A.; Daniel, R.; et al. CBF-1 Promotes the Establishment and Maintenance of HIV Latency by Recruiting Polycomb Repressive Complexes, PRC1 and PRC2, at HIV LTR. Viruses 2020, 12, 1040. [Google Scholar] [CrossRef]
- Sonti, S.; Sharma, A.L.; Tyagi, M. HIV-1 persistence in the CNS: Mechanisms of latency, pathogenesis and an update on eradication strategies. Virus Res. 2021, 303, 198523. [Google Scholar] [CrossRef]
- Sharma, A.L.; Shafer, D.; Netting, D.; Tyagi, M. Cocaine sensitizes the CD4(+) T cells for HIV infection by co-stimulating NFAT and AP-1. iScience 2022, 25, 105651. [Google Scholar] [CrossRef]
- Sonti, S.; Tyagi, K.; Pande, A.; Daniel, R.; Sharma, A.L.; Tyagi, M. Crossroads of Drug Abuse and HIV Infection: Neurotoxicity and CNS Reservoir. Vaccines 2022, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qin, L.; Zhang, L.; Safrit, J.; Ho, D.D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 1995, 332, 201–208. [Google Scholar] [CrossRef]
- Munoz, A.; Kirby, A.J.; He, Y.D.; Margolick, J.B.; Visscher, B.R.; Rinaldo, C.R.; Kaslow, R.A.; Phair, J.P. Long-term survivors with HIV-1 infection: Incubation period and longitudinal patterns of CD4+ lymphocytes. JAIDS J. Acquir. Immune Defic. Syndr. 1995, 8, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, G.; Menzo, S.; Vaccarezza, M.; Graziosi, C.; Cohen, O.J.; Demarest, J.F.; Montefiori, D.; Orenstein, J.M.; Fox, C.; Schrager, L.K.; et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N. Engl. J. Med. 1995, 332, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, H.W.; Lang, W.; Ascher, M.S.; Vittinghoff, E.; Winkelstein, W. The characterization of non-progressors: Long-term HIV-1 infection with stable CD4+ T-cell levels. AIDS 1993, 7, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Goudsmit, J.; Bogaards, J.A.; Jurriaans, S.; Schuitemaker, H.; Lange, J.M.; Coutinho, R.A.; Weverling, G.J. Naturally HIV-1 seroconverters with lowest viral load have best prognosis, but in time lose control of viraemia. AIDS 2002, 16, 791–793. [Google Scholar] [CrossRef]
- Lefrere, J.J.; Morand-Joubert, L.; Mariotti, M.; Bludau, H.; Burghoffer, B.; Petit, J.C.; Roudot-Thoraval, F. Even individuals considered as long-term nonprogressors show biological signs of progression after 10 years of human immunodeficiency virus infection. Blood 1997, 90, 1133–1140. [Google Scholar] [CrossRef]
- O’Brien, T.R.; Blattner, W.A.; Waters, D.; Eyster, E.; Hilgartner, M.W.; Cohen, A.R.; Luban, N.; Hatzakis, A.; Aledort, L.M.; Rosenberg, P.S.; et al. Serum HIV-1 RNA levels and time to development of AIDS in the Multicenter Hemophilia Cohort Study. JAMA 1996, 276, 105–110. [Google Scholar] [CrossRef]
- Rodes, B.; Toro, C.; Paxinos, E.; Poveda, E.; Martinez-Padial, M.; Benito, J.M.; Jimenez, V.; Wrin, T.; Bassani, S.; Soriano, V. Differences in disease progression in a cohort of long-term non-progressors after more than 16 years of HIV-1 infection. AIDS 2004, 18, 1109–1116. [Google Scholar] [CrossRef]
- Kayongo, A.; Gonzalo-Gil, E.; Gumusgoz, E.; Niwaha, A.J.; Semitala, F.; Kalyesubula, R.; Bagaya, B.S.; Joloba, M.L.; Sutton, R.E. Brief Report: Identification of Elite and Viremic Controllers from a Large Urban HIV Ambulatory Center in Kampala, Uganda. J. Acquir. Immune Defic. Syndr. 2018, 79, 394–398. [Google Scholar] [CrossRef]
- Lambotte, O.; Boufassa, F.; Madec, Y.; Nguyen, A.; Goujard, C.; Meyer, L.; Rouzioux, C.; Venet, A.; Delfraissy, J.F.; Group, S.-H.S. HIV controllers: A homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect Dis. 2005, 41, 1053–1056. [Google Scholar] [CrossRef]
- Laeyendecker, O.; Redd, A.D.; Lutalo, T.; Gray, R.H.; Wawer, M.; Ssempijja, V.; Gamiel, J.; Bwanika, J.B.; Makumbi, F.; Nalugoda, F.; et al. Frequency of long-term nonprogressors in HIV-1 seroconverters From Rakai Uganda. J. Acquir. Immune Defic. Syndr. 2009, 52, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Umbert, M.; Llano, A.; Bellido, R.; Olvera, A.; Ruiz-Riol, M.; Rocafort, M.; Fernandez, M.A.; Cobarsi, P.; Crespo, M.; Dorrell, L.; et al. Mechanisms of Abrupt Loss of Virus Control in a Cohort of Previous HIV Controllers. J. Virol. 2019, 93, e01436-18. [Google Scholar] [CrossRef] [PubMed]
- Pernas, M.; Tarancon-Diez, L.; Rodriguez-Gallego, E.; Gomez, J.; Prado, J.G.; Casado, C.; Dominguez-Molina, B.; Olivares, I.; Coiras, M.; Leon, A.; et al. Factors Leading to the Loss of Natural Elite Control of HIV-1 Infection. J. Virol. 2018, 92, e01805-17. [Google Scholar] [CrossRef]
- Sharma, A.L.; Hokello, J.; Tyagi, M. Circumcision as an Intervening Strategy against HIV Acquisition in the Male Genital Tract. Pathogens 2021, 10, 806. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, D.B.; Moss, G.B.; Willerford, D.M.; Hensel, M.; Emonyi, W.; Holmes, K.K.; Plummer, F.; Ndinya-Achola, J.; Roberts, P.L.; Hillier, S.; et al. Detection of HIV DNA in cervical and vaginal secretions. Prevalence and correlates among women in Nairobi, Kenya. JAMA 1993, 269, 2860–2864. [Google Scholar] [CrossRef] [PubMed]
- Bagasra, O.; Farzadegan, H.; Seshamma, T.; Oakes, J.W.; Saah, A.; Pomerantz, R.J. Detection of HIV-1 proviral DNA in sperm from HIV-1-infected men. AIDS 1994, 8, 1669–1674. [Google Scholar] [CrossRef]
- Barreto-de-Souza, V.; Arakelyan, A.; Margolis, L.; Vanpouille, C. HIV-1 vaginal transmission: Cell-free or cell-associated virus? Am. J. Reprod. Immunol. 2014, 71, 589–599. [Google Scholar] [CrossRef]
- Bracq, L.; Xie, M.; Benichou, S.; Bouchet, J. Mechanisms for Cell-to-Cell Transmission of HIV-1. Front. Immunol. 2018, 9, 260. [Google Scholar] [CrossRef]
- Ganor, Y.; Zhou, Z.; Tudor, D.; Schmitt, A.; Vacher-Lavenu, M.C.; Gibault, L.; Thiounn, N.; Tomasini, J.; Wolf, J.P.; Bomsel, M. Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans-T cell conjugates. Mucosal Immunol. 2010, 3, 506–522. [Google Scholar] [CrossRef]
- Kolodkin-Gal, D.; Hulot, S.L.; Korioth-Schmitz, B.; Gombos, R.B.; Zheng, Y.; Owuor, J.; Lifton, M.A.; Ayeni, C.; Najarian, R.M.; Yeh, W.W.; et al. Efficiency of cell-free and cell-associated virus in mucosal transmission of human immunodeficiency virus type 1 and simian immunodeficiency virus. J. Virol. 2013, 87, 13589–13597. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, N.; Carr, A.; Nam, D.S.; Moor-Jankowski, R.; Cooper, D.A.; Ho, D.D. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: Evidence for viral compartmentalization and selection during sexual transmission. J. Virol. 1996, 70, 3098–3107. [Google Scholar] [CrossRef]
- Nazli, A.; Chan, O.; Dobson-Belaire, W.N.; Ouellet, M.; Tremblay, M.J.; Gray-Owen, S.D.; Arsenault, A.L.; Kaushic, C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010, 6, e1000852. [Google Scholar] [CrossRef]
- Bomsel, M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 1997, 3, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.H.; Dizzell, S.; Nazli, A.; Kafka, J.K.; Mueller, K.; Nguyen, P.V.; Tremblay, M.J.; Cochrane, A.; Kaushic, C. Medroxyprogesterone Acetate Regulates HIV-1 Uptake and Transcytosis but Not Replication in Primary Genital Epithelial Cells, Resulting in Enhanced T-Cell Infection. J. Infect. Dis. 2015, 211, 1745–1756. [Google Scholar] [CrossRef]
- Stoddard, E.; Ni, H.; Cannon, G.; Zhou, C.; Kallenbach, N.; Malamud, D.; Weissman, D. gp340 promotes transcytosis of human immunodeficiency virus type 1 in genital tract-derived cell lines and primary endocervical tissue. J. Virol. 2009, 83, 8596–8603. [Google Scholar] [CrossRef]
- Bobardt, M.D.; Chatterji, U.; Selvarajah, S.; Van der Schueren, B.; David, G.; Kahn, B.; Gallay, P.A. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J. Virol. 2007, 81, 395–405. [Google Scholar] [CrossRef]
- Herrera, R.; Morris, M.; Rosbe, K.; Feng, Z.; Weinberg, A.; Tugizov, S. Human beta-defensins 2 and -3 cointernalize with human immunodeficiency virus via heparan sulfate proteoglycans and reduce infectivity of intracellular virions in tonsil epithelial cells. Virology 2016, 487, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S.M.; Herrera, R.; Veluppillai, P.; Greenspan, D.; Soros, V.; Greene, W.C.; Levy, J.A.; Palefsky, J.M. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology 2011, 409, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Richter, H.E.; Smith, P.D. Interactions between HIV-1 and mucosal cells in the female reproductive tract. Am. J. Reprod. Immunol. 2014, 71, 608–617. [Google Scholar] [CrossRef]
- Gonzalez, S.M.; Aguilar-Jimenez, W.; Su, R.C.; Rugeles, M.T. Mucosa: Key Interactions Determining Sexual Transmission of the HIV Infection. Front. Immunol. 2019, 10, 144. [Google Scholar] [CrossRef]
- Choi, R.Y.; Levinson, P.; Guthrie, B.L.; Lohman-Payne, B.; Bosire, R.; Liu, A.Y.; Hirbod, T.; Kiarie, J.; Overbaugh, J.; John-Stewart, G.; et al. Cervicovaginal HIV-1-neutralizing immunoglobulin A detected among HIV-1-exposed seronegative female partners in HIV-1-discordant couples. AIDS 2012, 26, 2155–2163. [Google Scholar] [CrossRef]
- Carrillo, J.; Restrepo, C.; Rallon, N.I.; Massanella, M.; del Romero, J.; Rodriguez, C.; Soriano, V.; Clotet, B.; Benito, J.M.; Blanco, J. HIV exposed seronegative individuals show antibodies specifically recognizing native HIV envelope glycoprotein. AIDS 2013, 27, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Hirbod, T.; Kong, X.; Kigozi, G.; Ndyanabo, A.; Serwadda, D.; Prodger, J.L.; Tobian, A.A.; Nalugoda, F.; Wawer, M.J.; Shahabi, K.; et al. HIV acquisition is associated with increased antimicrobial peptides and reduced HIV neutralizing IgA in the foreskin prepuce of uncircumcised men. PLoS Pathog. 2014, 10, e1004416. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, L.; Sabourin-Poirier, C.; Perraud, V.; Faucher, M.C.; Chagnon-Choquet, J.; Labbe, A.C.; Alary, M.; Guedou, F.; Poudrier, J.; Roger, M. Natural Immunity to HIV is associated with Low BLyS/BAFF levels and low frequencies of innate marginal zone like CD1c+ B-cells in the genital tract. PLoS Pathog. 2019, 15, e1007840. [Google Scholar] [CrossRef] [PubMed]
- Khamassi, M.; Xu, L.; Rey, J.; Duchemin, M.; Bouceba, T.; Tuffery, P.; Tudor, D.; Bomsel, M. The CH1alpha domain of mucosal gp41 IgA contributes to antibody specificity and antiviral functions in HIV-1 highly exposed Sero-Negative individuals. PLoS Pathog. 2020, 16, e1009103. [Google Scholar] [CrossRef] [PubMed]
- Devito, C.; Hinkula, J.; Kaul, R.; Lopalco, L.; Bwayo, J.J.; Plummer, F.; Clerici, M.; Broliden, K. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS 2000, 14, 1917–1920. [Google Scholar] [CrossRef]
- Hocini, H.; Bomsel, M. Infectious human immunodeficiency virus can rapidly penetrate a tight human epithelial barrier by transcytosis in a process impaired by mucosal immunoglobulins. J. Infect. Dis. 1999, 179 (Suppl. 3), S448–S453. [Google Scholar] [CrossRef]
- Collins, D.R.; Hitschfel, J.; Urbach, J.M.; Mylvaganam, G.H.; Ly, N.L.; Arshad, U.; Racenet, Z.J.; Yanez, A.G.; Diefenbach, T.J.; Walker, B.D. Cytolytic CD8(+) T cells infiltrate germinal centers to limit ongoing HIV replication in spontaneous controller lymph nodes. Sci. Immunol. 2023, 8, eade5872. [Google Scholar] [CrossRef]
- Kaul, R.; Plummer, F.A.; Kimani, J.; Dong, T.; Kiama, P.; Rostron, T.; Njagi, E.; MacDonald, K.S.; Bwayo, J.J.; McMichael, A.J.; et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 2000, 164, 1602–1611. [Google Scholar] [CrossRef]
- Furci, L.; Lopalco, L.; Loverro, P.; Sinnone, M.; Tambussi, G.; Lazzarin, A.; Lusso, P. Non-cytotoxic inhibition of HIV-1 infection by unstimulated CD8+ T lymphocytes from HIV-exposed-uninfected individuals. AIDS 2002, 16, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.X.; Luong, T.T.; Scott-Algara, D.; Versmisse, P.; David, A.; Perez-Bercoff, D.; Nguyen, N.V.; Tran, H.K.; Cao, C.T.; Fontanet, A.; et al. CD4 cell and CD8 cell-mediated resistance to HIV-1 infection in exposed uninfected intravascular drug users in Vietnam. AIDS 2003, 17, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Paxton, W.A.; Martin, S.R.; Tse, D.; O’Brien, T.R.; Skurnick, J.; VanDevanter, N.L.; Padian, N.; Braun, J.F.; Kotler, D.P.; Wolinsky, S.M.; et al. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat. Med. 1996, 2, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Paxton, W.A.; Liu, R.; Kang, S.; Wu, L.; Gingeras, T.R.; Landau, N.R.; Mackay, C.R.; Koup, R.A. Reduced HIV-1 infectability of CD4+ lymphocytes from exposed-uninfected individuals: Association with low expression of CCR5 and high production of beta-chemokines. Virology 1998, 244, 66–73. [Google Scholar] [CrossRef]
- Connor, R.I.; Paxton, W.A.; Sheridan, K.E.; Koup, R.A. Macrophages and CD4+ T lymphocytes from two multiply exposed, uninfected individuals resist infection with primary non-syncytium-inducing isolates of human immunodeficiency virus type 1. J. Virol. 1996, 70, 8758–8764. [Google Scholar] [CrossRef]
- Bushman, F.D.; Malani, N.; Fernandes, J.; D’Orso, I.; Cagney, G.; Diamond, T.L.; Zhou, H.; Hazuda, D.J.; Espeseth, A.S.; Konig, R.; et al. Host cell factors in HIV replication: Meta-analysis of genome-wide studies. PLoS Pathog. 2009, 5, e1000437. [Google Scholar] [CrossRef]
- MacPherson, J.I.; Dickerson, J.E.; Pinney, J.W.; Robertson, D.L. Patterns of HIV-1 protein interaction identify perturbed host-cellular subsystems. PLoS Comput. Biol. 2010, 6, e1000863. [Google Scholar] [CrossRef]
- Strebel, K.; Luban, J.; Jeang, K.T. Human cellular restriction factors that target HIV-1 replication. BMC Med. 2009, 7, 48. [Google Scholar] [CrossRef]
- Cherepanov, P.; Maertens, G.; Proost, P.; Devreese, B.; Van Beeumen, J.; Engelborghs, Y.; De Clercq, E.; Debyser, Z. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J. Biol. Chem. 2003, 278, 372–381. [Google Scholar] [CrossRef]
- Ciuffi, A.; Llano, M.; Poeschla, E.; Hoffmann, C.; Leipzig, J.; Shinn, P.; Ecker, J.R.; Bushman, F. A role for LEDGF/p75 in targeting HIV DNA integration. Nat. Med. 2005, 11, 1287–1289. [Google Scholar] [CrossRef]
- Van Maele, B.; Busschots, K.; Vandekerckhove, L.; Christ, F.; Debyser, Z. Cellular co-factors of HIV-1 integration. Trends Biochem. Sci. 2006, 31, 98–105. [Google Scholar] [CrossRef]
- Stremlau, M.; Owens, C.M.; Perron, M.J.; Kiessling, M.; Autissier, P.; Sodroski, J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 2004, 427, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Neil, S.J.; Zang, T.; Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008, 451, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, A.M.; Gaddis, N.C.; Choi, J.D.; Malim, M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 2002, 418, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Black, L.R.; Aiken, C. TRIM5alpha disrupts the structure of assembled HIV-1 capsid complexes in vitro. J. Virol. 2010, 84, 6564–6569. [Google Scholar] [CrossRef]
- Stremlau, M.; Perron, M.; Lee, M.; Li, Y.; Song, B.; Javanbakht, H.; Diaz-Griffero, F.; Anderson, D.J.; Sundquist, W.I.; Sodroski, J. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc. Natl. Acad. Sci. USA 2006, 103, 5514–5519. [Google Scholar] [CrossRef]
- Zhang, X.; Kondo, M.; Chen, J.; Miyoshi, H.; Suzuki, H.; Ohashi, T.; Shida, H. Inhibitory effect of human TRIM5alpha on HIV-1 production. Microbes Infect. 2010, 12, 768–777. [Google Scholar] [CrossRef]
- Speelmon, E.C.; Livingston-Rosanoff, D.; Desbien, A.L.; Lee, J.; Wick, W.D.; Hladik, F.; McElrath, M.J. Impaired viral entry cannot explain reduced CD4+ T cell susceptibility to HIV type 1 in certain highly exposed individuals. AIDS Res. Hum. Retrovir. 2008, 24, 1415–1427. [Google Scholar] [CrossRef]
- Gonzalez, S.M.; Taborda, N.A.; Feria, M.G.; Arcia, D.; Aguilar-Jimenez, W.; Zapata, W.; Rugeles, M.T. High Expression of Antiviral Proteins in Mucosa from Individuals Exhibiting Resistance to Human Immunodeficiency Virus. PLoS ONE 2015, 10, e0131139. [Google Scholar] [CrossRef]
- Mous, K.; Jennes, W.; Camara, M.; Seydi, M.; Daneau, G.; Mboup, S.; Kestens, L.; Van Ostade, X. Expression analysis of LEDGF/p75, APOBEC3G, TRIM5alpha, and tetherin in a Senegalese cohort of HIV-1-exposed seronegative individuals. PLoS ONE 2012, 7, e33934. [Google Scholar] [CrossRef]
- Lajoie, J.; Juno, J.; Burgener, A.; Rahman, S.; Mogk, K.; Wachihi, C.; Mwanjewe, J.; Plummer, F.A.; Kimani, J.; Ball, T.B.; et al. A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunol. 2012, 5, 277–287. [Google Scholar] [CrossRef]
- Poudrier, J.; Thibodeau, V.; Roger, M. Natural Immunity to HIV: A delicate balance between strength and control. Clin. Dev. Immunol. 2012, 2012, 875821. [Google Scholar] [CrossRef]
- Schellenberg, J.J.; Plummer, F.A. The Microbiological Context of HIV Resistance: Vaginal Microbiota and Mucosal Inflammation at the Viral Point of Entry. Int. J. Inflam. 2012, 2012, 131243. [Google Scholar] [CrossRef]
- Yao, X.D.; Omange, R.W.; Henrick, B.M.; Lester, R.T.; Kimani, J.; Ball, T.B.; Plummer, F.A.; Rosenthal, K.L. Acting locally: Innate mucosal immunity in resistance to HIV-1 infection in Kenyan commercial sex workers. Mucosal Immunol. 2014, 7, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.; Poudrier, J.; Massinga Loembe, M.; Guedou, F.; Leblond, F.; Labbe, A.C.; Alary, M.; Roger, M. Chemokine expression patterns in the systemic and genital tract compartments are associated with HIV-1 infection in women from Benin. J. Clin. Immunol. 2010, 30, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.; Poudrier, J.; Massinga-Loembe, M.; Guedou, F.; Agossa-Gbenafa, C.; Labbe, A.C.; Alary, M.; Roger, M. Differences in immunoregulatory cytokine expression patterns in the systemic and genital tract compartments of HIV-1-infected commercial sex workers in Benin. Mucosal Immunol. 2008, 1, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Ball, T.B.; Ji, H.; Kimani, J.; McLaren, P.; Marlin, C.; Hill, A.V.; Plummer, F.A. Polymorphisms in IRF-1 associated with resistance to HIV-1 infection in highly exposed uninfected Kenyan sex workers. AIDS 2007, 21, 1091–1101. [Google Scholar] [CrossRef]
- Ji, H.; Ball, T.B.; Ao, Z.; Kimani, J.; Yao, X.; Plummer, F.A. Reduced HIV-1 long terminal repeat transcription in subjects with protective interferon regulatory factor-1 genotype: A potential mechanism mediating resistance to infection by HIV-1. Scand. J. Infect. Dis. 2010, 42, 389–394. [Google Scholar] [CrossRef]
- Fowke, K.R.; Kaul, R.; Rosenthal, K.L.; Oyugi, J.; Kimani, J.; Rutherford, W.J.; Nagelkerke, N.J.; Ball, T.B.; Bwayo, J.J.; Simonsen, J.N.; et al. HIV-1-specific cellular immune responses among HIV-1-resistant sex workers. Immunol. Cell Biol. 2000, 78, 586–595. [Google Scholar] [CrossRef]
- McKinnon, L.R.; Nyanga, B.; Chege, D.; Izulla, P.; Kimani, M.; Huibner, S.; Gelmon, L.; Block, K.E.; Cicala, C.; Anzala, A.O.; et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J. Immunol. 2011, 187, 6032–6042. [Google Scholar] [CrossRef]
- McLaren, P.J.; Ball, T.B.; Wachihi, C.; Jaoko, W.; Kelvin, D.J.; Danesh, A.; Kimani, J.; Plummer, F.A.; Fowke, K.R. HIV-exposed seronegative commercial sex workers show a quiescent phenotype in the CD4+ T cell compartment and reduced expression of HIV-dependent host factors. J. Infect. Dis. 2010, 202 (Suppl. 3), S339–S344. [Google Scholar] [CrossRef]
- Ritchie, A.J.; Campion, S.L.; Kopycinski, J.; Moodie, Z.; Wang, Z.M.; Pandya, K.; Moore, S.; Liu, M.K.; Brackenridge, S.; Kuldanek, K.; et al. Differences in HIV-specific T cell responses between HIV-exposed and -unexposed HIV-seronegative individuals. J. Virol. 2011, 85, 3507–3516. [Google Scholar] [CrossRef]
- Murashev, B.V.; Nazarenko, O.V.; Akulova, E.B.; Artemyeva, A.K.; Verevochkin, S.V.; Shaboltas, A.V.; Skochilov, R.V.; Toussova, O.V.; Kozlov, A.P. The high frequency of HIV type 1-specific cellular immune responses in seronegative individuals with parenteral and/or heterosexual HIV type 1 exposure. AIDS. Res. Hum. Retrovir. 2012, 28, 1598–1605. [Google Scholar] [CrossRef]
- Cerutti, A.; Cols, M.; Puga, I. Marginal zone B cells: Virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013, 13, 118–132. [Google Scholar] [CrossRef]
- Victora, G.D.; Nussenzweig, M.C. Germinal centers. Annu. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef]
- He, B.; Qiao, X.; Klasse, P.J.; Chiu, A.; Chadburn, A.; Knowles, D.M.; Moore, J.P.; Cerutti, A. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J. Immunol. 2006, 176, 3931–3941. [Google Scholar] [CrossRef]
- Fontaine, J.; Chagnon-Choquet, J.; Valcke, H.S.; Poudrier, J.; Roger, M.; Montreal Primary, H.I.V.I.; Long-Term Non-Progressor Study, G. High expression levels of B lymphocyte stimulator (BLyS) by dendritic cells correlate with HIV-related B-cell disease progression in humans. Blood 2011, 117, 145–155. [Google Scholar] [CrossRef]
- Wada, N.I.; Jacobson, L.P.; Margolick, J.B.; Breen, E.C.; Macatangay, B.; Penugonda, S.; Martinez-Maza, O.; Bream, J.H. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015, 29, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Chagnon-Choquet, J.; Gauvin, J.; Roger, J.; Fontaine, J.; Poudrier, J.; Roger, M.; Montreal Primary, H.I.V.I.; Slow Progressor Study, G. HIV Nef promotes expression of B-lymphocyte stimulator by blood dendritic cells during HIV infection in humans. J. Infect. Dis. 2015, 211, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.M.; Ouellet, M.; Tremblay, M.J. HIV-1-triggered release of type I IFN by plasmacytoid dendritic cells induces BAFF production in monocytes. J. Immunol. 2015, 194, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Sjostrand, M.; Johansson, A.; Aqrawi, L.; Olsson, T.; Wahren-Herlenius, M.; Espinosa, A. The Expression of BAFF Is Controlled by IRF Transcription Factors. J. Immunol. 2016, 196, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.T.; Enghard, P.; Riemekasten, G.; Berek, C. In vitro and in vivo activation induces BAFF and APRIL expression in B cells. J. Immunol. 2007, 179, 5947–5957. [Google Scholar] [CrossRef] [PubMed]
- Chagnon-Choquet, J.; Fontaine, J.; Poudrier, J.; Roger, M.; Montreal Primary, H.I.V.I.S.G.; Slow Progressor Study, G. IL-10 and lymphotoxin-alpha expression profiles within marginal zone-like B-cell populations are associated with control of HIV-1 disease progression. PLoS ONE 2014, 9, e101949. [Google Scholar] [CrossRef] [PubMed]
- Sabourin-Poirier, C.; Fourcade, L.; Chagnon-Choquet, J.; Labbe, A.C.; Alary, M.; Guedou, F.; Poudrier, J.; Roger, M. Blood B Lymphocyte Stimulator (BLyS)/BAFF levels may reflect natural immunity to HIV in highly exposed uninfected Beninese Commercial Sex Workers. Sci. Rep. 2016, 6, 32318. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, L.; Poudrier, J.; Roger, M. Natural Immunity to HIV: A Template for Vaccine Strategies. Viruses 2018, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, T.L.; MacGregor, R.R.; Burger, H.; Mick, R.; Doms, R.W.; Collman, R.G. CCR5 genotypes in sexually active couples discordant for human immunodeficiency virus type 1 infection status. J. Infect. Dis. 1997, 176, 1093–1096. [Google Scholar] [CrossRef]
- Marmor, M.; Sheppard, H.W.; Donnell, D.; Bozeman, S.; Celum, C.; Buchbinder, S.; Koblin, B.; Seage, G.R., 3rd. HIV Network for Prevention Trials Vaccine Preparedness Protocol Team. Homozygous and heterozygous CCR5-Delta32 genotypes are associated with resistance to HIV infection. J. Acquir. Immune Defic. Syndr. 2001, 27, 472–481. [Google Scholar] [CrossRef]
- Munusamy Ponnan, S.; Thiruvengadam, K.; Tellapragada, C.; Ambikan, A.T.; Narayanan, A.; Kathirvel, S.; Mathayan, M.; Shankar, J.; Rajaraman, A.; Afshan Amanulla, M.; et al. Deciphering the Role of Mucosal Immune Responses and the Cervicovaginal Microbiome in Resistance to HIV Infection in HIV-Exposed Seronegative (HESN) Women. Microbiol. Spectr. 2021, 9, e0047021. [Google Scholar] [CrossRef]
- Tomescu, C.; Seaton, K.E.; Smith, P.; Taylor, M.; Tomaras, G.D.; Metzger, D.S.; Montaner, L.J. Innate activation of MDC and NK cells in high-risk HIV-1-exposed seronegative IV-drug users who share needles when compared with low-risk nonsharing IV-drug user controls. J. Acquir. Immune Defic. Syndr. 2015, 68, 264–273. [Google Scholar] [CrossRef]
- Saulle, I.; Biasin, M.; Gnudi, F.; Rainone, V.; Ibba, S.V.; Lo Caputo, S.; Mazzotta, F.; Trabattoni, D.; Clerici, M. Short Communication: Immune Activation Is Present in HIV-1-Exposed Seronegative Individuals and Is Independent of Microbial Translocation. AIDS Res. Hum. Retrovir. 2016, 32, 129–133. [Google Scholar] [CrossRef]
- Fenizia, C.; Rossignol, J.F.; Clerici, M.; Biasin, M. Genetic and immune determinants of immune activation in HIV-exposed seronegative individuals and their role in protection against HIV infection. Infect. Genet. Evol. 2018, 66, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Sandonis, V.; Casado, C.; Alvaro, T.; Pernas, M.; Olivares, I.; Garcia, S.; Rodriguez, C.; del Romero, J.; Lopez-Galindez, C. A combination of defective DNA and protective host factors are found in a set of HIV-1 ancestral LTNPs. Virology 2009, 391, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Mikhail, M.; Dyer, W.B.; Zaunders, J.J.; Kelleher, A.D.; Saksena, N.K. First demonstration of a lack of viral sequence evolution in a nonprogressor, defining replication-incompetent HIV-1 infection. Virology 2003, 312, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Casado, C.; Marrero-Hernandez, S.; Marquez-Arce, D.; Pernas, M.; Marfil, S.; Borras-Granana, F.; Olivares, I.; Cabrera-Rodriguez, R.; Valera, M.S.; de Armas-Rillo, L.; et al. Viral Characteristics Associated with the Clinical Nonprogressor Phenotype Are Inherited by Viruses from a Cluster of HIV-1 Elite Controllers. mBio 2018, 9, e02338-17. [Google Scholar] [CrossRef]
- Perez-Yanes, S.; Pernas, M.; Marfil, S.; Cabrera-Rodriguez, R.; Ortiz, R.; Urrea, V.; Rovirosa, C.; Estevez-Herrera, J.; Olivares, I.; Casado, C.; et al. The Characteristics of the HIV-1 Env Glycoprotein Are Linked with Viral Pathogenesis. Front. Microbiol. 2022, 13, 763039. [Google Scholar] [CrossRef] [PubMed]
- Crotti, A.; Neri, F.; Corti, D.; Ghezzi, S.; Heltai, S.; Baur, A.; Poli, G.; Santagostino, E.; Vicenzi, E. Nef alleles from human immunodeficiency virus type 1-infected long-term-nonprogressor hemophiliacs with or without late disease progression are defective in enhancing virus replication and CD4 down-regulation. J. Virol. 2006, 80, 10663–10674. [Google Scholar] [CrossRef]
- Learmont, J.C.; Geczy, A.F.; Mills, J.; Ashton, L.J.; Raynes-Greenow, C.H.; Garsia, R.J.; Dyer, W.B.; McIntyre, L.; Oelrichs, R.B.; Rhodes, D.I.; et al. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 1999, 340, 1715–1722. [Google Scholar] [CrossRef]
- Choudhary, S.K.; Choudhary, N.R.; Kimbrell, K.C.; Colasanti, J.; Ziogas, A.; Kwa, D.; Schuitemaker, H.; Camerini, D. R5 human immunodeficiency virus type 1 infection of fetal thymic organ culture induces cytokine and CCR5 expression. J. Virol. 2005, 79, 458–471. [Google Scholar] [CrossRef]
- Alexander, L.; Weiskopf, E.; Greenough, T.C.; Gaddis, N.C.; Auerbach, M.R.; Malim, M.H.; O’Brien, S.J.; Walker, B.D.; Sullivan, J.L.; Desrosiers, R.C. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J. Virol. 2000, 74, 4361–4376. [Google Scholar] [CrossRef]
- Blankson, J.N.; Bailey, J.R.; Thayil, S.; Yang, H.C.; Lassen, K.; Lai, J.; Gandhi, S.K.; Siliciano, J.D.; Williams, T.M.; Siliciano, R.F. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J. Virol. 2007, 81, 2508–2518. [Google Scholar] [CrossRef]
- Piacentini, L.; Biasin, M.; Fenizia, C.; Clerici, M. Genetic correlates of protection against HIV infection: The ally within. J. Intern. Med. 2009, 265, 110–124. [Google Scholar] [CrossRef]
- Jin, X.; Wu, H.; Smith, H. APOBEC3G levels predict rates of progression to AIDS. Retrovirology 2007, 4, 20. [Google Scholar] [CrossRef]
- Vazquez-Perez, J.A.; Ormsby, C.E.; Hernandez-Juan, R.; Torres, K.J.; Reyes-Teran, G. APOBEC3G mRNA expression in exposed seronegative and early stage HIV infected individuals decreases with removal of exposure and with disease progression. Retrovirology 2009, 6, 23. [Google Scholar] [CrossRef]
- Land, A.M.; Ball, T.B.; Luo, M.; Pilon, R.; Sandstrom, P.; Embree, J.E.; Wachihi, C.; Kimani, J.; Plummer, F.A. Human immunodeficiency virus (HIV) type 1 proviral hypermutation correlates with CD4 count in HIV-infected women from Kenya. J. Virol. 2008, 82, 8172–8182. [Google Scholar] [CrossRef]
- An, P.; Martin, M.P.; Nelson, G.W.; Carrington, M.; Smith, M.W.; Gong, K.; Vlahov, D.; O’Brien, S.J.; Winkler, C.A. Influence of CCR5 promoter haplotypes on AIDS progression in African-Americans. AIDS 2000, 14, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- van Manen, D.; Rits, M.A.; Beugeling, C.; van Dort, K.; Schuitemaker, H.; Kootstra, N.A. The effect of Trim5 polymorphisms on the clinical course of HIV-1 infection. PLoS Pathog. 2008, 4, e18. [Google Scholar] [CrossRef]
- Javanbakht, H.; An, P.; Gold, B.; Petersen, D.C.; O’Huigin, C.; Nelson, G.W.; O’Brien, S.J.; Kirk, G.D.; Detels, R.; Buchbinder, S.; et al. Effects of human TRIM5alpha polymorphisms on antiretroviral function and susceptibility to human immunodeficiency virus infection. Virology 2006, 354, 15–27. [Google Scholar] [CrossRef]
- Nissen, S.K.; Christiansen, M.; Helleberg, M.; Kjaer, K.; Jorgensen, S.E.; Gerstoft, J.; Katzenstein, T.L.; Benfield, T.; Kronborg, G.; Larsen, C.S.; et al. Whole Exome Sequencing of HIV-1 long-term non-progressors identifies rare variants in genes encoding innate immune sensors and signaling molecules. Sci. Rep. 2018, 8, 15253. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.R.; van der Burg, S.H.; Hovenkamp, E.; Holwerda, A.M.; Drijfhout, J.W.; Melief, C.J.; Miedema, F. Characterization of HLA-B57-restricted human immunodeficiency virus type 1 Gag- and RT-specific cytotoxic T lymphocyte responses. J. Gen. Virol. 1998, 79, 2191–2201. [Google Scholar] [CrossRef] [PubMed]
- Bello, G.; Casado, C.; Sandonis, V.; Alonso-Nieto, M.; Vicario, J.L.; Garcia, S.; Hernando, V.; Rodriguez, C.; Romero, J.D.; Lopez-Galindez, C. A subset of human immunodeficiency virus type 1 long-term non-progressors is characterized by the unique presence of ancestral sequences in the viral population. J. Gen. Virol. 2005, 86, 355–364. [Google Scholar] [CrossRef]
- Middleton, D.; Menchaca, L.; Rood, H.; Komerofsky, R. New allele frequency database: Http://www.allelefrequencies.net. Tissue Antigens 2003, 61, 403–407. [Google Scholar] [CrossRef]
- Migueles, S.A.; Connors, M. Long-term nonprogressive disease among untreated HIV-infected individuals: Clinical implications of understanding immune control of HIV. JAMA 2010, 304, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Walli, R.; Reinhart, B.; Luckow, B.; Lederer, E.; Loch, O.; Malo, A.; Wank, R.; Schlondorff, D.; Goebel, F.D. HIV-1-infected long-term slow progressors heterozygous for delta32-CCR5 show significantly lower plasma viral load than wild-type slow progressors. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1998, 18, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Cecilia, D.; Kleeberger, C.; Munoz, A.; Giorgi, J.V.; Zolla-Pazner, S. A longitudinal study of neutralizing antibodies and disease progression in HIV-1-infected subjects. J. Infect. Dis. 1999, 179, 1365–1374. [Google Scholar] [CrossRef]
- Gonzalez, N.; McKee, K.; Lynch, R.M.; Georgiev, I.S.; Jimenez, L.; Grau, E.; Yuste, E.; Kwong, P.D.; Mascola, J.R.; Alcami, J. Characterization of broadly neutralizing antibody responses to HIV-1 in a cohort of long term non-progressors. PLoS ONE 2018, 13, e0193773. [Google Scholar] [CrossRef]
- Pilgrim, A.K.; Pantaleo, G.; Cohen, O.J.; Fink, L.M.; Zhou, J.Y.; Zhou, J.T.; Bolognesi, D.P.; Fauci, A.S.; Montefiori, D.C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J. Infect. Dis. 1997, 176, 924–932. [Google Scholar] [CrossRef]
- Doria-Rose, N.A.; Klein, R.M.; Daniels, M.G.; O’Dell, S.; Nason, M.; Lapedes, A.; Bhattacharya, T.; Migueles, S.A.; Wyatt, R.T.; Korber, B.T.; et al. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: Clustering analysis and association with clinical variables. J. Virol. 2010, 84, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, F.; Addo, M.M.; Kaufmann, D.E.; Liu, Y.; Miura, T.; Rathod, A.; Baker, B.; Trocha, A.; Rosenberg, R.; Mackey, E.; et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 2008, 197, 563–571. [Google Scholar] [CrossRef]
- Braibant, M.; Agut, H.; Rouzioux, C.; Costagliola, D.; Autran, B.; Barin, F. Characteristics of the env genes of HIV type 1 quasispecies in long-term nonprogressors with broadly neutralizing antibodies. J. Acquir. Immune Defic. Syndr. 2008, 47, 274–284. [Google Scholar] [CrossRef]
- Mahalanabis, M.; Jayaraman, P.; Miura, T.; Pereyra, F.; Chester, E.M.; Richardson, B.; Walker, B.; Haigwood, N.L. Continuous viral escape and selection by autologous neutralizing antibodies in drug-naive human immunodeficiency virus controllers. J. Virol. 2009, 83, 662–672. [Google Scholar] [CrossRef]
- Sreepian, A.; Srisurapanon, S.; Horthongkham, N.; Tunsupasawasdikul, S.; Kaoriangudom, S.; Khusmith, S.; Sutthent, R. Conserved neutralizing epitopes of HIV type 1 CRF01_AE against primary isolates in long-term nonprogressors. AIDS Res. Hum. Retrovir. 2004, 20, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, L. Humoral immunity in HIV-1 exposure: Cause or effect of HIV resistance? Curr. HIV Res. 2004, 2, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Migueles, S.A.; Connors, M. Frequency and function of HIV-specific CD8(+) T cells. Immunol. Lett. 2001, 79, 141–150. [Google Scholar] [CrossRef]
- Betts, M.R.; Krowka, J.F.; Kepler, T.B.; Davidian, M.; Christopherson, C.; Kwok, S.; Louie, L.; Eron, J.; Sheppard, H.; Frelinger, J.A. Human immunodeficiency virus type 1-specific cytotoxic T lymphocyte activity is inversely correlated with HIV type 1 viral load in HIV type 1-infected long-term survivors. AIDS Res. Hum. Retrovir. 1999, 15, 1219–1228. [Google Scholar] [CrossRef]
- Geldmacher, C.; Currier, J.R.; Herrmann, E.; Haule, A.; Kuta, E.; McCutchan, F.; Njovu, L.; Geis, S.; Hoffmann, O.; Maboko, L.; et al. CD8 T-cell recognition of multiple epitopes within specific Gag regions is associated with maintenance of a low steady-state viremia in human immunodeficiency virus type 1-seropositive patients. J. Virol. 2007, 81, 2440–2448. [Google Scholar] [CrossRef]
- Ferre, A.L.; Lemongello, D.; Hunt, P.W.; Morris, M.M.; Garcia, J.C.; Pollard, R.B.; Yee, H.F., Jr.; Martin, J.N.; Deeks, S.G.; Shacklett, B.L. Immunodominant HIV-specific CD8+ T-cell responses are common to blood and gastrointestinal mucosa, and Gag-specific responses dominate in rectal mucosa of HIV controllers. J. Virol. 2010, 84, 10354–10365. [Google Scholar] [CrossRef]
- Moris, A.; Pereira, M.; Chakrabarti, L. A role for antibodies in natural HIV control. Curr. Opin. HIV AIDS 2019, 14, 265–272. [Google Scholar] [CrossRef]
- Lambotte, O.; Pollara, J.; Boufassa, F.; Moog, C.; Venet, A.; Haynes, B.F.; Delfraissy, J.F.; Saez-Cirion, A.; Ferrari, G. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLoS ONE 2013, 8, e74855. [Google Scholar] [CrossRef]
- Lambotte, O.; Ferrari, G.; Moog, C.; Yates, N.L.; Liao, H.X.; Parks, R.J.; Hicks, C.B.; Owzar, K.; Tomaras, G.D.; Montefiori, D.C.; et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 2009, 23, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, V.; Wines, B.D.; Amin, J.; Emery, S.; Group, E.S.; Lopez, E.; Kelleher, A.; Sydney, L.S.G.; Center, R.J.; Hogarth, P.M.; et al. HIV-1 Env- and Vpu-Specific Antibody-Dependent Cellular Cytotoxicity Responses Associated with Elite Control of HIV. J. Virol. 2017, 91, e00700-17. [Google Scholar] [CrossRef] [PubMed]
- de Mulder, M.; SenGupta, D.; Deeks, S.G.; Martin, J.N.; Pilcher, C.D.; Hecht, F.M.; Sacha, J.B.; Nixon, D.F.; Michaud, H.A. Anti-HERV-K (HML-2) capsid antibody responses in HIV elite controllers. Retrovirology 2017, 14, 41. [Google Scholar] [CrossRef]
- Berzofsky, J.A.; Bensussan, A.; Cease, K.B.; Bourge, J.F.; Cheynier, R.; Lurhuma, Z.; Salaun, J.J.; Gallo, R.C.; Shearer, G.M.; Zagury, D. Antigenic peptides recognized by T lymphocytes from AIDS viral envelope-immune humans. Nature 1988, 334, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Krowka, J.F.; Stites, D.P.; Jain, S.; Steimer, K.S.; George-Nascimento, C.; Gyenes, A.; Barr, P.J.; Hollander, H.; Moss, A.R.; Homsy, J.M.; et al. Lymphocyte proliferative responses to human immunodeficiency virus antigens in vitro. J. Clin. Investig. 1989, 83, 1198–1203. [Google Scholar] [CrossRef]
- Wahren, B.; Morfeldt-Mansson, L.; Biberfeld, G.; Moberg, L.; Sonnerborg, A.; Ljungman, P.; Werner, A.; Kurth, R.; Gallo, R.; Bolognesi, D. Characteristics of the specific cell-mediated immune response in human immunodeficiency virus infection. J. Virol. 1987, 61, 2017–2023. [Google Scholar] [CrossRef]
- Betts, M.R.; Ambrozak, D.R.; Douek, D.C.; Bonhoeffer, S.; Brenchley, J.M.; Casazza, J.P.; Koup, R.A.; Picker, L.J. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: Relationship to viral load in untreated HIV infection. J. Virol. 2001, 75, 11983–11991. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.E.; Boritz, E.; Blyveis, N.; Wilson, C.C. Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4(+) T cells and HIV-1-specific lymphoproliferation in HIV-1-infected subjects with active viral replication. J. Virol. 2002, 76, 5925–5936. [Google Scholar] [CrossRef] [PubMed]
- Younes, S.A.; Yassine-Diab, B.; Dumont, A.R.; Boulassel, M.R.; Grossman, Z.; Routy, J.P.; Sekaly, R.P. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 2003, 198, 1909–1922. [Google Scholar] [CrossRef] [PubMed]
- Dyer, W.B.; Zaunders, J.J.; Yuan, F.F.; Wang, B.; Learmont, J.C.; Geczy, A.F.; Saksena, N.K.; McPhee, D.A.; Gorry, P.R.; Sullivan, J.S. Mechanisms of HIV non-progression; robust and sustained CD4+ T-cell proliferative responses to p24 antigen correlate with control of viraemia and lack of disease progression after long-term transfusion-acquired HIV-1 infection. Retrovirology 2008, 5, 112. [Google Scholar] [CrossRef]
- McNeil, A.C.; Shupert, W.L.; Iyasere, C.A.; Hallahan, C.W.; Mican, J.A.; Davey, R.T., Jr.; Connors, M. High-level HIV-1 viremia suppresses viral antigen-specific CD4(+) T cell proliferation. Proc. Natl. Acad. Sci. USA 2001, 98, 13878–13883. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Billingsley, J.M.; Caliendo, A.M.; Boswell, S.L.; Sax, P.E.; Kalams, S.A.; Walker, B.D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 1997, 278, 1447–1450. [Google Scholar] [CrossRef]
- Harari, A.; Petitpierre, S.; Vallelian, F.; Pantaleo, G. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: Changes after antiretroviral therapy. Blood 2004, 103, 966–972. [Google Scholar] [CrossRef]
- Harari, A.; Vallelian, F.; Pantaleo, G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur. J. Immunol. 2004, 34, 3525–3533. [Google Scholar] [CrossRef]
- Potter, S.J.; Lacabaratz, C.; Lambotte, O.; Perez-Patrigeon, S.; Vingert, B.; Sinet, M.; Colle, J.H.; Urrutia, A.; Scott-Algara, D.; Boufassa, F.; et al. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: An ANRS EP36 study. J. Virol. 2007, 81, 13904–13915. [Google Scholar] [CrossRef]
- Tilton, J.C.; Luskin, M.R.; Johnson, A.J.; Manion, M.; Hallahan, C.W.; Metcalf, J.A.; McLaughlin, M.; Davey, R.T., Jr.; Connors, M. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. J. Virol. 2007, 81, 2713–2725. [Google Scholar] [CrossRef]
- Chen, H.; Li, C.; Huang, J.; Cung, T.; Seiss, K.; Beamon, J.; Carrington, M.F.; Porter, L.C.; Burke, P.S.; Yang, Y.; et al. CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J. Clin. Investig. 2011, 121, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.; Goepfert, P. Understanding the CD8 T-cell response in natural HIV control. F1000Res 2018, 7. [Google Scholar] [CrossRef]
- Koofhethile, C.K.; Ndhlovu, Z.M.; Thobakgale-Tshabalala, C.; Prado, J.G.; Ismail, N.; Mncube, Z.; Mkhize, L.; van der Stok, M.; Yende, N.; Walker, B.D.; et al. CD8+ T Cell Breadth and Ex Vivo Virus Inhibition Capacity Distinguish between Viremic Controllers with and without Protective HLA Class I Alleles. J. Virol. 2016, 90, 6818–6831. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Deleage, C.; Darko, S.; Ransier, A.; Truong, D.P.; Agarwal, D.; Japp, A.S.; Wu, V.H.; Kuri-Cervantes, L.; Abdel-Mohsen, M.; et al. Elite control of HIV is associated with distinct functional and transcriptional signatures in lymphoid tissue CD8(+) T cells. Sci. Transl. Med. 2019, 11, eaax4077. [Google Scholar] [CrossRef]
- Shacklett, B.L.; Ferre, A.L. Mucosal immunity in HIV controllers: The right place at the right time. Curr. Opin. HIV AIDS 2011, 6, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Simonetta, F.; Hua, S.; Lecuroux, C.; Gerard, S.; Boufassa, F.; Saez-Cirion, A.; Pancino, G.; Goujard, C.; Lambotte, O.; Venet, A.; et al. High eomesodermin expression among CD57+ CD8+ T cells identifies a CD8+ T cell subset associated with viral control during chronic human immunodeficiency virus infection. J. Virol. 2014, 88, 11861–11871. [Google Scholar] [CrossRef]
- Altfeld, M.; Kalife, E.T.; Qi, Y.; Streeck, H.; Lichterfeld, M.; Johnston, M.N.; Burgett, N.; Swartz, M.E.; Yang, A.; Alter, G.; et al. HLA Alleles Associated with Delayed Progression to AIDS Contribute Strongly to the Initial CD8(+) T Cell Response against HIV-1. PLoS Med. 2006, 3, e403. [Google Scholar] [CrossRef]
- International, H.I.V.C.S.; Pereyra, F.; Jia, X.; McLaren, P.J.; Telenti, A.; de Bakker, P.I.; Walker, B.D.; Ripke, S.; Brumme, C.J.; Pulit, S.L.; et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010, 330, 1551–1557. [Google Scholar] [CrossRef]
- Migueles, S.A.; Sabbaghian, M.S.; Shupert, W.L.; Bettinotti, M.P.; Marincola, F.M.; Martino, L.; Hallahan, C.W.; Selig, S.M.; Schwartz, D.; Sullivan, J.; et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 2000, 97, 2709–2714. [Google Scholar] [CrossRef]
- Moosa, Y.; Tanko, R.F.; Ramsuran, V.; Singh, R.; Madzivhandila, M.; Yende-Zuma, N.; Abrahams, M.R.; Selhorst, P.; Gounder, K.; Moore, P.L.; et al. Case report: Mechanisms of HIV elite control in two African women. BMC Infect. Dis. 2018, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.A.; Ibarrondo, F.J.; Sugar, C.A.; Hausner, M.A.; Shih, R.; Ng, H.L.; Detels, R.; Margolick, J.B.; Rinaldo, C.R.; Phair, J.; et al. Early HLA-B*57-restricted CD8+ T lymphocyte responses predict HIV-1 disease progression. J. Virol. 2012, 86, 10505–10516. [Google Scholar] [CrossRef] [PubMed]
- Monel, B.; McKeon, A.; Lamothe-Molina, P.; Jani, P.; Boucau, J.; Pacheco, Y.; Jones, R.B.; Le Gall, S.; Walker, B.D. HIV Controllers Exhibit Effective CD8(+) T Cell Recognition of HIV-1-Infected Non-activated CD4(+) T Cells. Cell Rep. 2019, 27, 142–153 e144. [Google Scholar] [CrossRef] [PubMed]
- Buckheit, R.W., 3rd; Siliciano, R.F.; Blankson, J.N. Primary CD8+ T cells from elite suppressors effectively eliminate non-productively HIV-1 infected resting and activated CD4+ T cells. Retrovirology 2013, 10, 68. [Google Scholar] [CrossRef]
- Jiang, C.; Lian, X.; Gao, C.; Sun, X.; Einkauf, K.B.; Chevalier, J.M.; Chen, S.M.Y.; Hua, S.; Rhee, B.; Chang, K.; et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 2020, 585, 261–267. [Google Scholar] [CrossRef]
- Lian, X.; Gao, C.; Sun, X.; Jiang, C.; Einkauf, K.B.; Seiger, K.W.; Chevalier, J.M.; Yuki, Y.; Martin, M.; Hoh, R.; et al. Signatures of immune selection in intact and defective proviruses distinguish HIV-1 elite controllers. Sci. Transl. Med. 2021, 13, eabl4097. [Google Scholar] [CrossRef]
- Julg, B.; Pereyra, F.; Buzon, M.J.; Piechocka-Trocha, A.; Clark, M.J.; Baker, B.M.; Lian, J.; Miura, T.; Martinez-Picado, J.; Addo, M.M.; et al. Infrequent recovery of HIV from but robust exogenous infection of activated CD4(+) T cells in HIV elite controllers. Clin. Infect. Dis. 2010, 51, 233–238. [Google Scholar] [CrossRef]
- Pereyra, F.; Palmer, S.; Miura, T.; Block, B.L.; Wiegand, A.; Rothchild, A.C.; Baker, B.; Rosenberg, R.; Cutrell, E.; Seaman, M.S.; et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J. Infect. Dis. 2009, 200, 984–990. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hokello, J.; Tyagi, P.; Dimri, S.; Sharma, A.L.; Tyagi, M. Comparison of the Biological Basis for Non-HIV Transmission to HIV-Exposed Seronegative Individuals, Disease Non-Progression in HIV Long-Term Non-Progressors and Elite Controllers. Viruses 2023, 15, 1362. https://doi.org/10.3390/v15061362
Hokello J, Tyagi P, Dimri S, Sharma AL, Tyagi M. Comparison of the Biological Basis for Non-HIV Transmission to HIV-Exposed Seronegative Individuals, Disease Non-Progression in HIV Long-Term Non-Progressors and Elite Controllers. Viruses. 2023; 15(6):1362. https://doi.org/10.3390/v15061362
Chicago/Turabian StyleHokello, Joseph, Priya Tyagi, Shelly Dimri, Adhikarimayum Lakhikumar Sharma, and Mudit Tyagi. 2023. "Comparison of the Biological Basis for Non-HIV Transmission to HIV-Exposed Seronegative Individuals, Disease Non-Progression in HIV Long-Term Non-Progressors and Elite Controllers" Viruses 15, no. 6: 1362. https://doi.org/10.3390/v15061362
APA StyleHokello, J., Tyagi, P., Dimri, S., Sharma, A. L., & Tyagi, M. (2023). Comparison of the Biological Basis for Non-HIV Transmission to HIV-Exposed Seronegative Individuals, Disease Non-Progression in HIV Long-Term Non-Progressors and Elite Controllers. Viruses, 15(6), 1362. https://doi.org/10.3390/v15061362







