Sustained Impact of RHDV2 on Wild Rabbit Populations across Australia Eight Years after Its Initial Detection
Abstract
1. Introduction
2. Methods
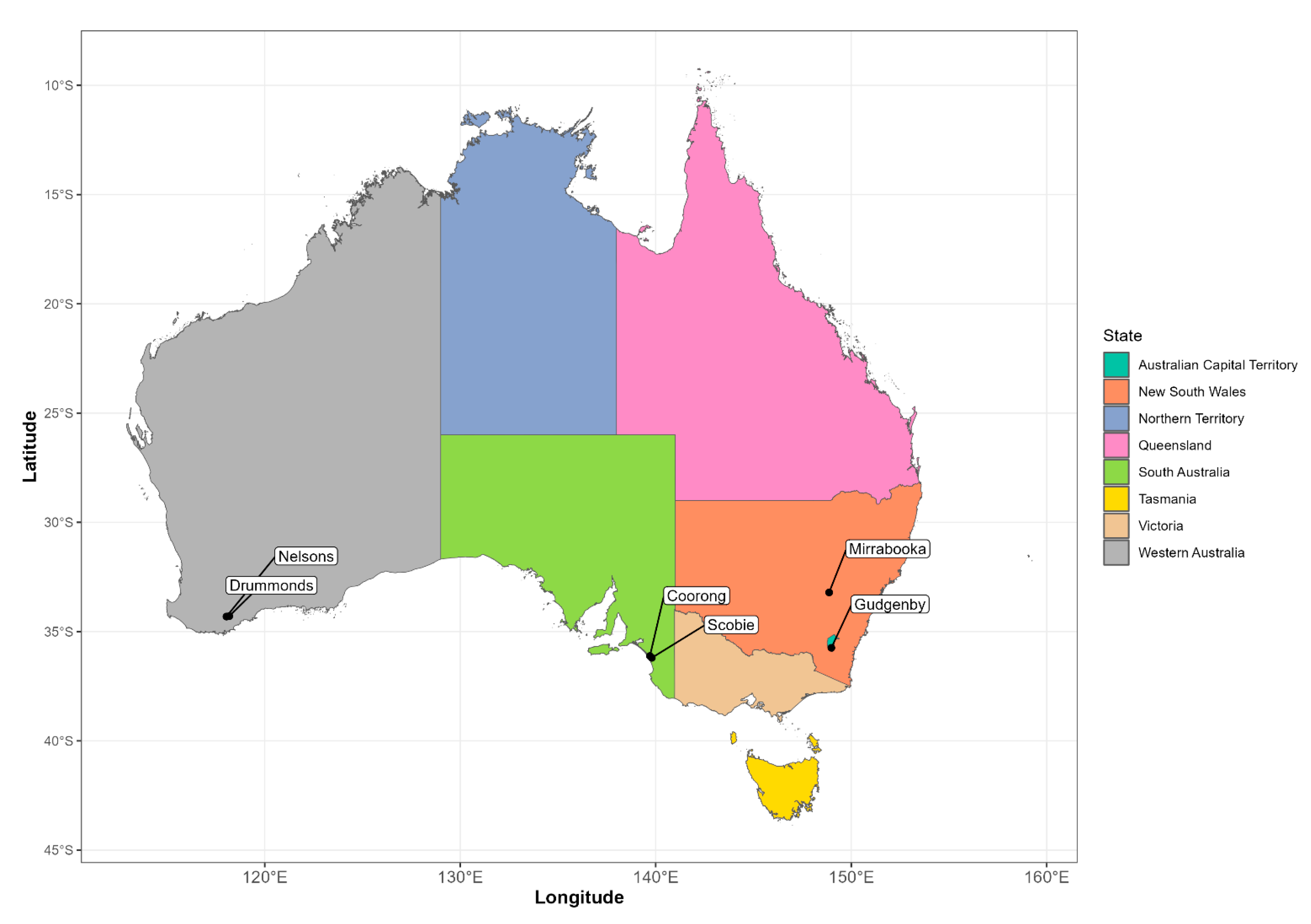
2.1. Spotlight Monitoring
2.2. Serology
2.3. Analysis of Rabbit Spotlight Data
2.4. Serological Analysis
2.5. Model Fitting
3. Results
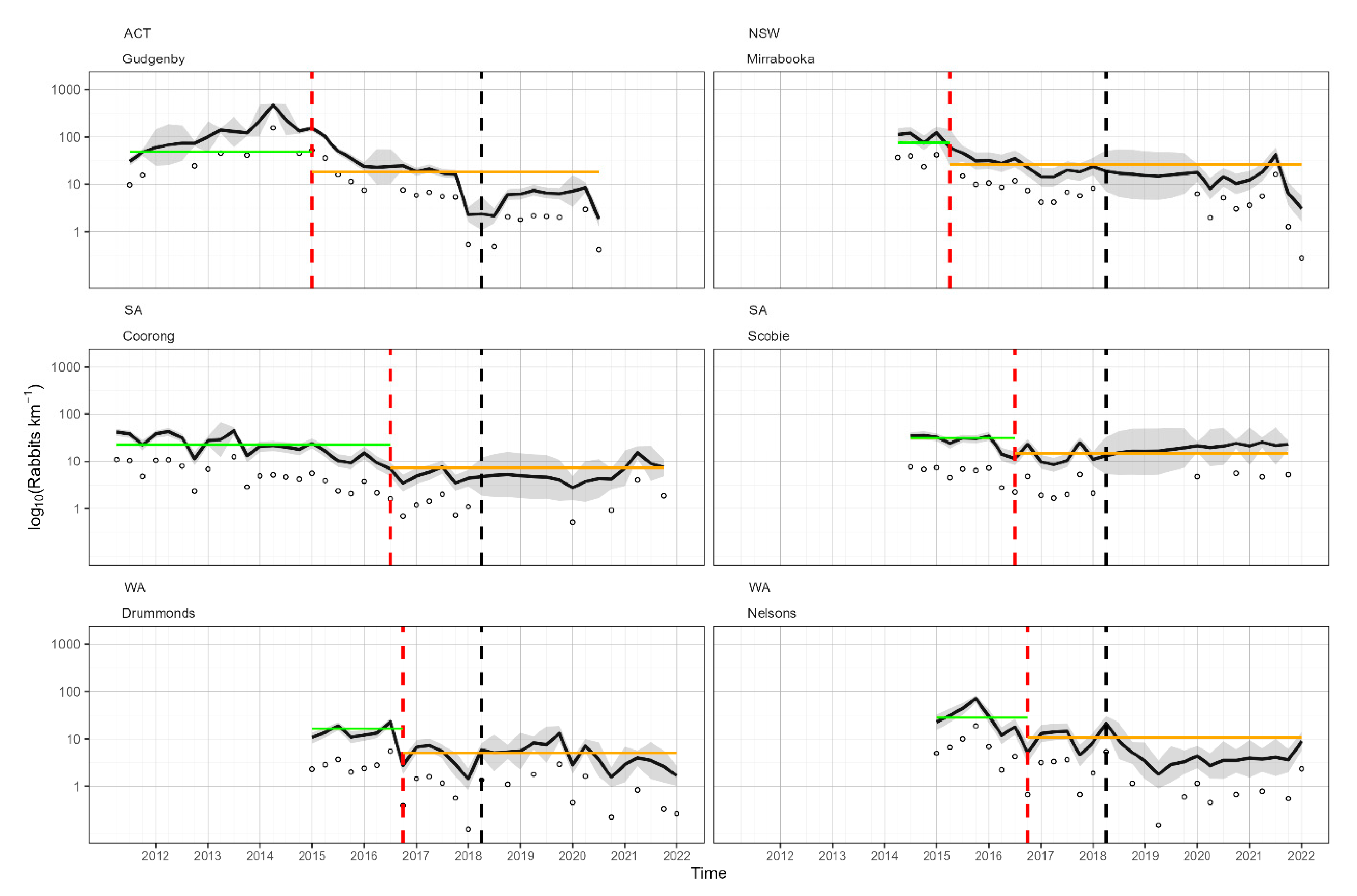
3.1. Spotlight Data
3.2. Serological Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalton, K.P.; Nicieza, I.; Balseiro, A.; Muguerza, M.A.; Rosell, J.M.; Casais, R.; Álvarez, Á.L.; Parra, F. Hemorrhagic Disease Virus in Young Rabbits, Spain. Emerg. Infect. Dis. 2012, 18, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Le Gall-Reculé, G.; Lavazza, A.; Marchandeau, S.; Bertagnoli, S.; Zwingelstein, F.; Cavadini, P.; Martinelli, N.; Lombardi, G.; Guérin, J.L.; Lemaitre, E.; et al. Emergence of a New Lagovirus Related to Rabbit Haemorrhagic Disease Virus. Vet. Res. 2013, 44, 81. [Google Scholar] [CrossRef] [PubMed]
- Rouco, C.; Aguayo-Adán, J.A.; Santoro, S.; Abrantes, J.; Delibes-Mateos, M. Worldwide Rapid Spread of the Novel Rabbit Haemorrhagic Disease Virus (GI.2/RHDV2/b). Transbound. Emerg. Dis. 2019, 66, 1762–1764. [Google Scholar] [CrossRef] [PubMed]
- Happi, A.N.; Ogunsanya, O.A.; Oguzie, J.U.; Oluniyi, P.E.; Olono, A.S.; Heeney, J.L.; Happi, C.T. Microbial Metagenomic Approach Uncovers the First Rabbit Haemorrhagic Disease Virus Genome in Sub-Saharan Africa. Sci. Rep. 2021, 11, 13689. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wei, H.; Fan, Z.; Song, Y.; Chen, M.; Qiu, R.; Zhu, W.; Xu, W.; Xue, J.; Wang, F. Emergence of Rabbit Haemorrhagic Disease Virus 2 in China in 2020. Vet. Med. Sci. 2021, 7, 236–239. [Google Scholar] [CrossRef]
- Asin, J.; Rejmanek, D.; Clifford, D.L.; Mikolon, A.B.; Henderson, E.E.; Nyaoke, A.C.; Macías-Rioseco, M.; Streitenberger, N.; Beingesser, J.; Woods, L.W.; et al. Early Circulation of Rabbit Haemorrhagic Disease Virus Type 2 in Domestic and Wild Lagomorphs in Southern California, USA (2020–2021). Transbound. Emerg. Dis. 2022, 69, e394–e405. [Google Scholar] [CrossRef]
- Hall, R.N.; King, T.; O’connor, T.W.; Read, A.J.; Vrankovic, S.; Piper, M.; Strive, T. Passive Immunisation against Rhdv2 Induces Protection against Disease but Not Infection. Vaccines 2021, 9, 1197. [Google Scholar] [CrossRef]
- Hall, R.N.; Peacock, D.E.; Kovaliski, J.; Mahar, J.E.; Mourant, R.; Piper, M.; Strive, T. Detection of RHDV2 in European Brown Hares (Lepus Europaeus) in Australia. Vet. Rec. 2017, 180, 121. [Google Scholar] [CrossRef]
- Neimanis, A.S.; Ahola, H.; Larsson Pettersson, U.; Lopes, A.M.; Abrantes, J.; Zohari, S.; Esteves, P.J.; Gavier-Widén, D. Overcoming Species Barriers: An Outbreak of Lagovirus Europaeus GI. 2/RHDV2 in an Isolated Population of Mountain Hares (Lepus Timidus). BMC Vet. Res. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Lankton, J.S.; Knowles, S.; Keller, S.; Shearn-Bochsler, V.I.; Ip, H.S. Pathology of Lagovirus Europaeus GI. 2/RHDV2/b (Rabbit Hemorrhagic Disease Virus 2) in Native North American Lagomorphs. J. Wildl. Dis. 2021, 57, 694–700. [Google Scholar] [CrossRef]
- Dalton, K.P.; Nicieza, I.; Abrantes, J.; Esteves, P.J.; Parra, F. Spread of New Variant RHDV in Domestic Rabbits on the Iberian Peninsula. Vet. Microbiol. 2014, 169, 67–73. [Google Scholar] [CrossRef]
- Peacock, D.; Kovaliski, J.; Sinclair, R.; Mutze, G.; Iannella, A.; Capucci, L. RHDV2 Overcoming RHDV Immunity in Wild Rabbits (Oryctolagus Cuniculus) in Australia. Vet. Rec. 2017, 180, 280. [Google Scholar] [CrossRef]
- Taggart, P.L.; O’Connor, T.W.; Cooke, B.; Read, A.J.; Kirkland, P.D.; Sawyers, E.; West, P.; Patel, K. Good Intentions with Adverse Outcomes When Conservation and Pest Management Guidelines Are Ignored: A Case Study in Rabbit Biocontrol. Conserv. Sci. Pract. 2022, 4, e12639. [Google Scholar] [CrossRef]
- Müller, C.; Ulrich, R.; Schinköthe, J.; Müller, M.; Köllner, B. Characterization of Protective Humoral and Cellular Immune Responses against RHDV2 Induced by a New Vaccine Based on Recombinant Baculovirus. Vaccine 2019, 37, 4195–4203. [Google Scholar] [CrossRef]
- Guerrero-Casado, J.; Carpio, A.J.; Tortosa, F.S. Recent Negative Trends of Wild Rabbit Populations in Southern Spain after the Arrival of the New Variant of the Rabbit Hemorrhagic Disease Virus RHDV2. Mamm. Biol. 2016, 81, 361–364. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A.; Hoskins, A.J.; Haubrock, P.J.; Cuthbert, R.N.; Diagne, C.; Leroy, B.; Andrews, L.; Page, B.; Cassey, P.; Sheppard, A.W. Detailed Assessment of the Reported Economic Costs of Invasive Species in Australia. NeoBiota 2021, 67, 511–550. [Google Scholar] [CrossRef]
- Finlayson, G.; Taggart, P.; Cooke, B. Recovering Australia’s Arid-Zone Ecosystems: Learning from Continental-Scale Rabbit Control Experiments. Restor. Ecol. 2022, 30, e13552. [Google Scholar] [CrossRef]
- Cooke, B.; Chudleigh, P.; Simpson, S.; Saunders, G. The Economic Benefits of the Biological Control of Rabbits in Australia, 1950–2011. Aust. Econ. Hist. Rev. 2013, 53, 91–107. [Google Scholar] [CrossRef]
- Mahar, J.E.; Hall, R.N.; Peacock, D.; Kovaliski, J.; Piper, M.; Mourant, R.; Huang, N.; Campbell, S.; Gu, X.; Read, A.; et al. Rabbit Hemorrhagic Disease Virus 2 (RHDV2; GI.2) Is Replacing Endemic Strains of RHDV in the Australian Landscape within 18 Months of Its Arrival. J. Virol. 2018, 92, e01374-17. [Google Scholar] [CrossRef]
- Strive, T.; Piper, M.; Huang, N.; Mourant, R.; Kovaliski, J.; Capucci, L.; Cox, T.E.; Smith, I. Retrospective Serological Analysis Reveals Presence of the Emerging Lagovirus RHDV2 in Australia in Wild Rabbits at Least Five Months Prior to Its First Detection. Transbound. Emerg. Dis. 2020, 67, 822–833. [Google Scholar] [CrossRef]
- Pacioni, C.; Hall, R.N.; Strive, T.; Ramsey, D.S.L.; Gill, M.S.; Vaughan, T.G. Comparative Epidemiology of Rabbit Haemorrhagic Disease Virus Strains from Viral Sequence Data. Viruses 2023, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Strive, T.; Cox, T.E. Lethal Biological Control of Rabbits—The Most Powerful Tools for Landscape-Scale Mitigation of Rabbit Impacts in Australia. Aust. Zool. 2019, 40, 118–128. [Google Scholar] [CrossRef]
- Ramsey, D.S.L.; Cox, T.; Strive, T.; Forsyth, D.M.; Stuart, I.; Hall, R.; Elsworth, P.; Campbell, S. Emerging RHDV2 Suppresses the Impact of Endemic and Novel Strains of RHDV on Wild Rabbit Populations. J. Appl. Ecol. 2020, 57, 630–641. [Google Scholar] [CrossRef]
- Taggart, P.L.; Hall, R.N.; Cox, T.E.; Kovaliski, J.; McLeod, S.R.; Strive, T. Changes in Virus Transmission Dynamics Following the Emergence of RHDV2 Shed Light on Its Competitive Advantage over Previously Circulating Variants. Transbound. Emerg. Dis. 2022, 69, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Mutze, G.; de Preu, N.; Mooney, T.; Koerner, D.; McKenzie, D.; Sinclair, R.; Kovaliskli, J.; Peacock, D. Substantial Numerical Decline in South Australian Rabbit Populations Following the Detection of Rabbit Haemorrhagic Disease Virus 2. Vet. Rec. 2018, 182, 574. [Google Scholar] [CrossRef]
- Lopes, A.M.; Correia, J.; Abrantes, J.; Melo, P.; Ramada, M.; Magalhães, M.J.; Alves, P.C.; Esteves, P.J. Is the New Variant RHDV Replacing Genogroup 1 in Portuguese Wild Rabbit Populations? Viruses 2014, 7, 27–36. [Google Scholar] [CrossRef]
- Augusteyn, R.C. On the Relationship between Rabbit Age and Lens Dry Weight: Improved Determination of the Age of Rabbits in the Wild. Mol. Vis. 2007, 13, 2030–2034. [Google Scholar]
- Dail, D.; Madsen, L. Models for Estimating Abundance from Repeated Counts of an Open Metapopulation. Biometrics 2011, 67, 577–587. [Google Scholar] [CrossRef]
- Dennis, B.; Ponciano, J.M.; Lele, S.R.; Taper, M.L.; Staples, D.F. Estimating Density Dependence, Process Noise, and Observation Error. Ecol. Monogr. 2006, 76, 323–341. [Google Scholar] [CrossRef]
- Ives, A.R.; Dennis, B.; Cottingham, K.L.; Carpenter, S.R. Estimating Community Stability and Ecological Interactions from Time-Series Data. Ecol. Monogr. 2003, 73, 301–330. [Google Scholar] [CrossRef]
- Carpenter, B.; Gelman, A.; Hoffman, M.D.; Lee, D.; Goodrich, B.; Betancourt, M.; Brubaker, M.; Guo, J.; Li, P.; Riddell, A. Stan: A Probabilistic Programming Language. J. Stat. Softw. 2017, 76, 1–32. [Google Scholar] [CrossRef]
- Lewandowski, D.; Kurowicka, D.; Joe, H. Generating Random Correlation Matrices Based on Vines and Extended Onion Method. J. Multivar. Anal. 2009, 100, 1989–2001. [Google Scholar] [CrossRef]
- Brooks, S.; Gelman, A. General Methods for Monitoring Convergence of Iterative Simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar]
- Ramsey, D. Dslramsey/RHDV_State_Space: Data and Code 2019. Available online: https://zenodo.org/record/3546398 (accessed on 17 April 2023).
- Patel, K.K.; Strive, T.; Hall, R.N.; Mutze, G.; Page, B.; Korcz, M.; Booth-Remmers, M.; Smith, I.L.; Huang, N.; Kovaliski, J.; et al. Cross-Protection, Infection and Case Fatality Rates in Wild European Rabbits Experimentally Challenged with Different Rabbit Haemorrhagic Disease Viruses. Transbound. Emerg. Dis. 2022, 69, e1959–e1971. [Google Scholar] [CrossRef]
- Strive, T.; Wright, J.D.; Robinson, A.J. Identification and Partial Characterisation of a New Lagovirus in Australian Wild Rabbits. Virology 2009, 384, 97–105. [Google Scholar] [CrossRef]
- Liu, J.; Fordham, D.A.; Cooke, B.D.; Cox, T.; Mutze, G.; Strive, T. Distribution and Prevalence of the Australian Non-Pathogenic Rabbit Calicivirus Is Correlated with Rainfall and Temperature. PLoS ONE 2014, 9, e113976. [Google Scholar] [CrossRef]
- Strive, T.; Elsworth, P.; Liu, J.; Wright, J.D.; Kovaliski, J.; Capucci, L. The non-pathogenic Australian rabbit calicivirus RCV-A1 provides temporal and partial cross protection to lethal Rabbit Haemorrhagic Disease Virus infection which is not dependent on antibody titres. Vet. Res. 2013, 44, 1–11. [Google Scholar] [CrossRef]
- O’Connor, T.W.; Read, A.J.; Hall, R.N.; Strive, T.; Kirkland, P.D. Immunological cross-protection between different rabbit hemorrhagic disease viruses—implications for rabbit biocontrol and vaccine development. Vaccines 2022, 10, 666. [Google Scholar] [CrossRef]
- Cooke, B.D.; Duncan, R.P.; McDonald, I.; Liu, J.; Capucci, L.; Mutze, G.J.; Strive, T. Prior exposure to non-pathogenic calicivirus RCV-A1 reduces both infection rate and mortality from rabbit haemorrhagic disease in a population of wild rabbits in Australia. Transbound. Emerg. Dis. 2018, 65, 470–477. [Google Scholar] [CrossRef]
- Saunders, G.; Cooke, B.; McColl, K.; Shine, R.; Peacock, T. Modern approaches for the biological control of vertebrate pests: An Australian perspective. Biol. Control 2010, 52, 288–295. [Google Scholar] [CrossRef]
- Mutze, G.J.; Sinclair, R.G.; Peacock, D.E.; Capucci, L.; Kovaliski, J. Is Increased Juvenile Infection the Key to Recovery of Wild Rabbit Populations from the Impact of Rabbit Haemorrhagic Disease? Eur. J. Wildl. Res. 2014, 60, 489–499. [Google Scholar] [CrossRef]
- Di Giallonardo, F.; Holmes, E.C. Viral biocontrol: Grand experiments in disease emergence and evolution. Trends Microbiol. 2015, 23, 83–90. [Google Scholar] [CrossRef] [PubMed]

| Scheme. | LCL() | UCL() | |||||
|---|---|---|---|---|---|---|---|
| Coorong | 20.3 | 4.7 | 7.3 | 2.4 | −0.64 | −0.85 | −0.34 |
| Drummonds | 15.4 | 5.0 | 5.8 | 1.9 | −0.61 | −0.86 | −0.32 |
| Gudgenby | 137.3 | 59.2 | 23.6 | 11.3 | −0.82 | −0.96 | −0.55 |
| Mirrabooka | 66.5 | 32.9 | 21.1 | 8.0 | −0.68 | −0.92 | −0.24 |
| Nelsons | 27.5 | 10.5 | 8.7 | 3.6 | −0.67 | −0.89 | −0.33 |
| Scobie | 31.2 | 11.0 | 15.6 | 6.0 | −0.52 | −0.80 | 0.17 |
| Average | 39.9 | 30.2 | 15.4 | 11.3 | −0.64 | −0.91 | −0.01 |
| Variant | RCVA | RHDV1 | RHDV2 |
|---|---|---|---|
| RCVA | 0.285 [0.162, 0.406] | 0.021 [−0.156, 0.193] | −0.215 [−0.319, −0.119] |
| RHDV1 | 0.128 [0.023, 0.235] | 0.112 [−0.051, 0.278] | −0.164 [−0.251, −0.082] |
| RHDV2 | −0.166 [−0.295, −0.048] | 0.271 [ 0.103, 0.45] | 0.749 [0.649, 0.844] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramsey, D.S.; Patel, K.K.; Campbell, S.; Hall, R.N.; Taggart, P.L.; Strive, T. Sustained Impact of RHDV2 on Wild Rabbit Populations across Australia Eight Years after Its Initial Detection. Viruses 2023, 15, 1159. https://doi.org/10.3390/v15051159
Ramsey DS, Patel KK, Campbell S, Hall RN, Taggart PL, Strive T. Sustained Impact of RHDV2 on Wild Rabbit Populations across Australia Eight Years after Its Initial Detection. Viruses. 2023; 15(5):1159. https://doi.org/10.3390/v15051159
Chicago/Turabian StyleRamsey, David S., Kandarp K. Patel, Susan Campbell, Robyn N. Hall, Patrick L. Taggart, and Tanja Strive. 2023. "Sustained Impact of RHDV2 on Wild Rabbit Populations across Australia Eight Years after Its Initial Detection" Viruses 15, no. 5: 1159. https://doi.org/10.3390/v15051159
APA StyleRamsey, D. S., Patel, K. K., Campbell, S., Hall, R. N., Taggart, P. L., & Strive, T. (2023). Sustained Impact of RHDV2 on Wild Rabbit Populations across Australia Eight Years after Its Initial Detection. Viruses, 15(5), 1159. https://doi.org/10.3390/v15051159






