Impact of Administering Intravenous Azithromycin within 7 Days of Hospitalization for Influenza Virus Pneumonia: A Propensity Score Analysis Using a Nationwide Administrative Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics
2.3. Data Source
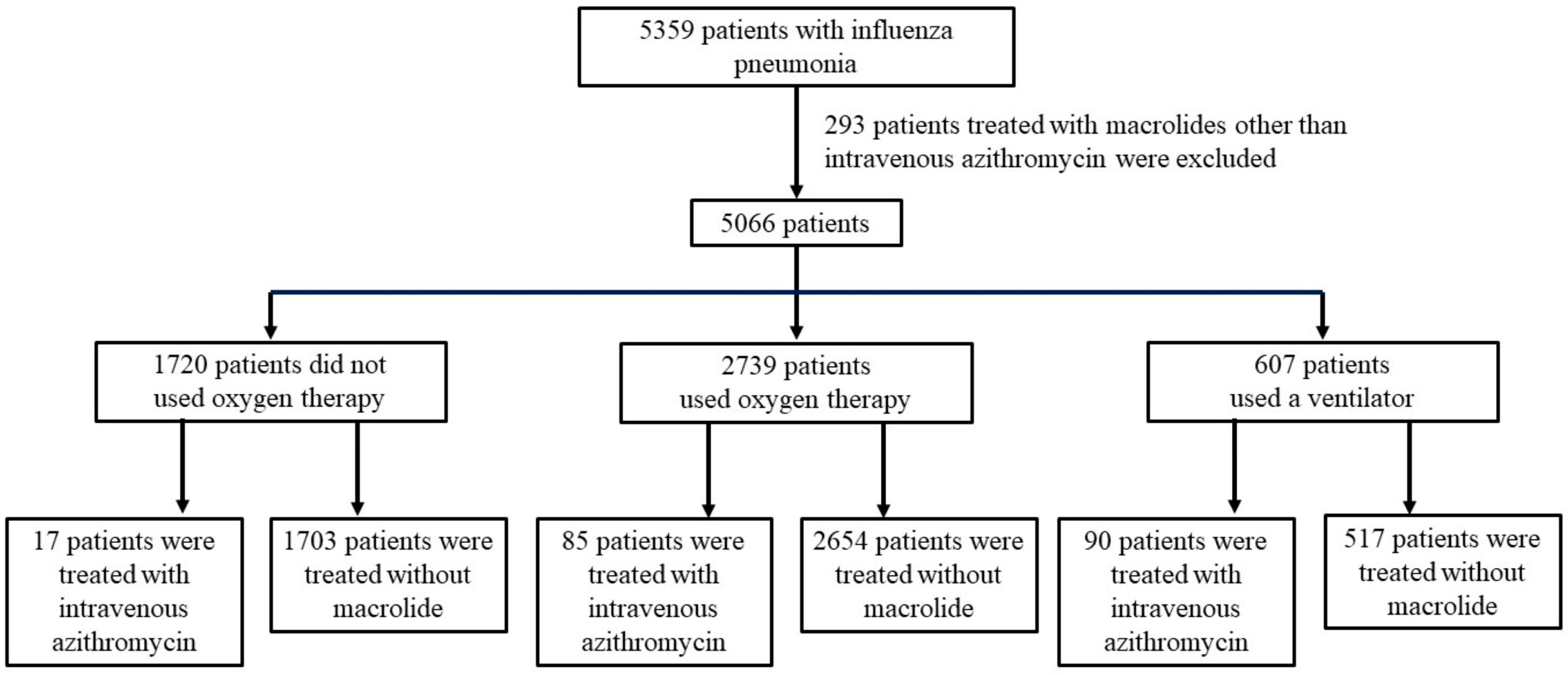
2.4. Patient Selection and Definitions
2.5. Variables
2.6. Outcomes
2.7. Statistical Analyses
3. Results
3.1. Mild Group
3.2. Moderate Group
3.3. Severe Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicholson, K.G.; Wood, J.M.; Zambon, M. Influenza. Lancet 2003, 362, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.S.; Swerdlow, D.L.; Borse, R.H.; Prabhu, V.S.; Finelli, L.; Atkins, C.Y.; Owusu-Edusei, K.; Bell, B.; Mead, P.S.; Biggerstaff, M.; et al. Estimating the Burden of 2009 Pandemic Influenza A (H1N1) in the United States (April 2009–April 2010). Clin. Infect. Dis. 2011, 52 (Suppl. S1), S75–S82. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of Global Seasonal Influenza-associated Respiratory Mortality: A Modelling Study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Krauland, M.G.; Galloway, D.D.; Raviotta, J.M.; Zimmerman, R.K.; Roberts, M.S. Impact of Low Rates of Influenza on Next-season Influenza Infections. Am. J. Prev. Med. 2022, 62, 503–510. [Google Scholar] [CrossRef]
- Lee, K.; Jalal, H.; Raviotta, J.M.; Krauland, M.G.; Zimmerman, R.K.; Burke, D.S.; Roberts, M.S. Estimating the Impact of Low Influenza Activity in 2020 on Population Immunity and Future Influenza Seasons in the United States. Open Forum Infect. Dis. 2021, 9, ofab607. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, C.; Omer, S.B.; Malani, P.N. Winter of Omicron-The Evolving COVID-19 Pandemic. JAMA 2022, 327, 319–320. [Google Scholar] [CrossRef]
- Hong, W.C.; Sun, S.F.; Hsu, C.W.; Lee, D.L.; Lee, C.H. Clinical Characteristics and Predictors of Mortality in Critically Ill Adult Patients with Influenza Infection. Int. J. Environ. Res. Public Health 2021, 18, 3682. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Vale, C.L.; Godolphin, P.J.; Fisher, D.; Higgins, J.P.T.; Spiga, F.; Savovic, J.; Tierney, J.; Baron, G.; Benbenishty, J.S.; et al. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-Analysis. JAMA 2021, 326, 499–518. [Google Scholar] [CrossRef]
- de Jong, M.D.; Ison, M.G.; Monto, A.S.; Metev, H.; Clark, C.; O’Neil, B.; Elder, J.; McCullough, A.; Collis, P.; Sheridan, W.P. Evaluation of Intravenous Peramivir for Treatment of Influenza in Hospitalized Patients. Clin. Infect. Dis. 2014, 59, e172–e185. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Kida, H.; Mizuguchi, M.; Hirotsu, N.; Ishida, T.; Kadota, J.; Shimada, J. Intravenous Peramivir for Treatment of Influenza A and B Virus Infection in High-risk Patients. Antimicrob. Agents Chemother. 2011, 55, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Okuno, D.; Kido, T.; Muramatsu, K.; Tokutsu, K.; Moriyama, S.; Miyamura, T.; Hara, A.; Ishimoto, H.; Yamaguchi, H.; Miyazaki, T.; et al. Impact of Corticosteroid Administration Within 7 Days of the Hospitalization for Influenza Pneumonia with Respiratory Failure: A Propensity Score Analysis Using a Nationwide Administrative Database. J. Clin. Med. 2021, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.N.; Chen, G.; Sun, J.; Liang, B.M.; Liang, Z.A. The Effect of Corticosteroids on Mortality of Patients with Influenza Pneumonia: A Systematic Review and Meta-analysis. Crit. Care 2019, 23, 99. [Google Scholar] [CrossRef] [PubMed]
- Horita, N.; Otsuka, T.; Haranaga, S.; Namkoong, H.; Miki, M.; Miyashita, N.; Higa, F.; Takahashi, H.; Yoshida, M.; Kohno, S.; et al. Beta-lactam Plus Macrolides or Beta-lactam Alone for Community-acquired Pneumonia: A Systematic Review and Meta-analysis. Respirology 2016, 21, 1193–1200. [Google Scholar] [CrossRef]
- Walkey, A.J.; Wiener, R.S. Macrolide Antibiotics and Survival in Patients with Acute Lung Injury. Chest 2012, 141, 1153–1159. [Google Scholar] [CrossRef]
- Lee, N.; Wong, C.K.; Chan, M.C.W.; Yeung, E.S.L.; Tam, W.W.S.; Tsang, O.T.Y.; Choi, K.W.; Chan, P.K.S.; Kwok, A.; Lui, G.C.Y.; et al. Anti-inflammatory Effects of Adjunctive Macrolide Treatment in Adults Hospitalized with Influenza: A Randomized Controlled Trial. Antivir. Res. 2017, 144, 48–56. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Hayashida, K.; Murakami, G.; Matsuda, S.; Fushimi, K. History and Profile of Diagnosis Procedure Combination (DPC): Development of a Real Data Collection System for Acute Inpatient Care in Japan. J. Epidemiol. 2021, 31, 1–11. [Google Scholar] [CrossRef]
- Kido, T.; Muramatsu, K.; Asakawa, T.; Otsubo, H.; Ogoshi, T.; Oda, K.; Kubo, T.; Fujino, Y.; Matsuda, S.; Mayumi, T.; et al. The Relationship Between High-dose Corticosteroid Treatment and Mortality in Acute Respiratory Distress Syndrome: A Retrospective and Observational Study Using a Nationwide Administrative Database in Japan. BMC Pulm. Med. 2018, 18, 28. [Google Scholar] [CrossRef]
- Kido, T.; Muramatsu, K.; Yatera, K.; Asakawa, T.; Otsubo, H.; Kubo, T.; Fujino, Y.; Matsuda, S.; Mayumi, T.; Mukae, H. Efficacy of Early Sivelestat Administration on Acute Lung Injury and Acute Respiratory Distress Syndrome. Respirology 2017, 22, 708–713. [Google Scholar] [CrossRef]
- Iwagami, M.; Yasunaga, H.; Doi, K.; Horiguchi, H.; Fushimi, K.; Matsubara, T.; Yahagi, N.; Noiri, E. Postoperative Polymyxin B Hemoperfusion and Mortality in Patients with Abdominal Septic Shock: A Propensity-matched Analysis. Crit. Care Med. 2014, 42, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. The Performance of Different Propensity Score Methods for Estimating Marginal Hazard Ratios. Stat Med. 2013, 32, 2837–2849. [Google Scholar] [CrossRef] [PubMed]
- Chesnaye, N.C.; Stel, V.S.; Tripepi, G.; Dekker, F.W.; Fu, E.L.; Zoccali, C.; Jager, K.J. An Introduction to Inverse Probability of Treatment Weighting in Observational Research. Clin. Kidney J. 2021, 15, 14–20. [Google Scholar] [CrossRef]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and Outcomes of Acute Lung Injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ishida, T.; Tachibana, H.; Tokumasu, H.; Yamazaki, A.; Washio, Y. Azithromycin Combination Therapy for Community-acquired Pneumonia: Propensity Score Analysis. Sci. Rep. 2019, 9, 18406. [Google Scholar] [CrossRef] [PubMed]
- Al-Salloum, J.; Gillani, S.W.; Mahmood, R.K.; Gulam, S.M. Comparative Efficacy of Azithromycin Versus Clarithromycin in Combination with Beta-lactams to Treat Community-acquired Pneumonia in Hospitalized Patients: A Systematic Review. J. Int. Med. Res. 2021, 49, 3000605211049943. [Google Scholar] [CrossRef]
- Falcone, M.; Russo, A.; Shindo, Y.; Farcomeni, A.; Pieralli, F.; Cangemi, R.; Liu, J.; Xia, J.; Okumura, J.; Sano, M.; et al. A Hypothesis-Generating Study of the Combination of Aspirin plus Macrolides in Patients with Severe Community-Acquired Pneumonia. Antimicrob. Agents Chemother. 2019, 63, e01556-18. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, R.; Liu, D.; Liu, X.; Zhang, J.; Zhang, Z.; Chen, S.; He, W.; Li, Y.; Xu, Y.; et al. Acute Respiratory Distress Syndrome Caused by Human Adenovirus in Adults: A Prospective Observational Study in Guangdong, China. Front. Med. 2021, 8, 791163. [Google Scholar] [CrossRef]
- Kawamura, K.; Ichikado, K.; Takaki, M.; Eguchi, Y.; Anan, K.; Suga, M. Adjunctive therapy with azithromycin for moderate and severe acute respiratory distress syndrome: A retrospective, propensity score-matching analysis of prospectively collected data at a single center. Int. J. Antimicrob. Agents 2018, 51, 918–924. [Google Scholar] [CrossRef]
- Kawamura, K.; Ichikado, K.; Suga, M.; Yoshioka, M. Efficacy of Azithromycin for Treatment of Acute Exacerbation of Chronic Fibrosing Interstitial Pneumonia: A Prospective, Open-label Study with Historical Controls. Respiration 2014, 87, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Ichikado, K.; Yasuda, Y.; Anan, K.; Suga, M. Azithromycin for Idiopathic Acute Exacerbation of Idiopathic Pulmonary Fibrosis: A Retrospective Single-center Study. BMC Pulm. Med. 2017, 17, 94. [Google Scholar] [CrossRef]
- Hung, I.F.N.; To, K.K.W.; Chan, J.F.W.; Cheng, V.C.C.; Liu, K.S.H.; Tam, A.; Chan, T.C.; Zhang, A.J.; Li, P.; Wong, T.L.; et al. Efficacy of Clarithromycin-naproxen-oseltamivir Combination in the Treatment of Patients Hospitalized for Influenza A(H3N2) Infection: An Open-label Randomized, Controlled, Phase Iib/III trial. Chest 2017, 151, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Higashi, F.; Kubo, H.; Yasuda, H.; Nukiwa, T.; Yamaya, M. Additional Treatment with Clarithromycin Reduces Fever Duration in Patients with Influenza. Respir. Investig. 2014, 52, 302–309. [Google Scholar] [CrossRef]
- Yatera, K.; Umeki, K.; Yamasaki, K.; Noguchi, S.; Nishida, C.; Ishimoto, H.; Sakamoto, N.; Ishii, H.; Kadota, J.I.; Mukae, H. The Additive Effect of Clarithromycin on Influenza A Infection in the Elderly Patients and Patients with Comorbid Diseases. Respir. Investig. 2017, 55, 380–383. [Google Scholar] [CrossRef]
- Kakeya, H.; Seki, M.; Izumikawa, K.; Kosai, K.; Morinaga, Y.; Kurihara, S.; Nakamura, S.; Imamura, Y.; Miyazaki, T.; Tsukamoto, M.; et al. Efficacy of Combination Therapy with Oseltamivir Phosphate and Azithromycin for Influenza: A Multicenter, Open-label, Randomized Study. PLoS ONE 2014, 9, e91293. [Google Scholar] [CrossRef] [PubMed]
- Martín-Loeches, I.; Bermejo-Martin, J.F.; Vallés, J.; Granada, R.; Vidaur, L.; Vergara-Serrano, J.C.; Martín, M.; Figueira, J.C.; Sirvent, J.M.; Blanquer, J.; et al. Macrolide-based Regimens in Absence of Bacterial Co-infection in Critically Ill H1N1 Patients with Primary Viral Pneumonia. Intensive Care Med. 2013, 39, 693–702. [Google Scholar] [CrossRef]
- Poddighe, D.; Aljofan, M. Clinical Evidences on the Antiviral Properties of Macrolide Antibiotics in the COVID-19 Era and Beyond. Antivir. Chem. Chemother. 2020, 28, 2040206620961712. [Google Scholar] [CrossRef]
- Firth, A.; Prathapan, P. Azithromycin: The First Broad-spectrum Therapeutic. Eur. J. Med. Chem. 2020, 207, 112739. [Google Scholar] [CrossRef]
- Kanoh, S.; Rubin, B.K. Mechanisms of Action and Clinical Application of Macrolides as Immunomodulatory Medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef]
- Pani, A.; Lauriola, M.; Romandini, A.; Scaglione, F. Macrolides and Viral Infections: Focus on Azithromycin in COVID-19 Pathology. Int. J. Antimicrob. Agents 2020, 56, 106053. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zuo, X.; Meng, F.; Han, C.; Ouyang, W.; Han, Y.; Ouyang, W.; Han, Y.; Gu, Y.; Zhao, X.; et al. Direct Inhibitory Effect on Viral Entry of Influenza A and SARS-CoV-2 Viruses by Azithromycin. Cell Prolif. 2021, 54, e12953. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.E.; Hinks, T.S.C. Azithromycin in Viral Infections. Rev. Med. Virol. 2021, 31, e2163. [Google Scholar] [CrossRef] [PubMed]
- Abaleke, E.; Abbas, M.; Abbasi, S.; Abbott, A.; Abdelaziz, A.; Abdelbadiee, S.; Abdelfattah, M.; Abdul, B.; Rasheed, A.A.; Abdul-Kadir, R.; et al. Azithromycin in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-label, Platform Trial. Lancet 2021, 397, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Hinks, T.S.C.; Cureton, L.; Knight, R.; Wang, A.; Cane, J.L.; Barber, V.S.; Black, J.; Dutton, S.J.; Melhorn, J.; Jabeen, M.; et al. Azithromycin Versus Standard Care in Patients with Mild-to-Moderate COVID-19 (ATOMIC2): An Open-label, Randomised Trial. Lancet Respir. Med. 2021, 9, 1130–1140. [Google Scholar] [CrossRef]
- Melamed, K.H.; Williams, J.; Wang, X.; Hu, S.; Nguyen, C.; Cui, J.; Deng, J.C. Development of Secondary Bacterial Pneumonia in Adults Presenting with Influenza Versus Noninfluenza Viral Respiratory Infection. Ther. Adv. Respir. Dis. 2020, 14, 1753466620963026. [Google Scholar] [CrossRef]
- Shieh, W.J.; Blau, D.M.; Denison, A.M.; Deleon-Carnes, M.; Adem, P.; Bhatnagar, J.; Sumner, J.; Liu, L.; Patel, M.; Batten, B.; et al. 2009 Pandemic Influenza A (H1N1): Pathology and Pathogenesis of 100 Fatal Cases in the United States. Am. J. Pathol. 2010, 177, 166–175. [Google Scholar] [CrossRef]
- Manohar, P.; Loh, B.; Nachimuthu, R.; Hua, X.; Welburn, S.C.; Leptihn, S. Secondary Bacterial Infections in Patients with Viral Pneumonia. Front. Med. 2020, 7, 420. [Google Scholar] [CrossRef]
- The Japanese Respiratory Society. The JRS Guidelines for the Management of Pneumonia in Adults 2017; The Japanese Respiratory Society: Tokyo, Japan, 2017; Volume 1, pp. 17–19. (In Japanese) [Google Scholar]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary Bacterial Infections Associated with Influenza Pandemics. Front. Microbiol. 2017, 8, 1041. [Google Scholar] [CrossRef]
- McCullers, J.A. Insights into the Interaction Between Influenza Virus and Pneumococcus. Clin. Microbiol. Rev. 2006, 19, 571–582. [Google Scholar] [CrossRef]
- Shorr, A.F.; Zilberberg, M.D.; Kan, J.; Hoffman, J.; Micek, S.T.; Kollef, M.H. Azithromycin and Survival in Streptococcus pneumoniae Pneumonia: A Retrospective Study. BMJ Open 2013, 3, e002898. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M.; Shebelski, S.D.; Hesje, C.K. Killing of Streptococcus pneumoniae by Azithromycin, Clarithromycin, Erythromycin, Telithromycin and Gemifloxacin Using Drug Minimum Inhibitory Concentrations and Mutant Prevention Concentrations. Int. J. Antimicrob. Agents 2015, 45, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Simmons, J.; Hampton, N.; Micek, S.T.; Kollef, M.H. Pneumococal Community-acquired Pneumonia in the Intensive Care Unit: Azithromycin Remains Protective Despite Macrolide Resistance. Respir. Med. 2021, 177, 106307. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, K.; Izumikawa, K.; Higa, F.; Tateyama, M.; Tokimatsu, I.; Hiramatsu, K.; Fujita, J.; Kadota, J.; Kohno, S. Efficacy of Azithromycin in the Treatment of Community-acquired Pneumonia, Including Patients with Macrolide-resistant Streptococcus pneumoniae Infection. Intern. Med. 2009, 48, 527–535. [Google Scholar] [CrossRef]
- Parnham, M.J.; Erakovic Haber, V.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of Action and Their Relevance for Clinical Applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef]
- Mortensen, E.M.; Halm, E.A.; Pugh, M.J.; Copeland, L.A.; Metersky, M.; Fine, M.J.; Johnson, C.S.; Alvarez, C.A.; Frei, C.R.; Good, C.; et al. Association of Azithromycin with Mortality and Cardiovascular Events Among Older Patients Hospitalized with Pneumonia. JAMA 2014, 311, 2199–2208. [Google Scholar] [CrossRef]
| Before Adjustment | After Adjustment | |||||
|---|---|---|---|---|---|---|
| Variables | Intravenous Azithromycin (n = 85) | Without Macrolide (n = 2654) | p-Value | Intravenous Azithromycin (n = 85) | Without Macrolide (n = 2654) | p-Value |
| Sex (female) | 37.6 | 45.5 | 0.151 | 33.39 | 45.29 | 0.061 |
| Age (years) | 72.38 ± 2.1 | 70.84 ± 0.5 | 0.087 | 67.92 ± 3.2 | 70.87 ± 0.5 | 0.353 |
| Hospital volume per year | 11.79 ± 0.9 | 14.65 ± 0.5 | 0.092 | 10.97 ± 1.3 | 14.55 ± 0.5 | 0.010 |
| Emergency admission | 78.8 | 68.2 | 0.037 | 73.76 | 68.54 | 0.469 |
| Emergency transport | 65.9 | 55.3 | 0.054 | 60.85 | 55.60 | 0.488 |
| Home healthcare | 4.7 | 10.2 | 0.098 | 6.17 | 10.00 | 0.206 |
| Smoking | 50.6 | 32.7 | 0.001 | 29.17 | 33.20 | 0.464 |
| Diabetes mellitus | 18.8 | 17.0 | 0.652 | 13.59 | 17.00 | 0.441 |
| Chronic kidney disease | 12.9 | 8.2 | 0.121 | 5.91 | 8.34 | 0.350 |
| Liver dysfunction | 4.7 | 1.8 | 0.049 | 4.78 | 1.91 | 0.353 |
| Cerebrovascular disease | 5.9 | 10.7 | 0.155 | 18.53 | 10.55 | 0.350 |
| Heart failure | 12.9 | 15.4 | 0.534 | 10.72 | 15.33 | 0.218 |
| Cardiovascular disease | 4.7 | 4.2 | 0.827 | 3.89 | 4.23 | 0.911 |
| Hypertension | 24.7 | 25.4 | 0.892 | 20.26 | 25.37 | 0.307 |
| Cancer | 15.3 | 8.9 | 0.043 | 5.92 | 9.10 | 0.161 |
| COPD | 5.9 | 7.4 | 0.593 | 6.97 | 7.35 | 0.914 |
| Asthma | 12.9 | 13.7 | 0.846 | 11.52 | 13.65 | 0.495 |
| Interstitial lung disease | 27.1 | 12.3 | <0.001 | 16.28 | 12.77 | 0.424 |
| Chronic respiratory failure | 4.7 | 1.8 | 0.060 | 1.72 | 1.92 | 0.770 |
| Neurological dysfunction | 2.4 | 2.7 | 0.856 | 9.78 | 2.68 | 0.361 |
| Vasopressor | 11.8 | 4.5 | 0.002 | 13.82 | 4.84 | 0.267 |
| Maintenance hemodialysis | 1.2 | 2.3 | 0.494 | 0.72 | 2.26 | 0.319 |
| Emergency hemodialysis | 2.4 | 0.4 | 0.010 | 0.31 | 0.48 | 0.270 |
| Platelet transfusion | 0.0 | 0.0 | - | 0.0 | 0.0 | - |
| Red blood cell transfusion | 2.4 | 1.6 | 0.601 | 2.96 | 1.68 | 0.682 |
| Antithrombin III | 0.0 | 0.0 | - | 0.0 | 0.0 | - |
| rhTM | 5.9 | 0.7 | <0.001 | 2.15 | 0.94 | 0.391 |
| Heparin | 14.1 | 5.3 | 0.001 | 8.27 | 5.66 | 0.537 |
| Insulin | 25.9 | 12.3 | <0.001 | 12.98 | 12.74 | 0.951 |
| Albumin | 4.7 | 1.0 | 0.002 | 1.00 | 1.17 | 0.751 |
| Sivelestat | 0.0 | 0.0 | - | 0.0 | 0.0 | - |
| Immunoglobulin | 0.0 | 0.0 | - | 0.0 | 0.0 | - |
| Oseltamivir | 9.4 | 10.4 | 0.769 | 8.25 | 10.35 | 0.579 |
| Laninamivir | 1.2 | 2.6 | 0.413 | 0.47 | 2.55 | <0.001 |
| Peramivir | 71.8 | 59.0 | 0.018 | 63.22 | 59.36 | 0.635 |
| Penicillin antibiotics | 37.6 | 37.1 | 0.920 | 39.16 | 37.15 | 0.808 |
| Cephalosporin (1st generation, iv) | 1.2 | 1.0 | 0.857 | 0.72 | 0.99 | 0.858 |
| Cephalosporin (2nd generation, iv) | 1.2 | 1.7 | 0.697 | 0.47 | 1.71 | 0.019 |
| Cephalosporin (3rd generation, iv) | 44.7 | 31.7 | 0.012 | 27.29 | 32.06 | 0.415 |
| Cephalosporin (4th generation, iv) | 3.5 | 1.4 | 0.096 | 1.04 | 1.40 | 0.573 |
| Carbapenem | 20.0 | 8.9 | <0.001 | 11.67 | 9.31 | 0.639 |
| Clindamycin | 1.2 | 0.5 | 0.339 | 0.51 | 0.48 | 0.919 |
| New quinolone | 5.9 | 5.7 | 0.928 | 14.90 | 5.68 | 0.121 |
| Anti-MRSA drug | 3.5 | 0.7 | 0.003 | 1.51 | 0.77 | 0.712 |
| Minocycline | 4.7 | 1.9 | 0.065 | 2.80 | 1.97 | 0.437 |
| Steroid use | 29.4 | 20.1 | 0.036 | 16.25 | 20.40 | 0.376 |
| PMX | 0.0 | 0.0 | - | 0.0 | 0.0 | - |
| ICU admission rate | 2.4 | 1.0 | 0.236 | 0.76 | 1.06 | 0.583 |
| Outcomes | Intravenous Azithromycin (n = 85) | Without Macrolide (n = 2654) | p-Value |
|---|---|---|---|
| 30-day mortality (%) | 14.89 | 9.23 | 0.477 |
| 90-day mortality (%) | 15.06 | 11.16 | 0.622 |
| In-hospital mortality (%) | 16.56 | 11.45 | 0.516 |
| ICU management, mean days (±SE) | 0.050 ± 0.03 | 0.069 ± 0.01 | 0.553 |
| IMV, mean days (±SE) | 0.028 ± 0.02 | 0.154 ± 0.05 | 0.026 |
| Hospital stay, mean days (±SE) | 17.55 ± 2.9 | 17.95 ± 0.4 | 0.891 |
| Before Adjustment | After Adjustment | |||||
|---|---|---|---|---|---|---|
| Variables | Intravenous Azithromycin (n = 90) | Without Macrolide (n = 517) | p-Value | Intravenous Azithromycin (n = 90) | Without Macrolide (n = 517) | p-Value |
| Sex (female) | 34.44 | 38.49 | 0.465 | 43.14 | 38.24 | 0.424 |
| Age (years) | 67.99 ± 1.7 | 68.03 ± 1.0 | 0.075 | 69.91 ± 1.7 | 68.05 ± 0.9 | 0.282 |
| Hospital volume per year | 9.19 ± 0.8 | 11.85 ± 0.8 | 0.433 | 10.11 ± 1.1 | 11.40 ± 0.7 | 0.298 |
| Emergency admission | 90.0 | 89.17 | 0.814 | 92.07 | 89.49 | 0.295 |
| Emergency transport | 78.9 | 75.0 | 0.434 | 80.81 | 75.92 | 0.101 |
| Home healthcare | 4.4 | 9.5 | 0.119 | 10.12 | 8.71 | 0.775 |
| Smoking | 51.1 | 47.8 | 0.559 | 50.21 | 48.83 | 0.727 |
| Diabetes mellitus | 24.4 | 19.5 | 0.285 | 23.32 | 20.35 | 0.539 |
| Chronic kidney disease | 17.8 | 15.7 | 0.614 | 13.23 | 15.87 | 0.440 |
| Liver dysfunction | 5.6 | 2.1 | 0.061 | 3.27 | 2.56 | 0.673 |
| Cerebrovascular disease | 8.9 | 8.7 | 0.954 | 11.02 | 8.76 | 0.556 |
| Heart failure | 15.6 | 23.6 | 0.091 | 26.54 | 22.50 | 0.541 |
| Cardiovascular disease | 6.7 | 6.4 | 0.919 | 51.49 | 63.06 | 0.519 |
| Hypertension | 18.9 | 21.9 | 0.526 | 27.33 | 21.29 | 0.368 |
| Cancer | 7.8 | 5.2 | 0.331 | 8.68 | 5.79 | 0.295 |
| COPD | 2.2 | 7.0 | 0.087 | 4.35 | 6.25 | 0.336 |
| Asthma | 4.4 | 6.6 | 0.441 | 3.90 | 6.17 | 0.130 |
| Interstitial lung disease | 20.0 | 15.9 | 0.329 | 12.90 | 16.19 | 0.241 |
| Chronic respiratory failure | 1.1 | 3.3 | 0.261 | 1.71 | 2.96 | 0.419 |
| Neurological dysfunction | 14.4 | 22.4 | 0.087 | 17.70 | 21.45 | 0.335 |
| Vasopressor | 63.3 | 56.3 | 0.212 | 66.75 | 57.89 | 0.158 |
| Maintenance hemodialysis | 1.1 | 3.1 | 0.293 | 0.80 | 2.78 | 0.006 |
| Emergency hemodialysis | 1.1 | 3.1 | 0.293 | 1.57 | 2.79 | 0.443 |
| Platelet transfusion | 11.1 | 7.4 | 0.222 | 8.26 | 7.82 | 0.835 |
| Red blood cell transfusion | 22.2 | 11.6 | 0.006 | 15.57 | 13.60 | 0.530 |
| Antithrombin III | 12.2 | 5.0 | 0.008 | 7.92 | 6.13 | 0.491 |
| rhTM | 20.0 | 7.9 | <0.001 | 10.56 | 9.41 | 0.667 |
| Heparin | 62.2 | 46.6 | 0.006 | 53.53 | 48.76 | 0.434 |
| Insulin | 57.8 | 37.7 | <0.001 | 47.64 | 40.85 | 0.220 |
| Albumin | 34.4 | 20.5 | 0.004 | 26.11 | 22.92 | 0.579 |
| Sivelestat | 5.6 | 2.9 | 0.193 | 55.85 | 34.58 | 0.539 |
| Immunoglobulin | 14.4 | 6.2 | 0.006 | 9.85 | 7.45 | 0.428 |
| Oseltamivir | 6.7 | 6.2 | 0.863 | 4.68 | 6.12 | 0.283 |
| Laninamivir | 1.1 | 1.2 | 0.968 | 0.47 | 1.10 | 0.078 |
| Peramivir | 86.7 | 74.1 | 0.010 | 82.26 | 76.05 | 0.153 |
| Penicillin antibiotics | 34.4 | 34.4 | 0.998 | 37.27 | 34.46 | 0.589 |
| Cephalosporins (1st generation, iv) | 2.2 | 2.1 | 0.954 | 2.15 | 2.03 | 0.924 |
| Cephalosporins (2nd generation, iv) | 0.0 | 0.0 | - | 0.0 | 0.0 | - |
| Cephalosporins (3rd generation, iv) | 34.4 | 33.3 | 0.827 | 33.35 | 33.32 | 0.995 |
| Cephalosporins (4th generation, iv) | 3.3 | 2.3 | 0.023 | 2.22 | 2.42 | 0.830 |
| Carbapenem | 41.1 | 27.7 | 0.010 | 35.96 | 30.12 | 0.337 |
| Clindamycin | 1.1 | 1.2 | 0.968 | 0.94 | 1.12 | 0.723 |
| New quinolone | 12.2 | 19.3 | 0.107 | 15.27 | 18.28 | 0.550 |
| Anti-MRSA drug | 15.6 | 5.4 | <0.001 | 9.27 | 6.98 | 0.346 |
| Minocycline | 3.3 | 2.1 | 0.482 | 4.45 | 2.36 | 0.489 |
| Steroid use | 62.2 | 39.5 | <0.001 | 45.43 | 42.78 | 0.599 |
| PMX | 1.1 | 0.6 | 0.566 | 0.45 | 0.62 | 0.471 |
| Oxygen therapy | 41.1 | 45.5 | 0.444 | 49.96 | 44.85 | 0.397 |
| NPPV | 5.6 | 4.3 | 0.581 | 3.77 | 4.25 | 0.750 |
| IMV wearing days | 5.18 | 4.21 | <0.001 | 4.61 | 4.35 | 0.183 |
| ICU admission rate | 38.9 | 28.4 | 0.046 | 38.03 | 30.26 | 0.119 |
| Outcomes | Intravenous Azithromycin (n = 90) | Without Macrolide (n = 517) | p-Value |
|---|---|---|---|
| 30-day mortality (%) | 26.49 | 36.65 | 0.038 |
| 90-day mortality (%) | 33.78 | 40.37 | 0.234 |
| In-hospital mortality (%) | 33.78 | 40.97 | 0.194 |
| ICU management, mean days (±SE) | 2.96 ± 0.4 | 2.59 ± 0.2 | 0.407 |
| IMV, mean days (±SE) | 10.82 ± 1.2 | 11.69 ± 0.8 | 0.534 |
| Hospital stay, mean days (±SE) | 28.32 ± 2.3 | 27.33 ± 1.3 | 0.699 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokito, T.; Kido, T.; Muramatsu, K.; Tokutsu, K.; Okuno, D.; Yura, H.; Takemoto, S.; Ishimoto, H.; Takazono, T.; Sakamoto, N.; et al. Impact of Administering Intravenous Azithromycin within 7 Days of Hospitalization for Influenza Virus Pneumonia: A Propensity Score Analysis Using a Nationwide Administrative Database. Viruses 2023, 15, 1142. https://doi.org/10.3390/v15051142
Tokito T, Kido T, Muramatsu K, Tokutsu K, Okuno D, Yura H, Takemoto S, Ishimoto H, Takazono T, Sakamoto N, et al. Impact of Administering Intravenous Azithromycin within 7 Days of Hospitalization for Influenza Virus Pneumonia: A Propensity Score Analysis Using a Nationwide Administrative Database. Viruses. 2023; 15(5):1142. https://doi.org/10.3390/v15051142
Chicago/Turabian StyleTokito, Takatomo, Takashi Kido, Keiji Muramatsu, Kei Tokutsu, Daisuke Okuno, Hirokazu Yura, Shinnosuke Takemoto, Hiroshi Ishimoto, Takahiro Takazono, Noriho Sakamoto, and et al. 2023. "Impact of Administering Intravenous Azithromycin within 7 Days of Hospitalization for Influenza Virus Pneumonia: A Propensity Score Analysis Using a Nationwide Administrative Database" Viruses 15, no. 5: 1142. https://doi.org/10.3390/v15051142
APA StyleTokito, T., Kido, T., Muramatsu, K., Tokutsu, K., Okuno, D., Yura, H., Takemoto, S., Ishimoto, H., Takazono, T., Sakamoto, N., Obase, Y., Ishimatsu, Y., Fujino, Y., Yatera, K., Fushimi, K., Matsuda, S., & Mukae, H. (2023). Impact of Administering Intravenous Azithromycin within 7 Days of Hospitalization for Influenza Virus Pneumonia: A Propensity Score Analysis Using a Nationwide Administrative Database. Viruses, 15(5), 1142. https://doi.org/10.3390/v15051142





