Virome Profiling of Chickens with Hepatomegaly Rupture Syndrome Reveals Coinfection of Multiple Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Pretreatment and Viral Metagenomic Sequencing
2.3. Virome Annotation
2.4. PCR/RT-PCR Detection
2.5. Histological Section Preparation
2.6. Virus Isolation Using Chicken Embryos
2.7. Experimental Animal Infection
2.8. Phylogenetic Analyses
3. Results
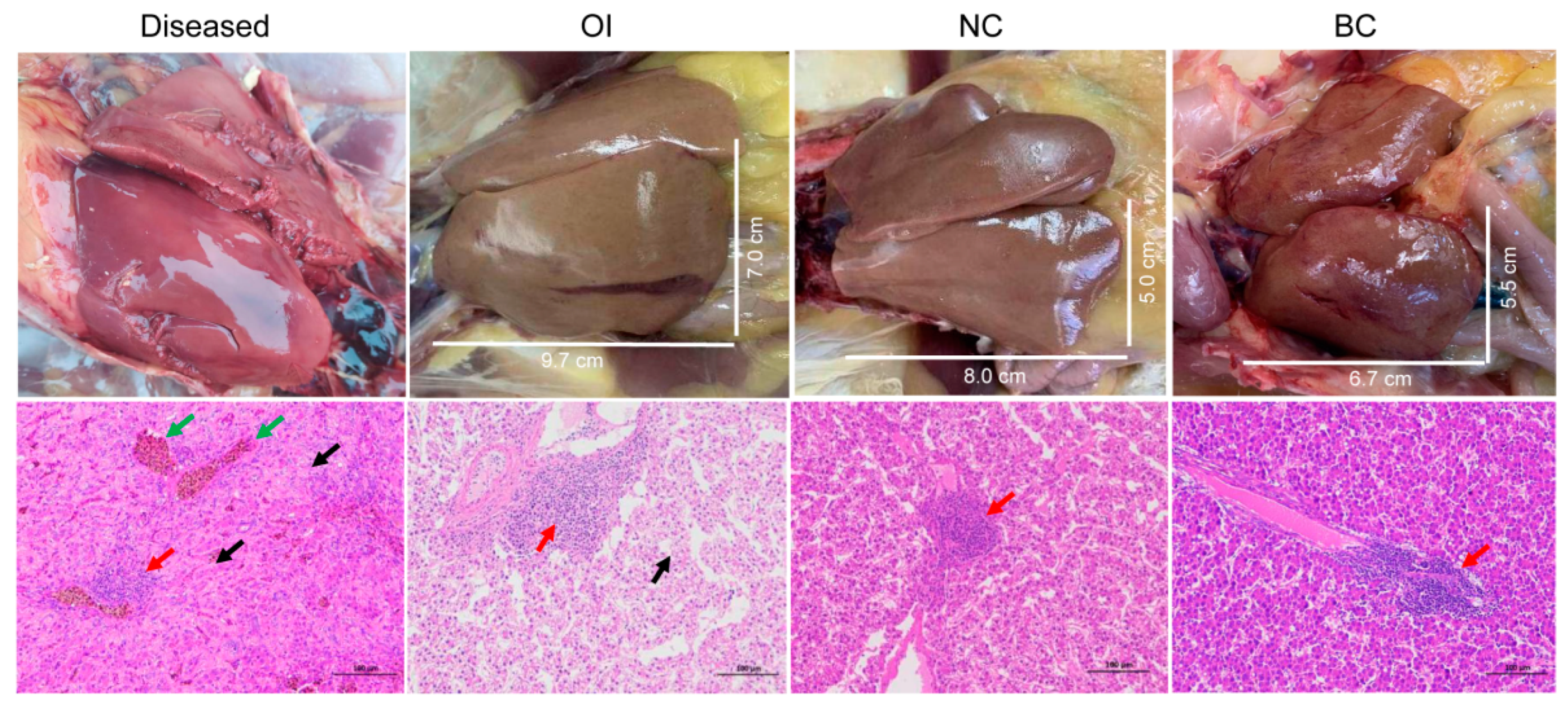
3.1. Overview of the Disease Outbreak
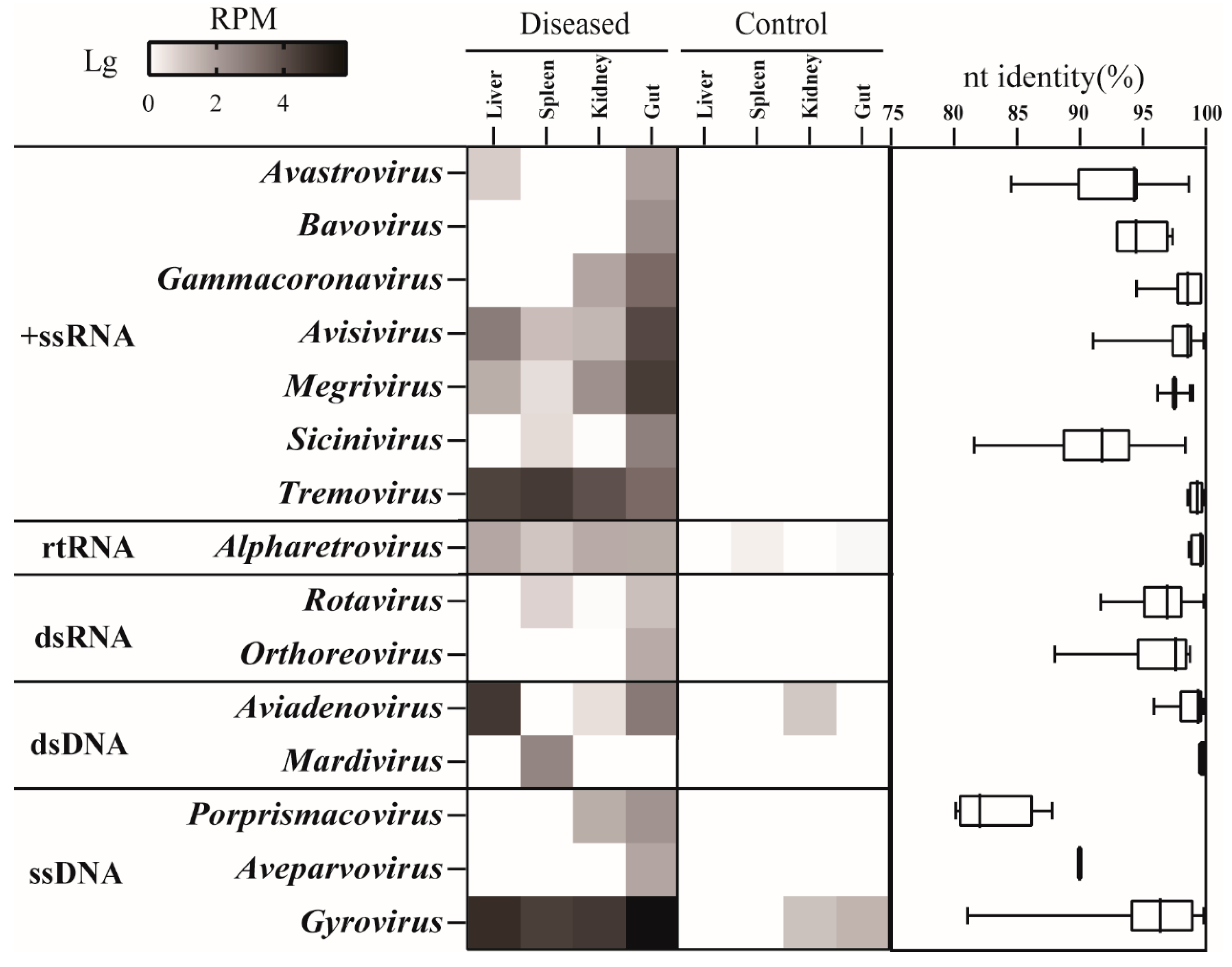
3.2. Virome Profile of Different Organs
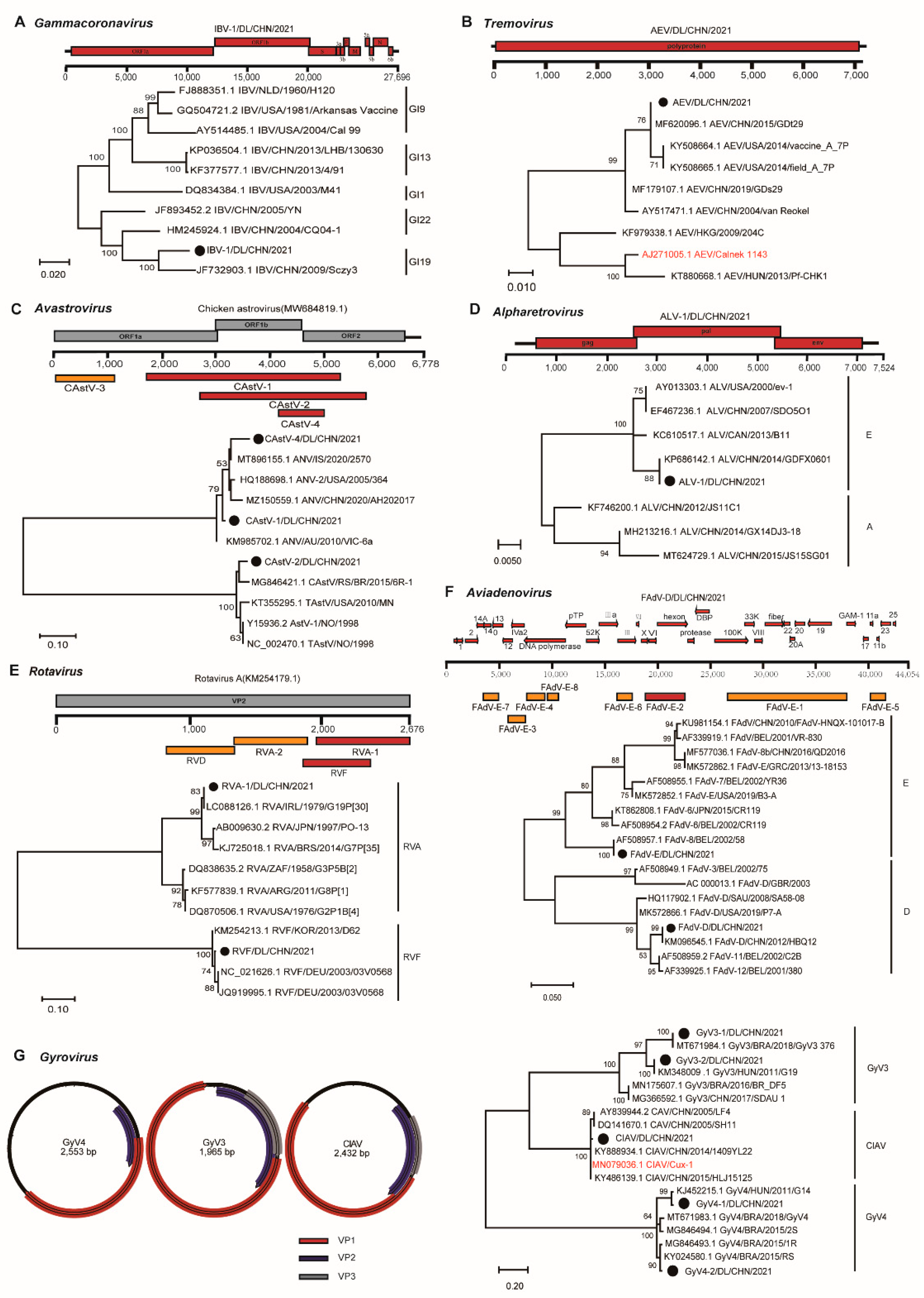
3.3. Phylogenetic Diversity
3.4. PCR/RT-PCR Detections
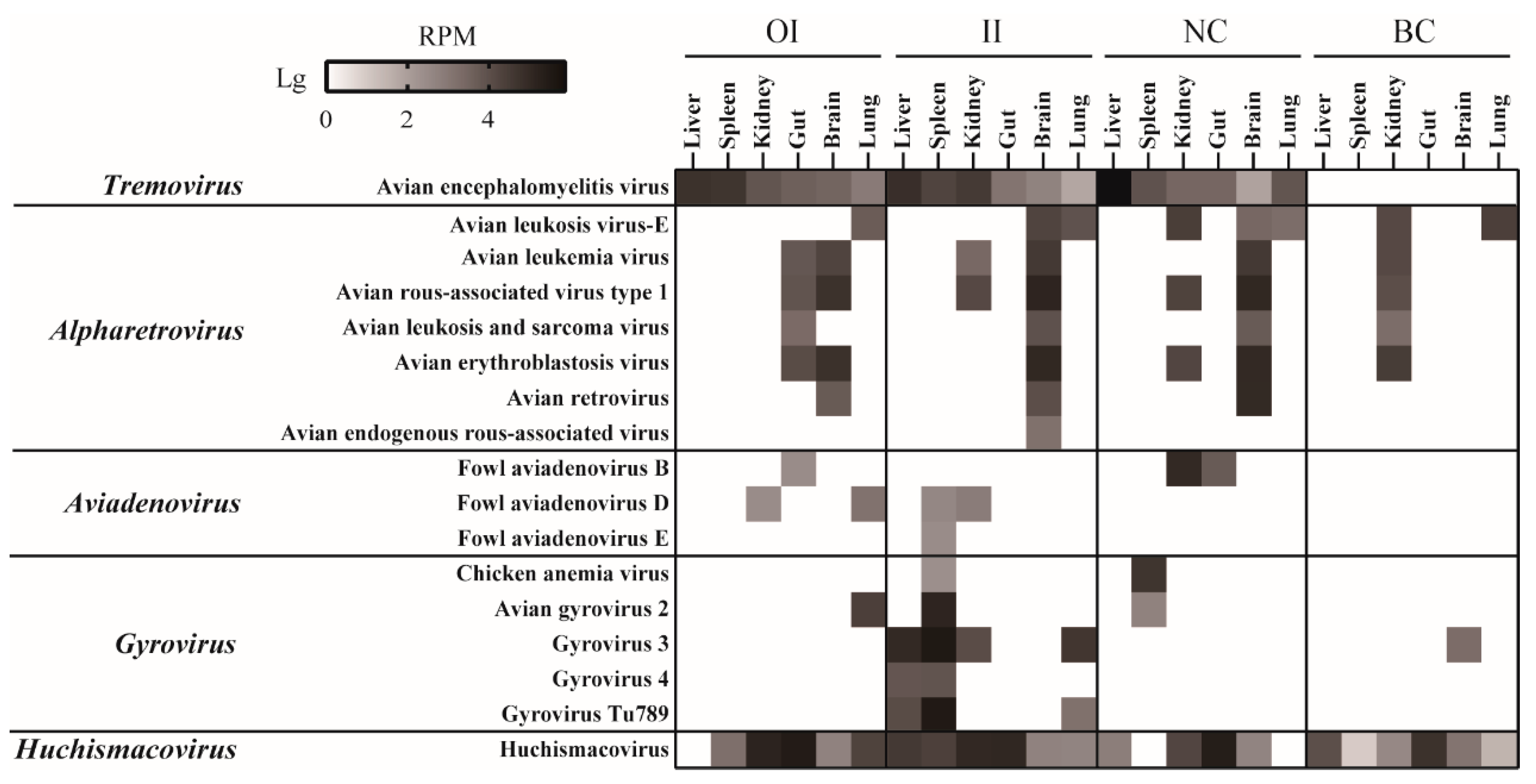
3.5. Experimental Animal Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, X.J. Recent advances in Hepatitis E virus. J. Viral Hepat. 2010, 17, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Morrow, C.J.; Samu, G.; Mátrai, E.; Klausz, A.; Wood, A.M.; Richter, S.; Jaskulska, B.; Hess, M. Avian hepatitis E virus infection and possible associated clinical disease in broiler breeder flocks in Hungary. Avian Pathol. 2008, 37, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.K.; Allan, G.M.; Bryson, D.G.; Williams, W.; Todd, D.; Mackie, D.P.; McFerran, J.B. Big liver and spleen disease of broiler breeders. Avian Pathol. 1990, 19, 41–50. [Google Scholar] [CrossRef]
- Handlinger, J.H.; Williams, W. An egg drop associated with splenomegaly in broiler breeders. Avian Dis. 1988, 32, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.J.; Riddell, C. British Columbia. “Hepatitis-splenomegaly” syndrome in commercial egg laying hens. Can. Vet. J. 1991, 32, 500–501. [Google Scholar]
- Su, Q.; Li, Y.; Meng, F.; Cui, Z.; Chang, S.; Zhao, P. Hepatic rupture hemorrhage syndrome in chickens caused by a novel genotype avian hepatitis E virus. Vet. Microbiol. 2018, 222, 91–97. [Google Scholar] [CrossRef]
- Su, Q.; Li, Y.; Zhang, Y.; Zhang, Z.; Meng, F.; Cui, Z.; Chang, S.; Zhao, P. Characterization of the novel genotype avian hepatitis E viruses from outbreaks of hepatic rupture haemorrhage syndrome in different geographical regions of China. Transbound. Emerg. Dis. 2018, 65, 2017–2026. [Google Scholar] [CrossRef]
- Tablante, N.L.; Vaillancourt, J.P.; Julian, R.J. Necrotic, haemorrhagic, hepatomegalic hepatitis associated with vasculitis and amyloidosis in commercial laying hens. Avian Pathol. 1994, 23, 725–732. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, Z.; Zhang, Y.; Cui, Z.; Chang, S.; Zhao, P. Complete genome analysis of avian hepatitis E virus from chicken with hepatic rupture hemorrhage syndrome. Vet. Microbiol. 2020, 242, 108577. [Google Scholar] [CrossRef]
- Crerar, S.K.; Cross, G.M. The experimental production of big liver and spleen disease in broiler breeder hens. Aust. Vet. J. 1994, 71, 414–417. [Google Scholar] [CrossRef]
- Billam, P.; Sun, Z.F.; Meng, X.J. Analysis of the complete genomic sequence of an apparently avirulent strain of avian hepatitis E virus (avian HEV) identified major genetic differences compared with the prototype pathogenic strain of avian HEV. J. Gen. Virol. 2007, 88, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Hsu, I.W.; Tsai, H.J. Avian hepatitis E virus in chickens, Taiwan, 2013. Emerg. Infect. Dis. 2014, 20, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.F.; Haqshenas, G.; Guenette, D.K.; Halbur, P.G.; Schommer, S.K.; Pierson, F.W.; Toth, T.E.; Meng, X.J. Detection by reverse transcription-PCR and genetic characterization of field isolates of swine hepatitis E virus from pigs in different geographic regions of the United States. J. Clin. Microbiol. 2002, 40, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.F.; Larsen, C.T.; Dunlop, A.; Huang, F.F.; Pierson, F.W.; Toth, T.E.; Meng, X.J. Genetic identification of avian hepatitis E virus (HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographical regions of the United States. J. Gen. Virol. 2004, 85, 693–700. [Google Scholar] [CrossRef]
- Hess, M. Detection and differentiation of avian adenoviruses: A review. Avian Pathol. 2000, 29, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Wang, H.; Yan, Y.; Liu, F.; Huang, M.; Chen, R. Pathogenicity of a fowl adenovirus serotype 4 isolated from chickens associated with hydropericardium-hepatitis syndrome in China. Poult. Sci. 2019, 98, 2765–2771. [Google Scholar] [CrossRef]
- Adedeji, A.; Abdu, P.; Akanbi, O.; Luka, P. Molecular and pathological investigations of Marek’s disease outbreaks in vaccinated poultry farms in Plateau State, North Central-Nigeria. Vet. Ital. 2022, 58, 77–85. [Google Scholar]
- Arshad, S.S.; Bland, A.P.; Hacker, S.M.; Payne, L.N. A low incidence of histiocytic sarcomatosis associated with infection of chickens with the HPRS-103 strain of subgroup J avian leukosis virus. Avian Dis. 1997, 41, 947–956. [Google Scholar] [CrossRef]
- Li, Y.; Yan, N.; Wang, Y.; Liu, A.; Liu, C.; Lan, X.; Yang, B.; Gao, Y.; Gao, H.; Qi, X.; et al. Molecular evolution and pathogenicity of chicken anemia virus isolates in China. Arch. Virol. 2021, 166, 439–449. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, Y.; Li, Y.; Cui, Z.; Chang, S.; Zhao, P. Epidemiological investigation of the novel genotype avian hepatitis E virus and co-infected immunosuppressive viruses in farms with hepatic rupture haemorrhage syndrome, recently emerged in China. Transbound. Emerg. Dis. 2019, 66, 776–784. [Google Scholar] [CrossRef]
- Yugo, D.M.; Hauck, R.; Shivaprasad, H.L.; Meng, X.J. Hepatitis Virus Infections in Poultry. Avian Dis. 2016, 60, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.J.; Ellis, T.M.; Plant, S.L.; Gregory, A.R.; Wilcox, G.E. Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Vet. Microbiol. 1999, 68, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Marek, A.; Kaján, G.L.; Kosiol, C.; Benkő, M.; Schachner, A.; Hess, M. Genetic diversity of species Fowl aviadenovirus D and Fowl aviadenovirus E. J. Gen. Virol. 2016, 97, 2323–2332. [Google Scholar] [CrossRef]
- Xu, M.; Hang, F.; Qian, K.; Shao, H.; Ye, J.; Qin, A. Chicken hepatomegaly and splenomegaly associated with novel subgroup J avian leukosis virus infection. BMC Vet. Res. 2022, 18, 32. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Chi, Z.; Cui, Z.; Chang, S.; Wang, Y.; Zhao, P. Isolation, identification and genome analysis of an avian hepatitis E virus from white-feathered broilers in China. Poult. Sci. 2022, 101, 101633. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Gong, W.; Yan, X.; Zhao, Z.; Yang, L.; Tan, Z.; Xu, L.; Zhu, A.; Zhang, J.; Rao, J.; et al. Viral Metagenome-Based Precision Surveillance of Pig Population at Large Scale Reveals Viromic Signatures of Sample Types and Influence of Farming Management on Pig Virome. mSystems 2021, 6, e0042021. [Google Scholar] [CrossRef]
- Sun, Y.; Qu, Y.; Yan, X.; Yan, G.; Chen, J.; Wang, G.; Zhao, Z.; Liu, Y.; Tu, C.; He, B. Comprehensive Evaluation of RNA and DNA Viromic Methods Based on Species Richness and Abundance Analyses Using Marmot Rectal Samples. mSystems 2022, 7, e0043022. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Y.; Yan, X.; Ren, Z.; Wang, G.; Liu, Y.; Zhao, Z.; Yi, L.; Tu, C.; He, B. Elimination of Foreign Sequences in Eukaryotic Viral Reference Genomes Improves the Accuracy of Virome Analysis. mSystems 2022, 7, e0090722. [Google Scholar] [CrossRef]
- Miłek, J.; Blicharz-Domańska, K. Coronaviruses in Avian Species–Review with Focus on Epidemiology and Diagnosis in Wild Birds. J. Vet. Res. 2018, 62, 249–255. [Google Scholar] [CrossRef]
- Zou, N.L.; Zhao, F.F.; Wang, Y.P.; Liu, P.; Cao, S.J.; Wen, X.T.; Huang, Y. Genetic analysis revealed LX4 genotype strains of avian infectious bronchitis virus became predominant in recent years in Sichuan area, China. Virus Genes 2010, 41, 202–209. [Google Scholar] [CrossRef]
- Payne, L.N.; Brown, S.R.; Bumstead, N.; Howes, K.; Frazier, J.A.; Thouless, M.E. A novel subgroup of exogenous avian leukosis virus in chickens. J. Gen. Virol. 1991, 72, 801–807. [Google Scholar] [CrossRef]
- Dhama, K.; Saminathan, M.; Karthik, K.; Tiwari, R.; Shabbir, M.Z.; Kumar, N.; Malik, Y.S.; Singh, R.K. Avian rotavirus enteritis–an updated review. Vet. Q. 2015, 35, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, W.; Gao, D.; Li, Y.; Yang, X.; Liu, H.; Yao, H.; Chen, L.; Wang, C.; Zhao, J. Genetic characterization of novel fowl aviadenovirus 4 isolates from outbreaks of hepatitis-hydropericardium syndrome in broiler chickens in China. Emerg. Microbes Infect. 2016, 5, e117. [Google Scholar] [CrossRef] [PubMed]
- Loiko, M.R.; Varela, A.P.M.; Tochetto, C.; Lopes, B.C.; Scheffer, C.M.; Morel, A.P.; Vidaletti, M.R.; Lima, D.A.; Cerva, C.; Mayer, F.Q.; et al. Novel Gyrovirus genomes recovered from free-living pigeons in Southern Brazil. Virology 2020, 548, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.; McNulty, M.S.; Adair, B.M.; Allan, G.M. Animal circoviruses. Adv. Virus Res. 2001, 57, 1–70. [Google Scholar]
- Alvarado, I.R.; Villegas, P.; El-Attrache, J.; Jensen, E.; Rosales, G.; Perozo, F.; Purvis, L.B. Genetic characterization, pathogenicity, and protection studies with an avian adenovirus isolate associated with inclusion body hepatitis. Avian Dis. 2007, 51, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Domanska-Blicharz, K.; Tomczyk, G.; Smietanka, K.; Kozaczynski, W.; Minta, Z. Molecular characterization of fowl adenoviruses isolated from chickens with gizzard erosions. Poult. Sci. 2011, 90, 983–989. [Google Scholar] [CrossRef]
- Marek, A.; Schulz, E.; Hess, C.; Hess, M. Comparison of the fibers of Fowl adenovirus A serotype 1 isolates from chickens with gizzard erosions in Europe and apathogenic reference strains. J. Vet. Diagn. Investig. 2010, 22, 937–941. [Google Scholar] [CrossRef]
- Mase, M.; Nakamura, K.; Minami, F. Fowl adenoviruses isolated from chickens with inclusion body hepatitis in Japan, 2009–2010. J. Vet. Med. Sci. 2012, 74, 1087–1089. [Google Scholar] [CrossRef]
- Olitsky, P.K. Experimental Studies of the Virus Of Infectious Avian Encephalomyelitis. J. Exp. Med. 1939, 70, 565–582. [Google Scholar] [CrossRef]
- Shafren, D.R.; Tannock, G.A.; Groves, P.J. Antibody responses to avian encephalomyelitis virus vaccines when administered by different routes. Aust. Vet. J. 1992, 69, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Westbury, H.A.; Sinkovic, B. The pathogenesis of infectious avian encephalomyelitis. 1. The effect of the age of the chicken and the route of administration of the virus. Aust. Vet. J. 1978, 54, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Tannock, G.A.; Shafren, D.R. Avian encephalomyelitis: A review. Avian Pathol. 1994, 23, 603–620. [Google Scholar] [CrossRef]
- Fan, X.; Gao, X.; Wang, Q.; Shen, J.; Zhou, L.; Xie, J. Complete genome sequence analysis of the novel mycobacteriophage Shandong1. Arch. Virol. 2017, 162, 3903–3905. [Google Scholar] [CrossRef] [PubMed]
- Squires, E.J.; Leeson, S. Aetiology of fatty liver syndrome in laying hens. Br. Vet. J. 1988, 144, 602–609. [Google Scholar] [CrossRef]
- Pearson, A.W.; Curtis, M.J.; Butler, E.J. Bacterial endotoxins and the pathogenesis of fatty liver--haemorrhagic syndrome in the laying hen. Res. Vet. Sci. 1981, 31, 259–261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; He, Y.; Yan, X.; Sun, Y.; Yi, L.; Tu, C.; He, B. Virome Profiling of Chickens with Hepatomegaly Rupture Syndrome Reveals Coinfection of Multiple Viruses. Viruses 2023, 15, 1249. https://doi.org/10.3390/v15061249
Wang G, He Y, Yan X, Sun Y, Yi L, Tu C, He B. Virome Profiling of Chickens with Hepatomegaly Rupture Syndrome Reveals Coinfection of Multiple Viruses. Viruses. 2023; 15(6):1249. https://doi.org/10.3390/v15061249
Chicago/Turabian StyleWang, Guoshuai, Yaqi He, Xiaomin Yan, Yue Sun, Le Yi, Changchun Tu, and Biao He. 2023. "Virome Profiling of Chickens with Hepatomegaly Rupture Syndrome Reveals Coinfection of Multiple Viruses" Viruses 15, no. 6: 1249. https://doi.org/10.3390/v15061249
APA StyleWang, G., He, Y., Yan, X., Sun, Y., Yi, L., Tu, C., & He, B. (2023). Virome Profiling of Chickens with Hepatomegaly Rupture Syndrome Reveals Coinfection of Multiple Viruses. Viruses, 15(6), 1249. https://doi.org/10.3390/v15061249






