Seroprevalence and Vaginal Shedding of Herpes Simplex Virus Type 2 in Pregnant Adolescents and Young Women from Morelos, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Considerations
2.3. Vaginal and Blood Samples Collection
2.4. HSV-2 Seroprevalence
2.5. Vaginal HSV-2 Shedding
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Study Population
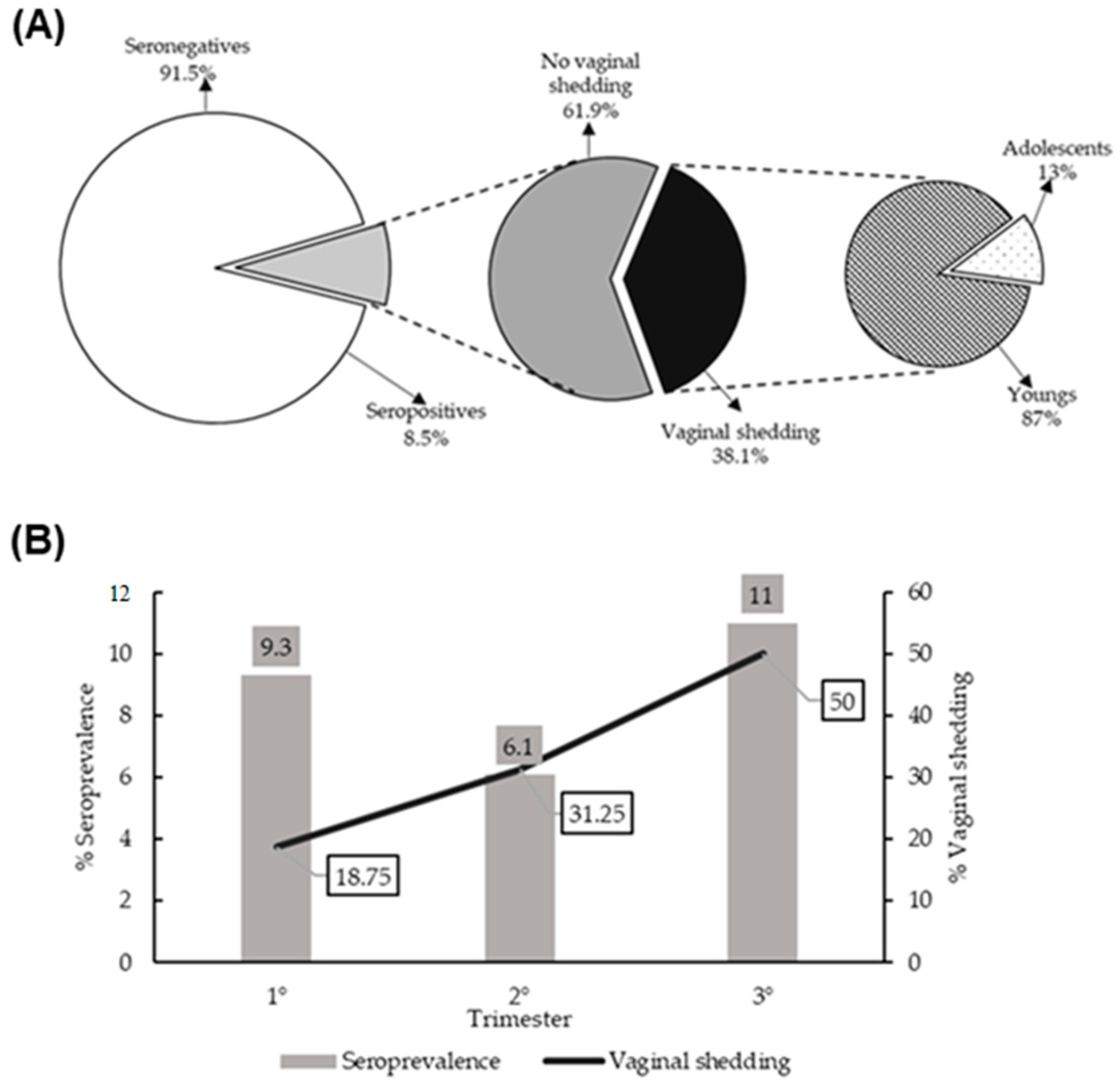
3.2. Seroprevalence of HSV-2 and Genital Shedding
3.3. Asociated Factors with Seroprevalence of HSV-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Herpes Simplex Virus. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 15 March 2022).
- Tata, S.; Johnston, C.; Huang, M.L.; Selke, S.; Magaret, A.; Corey, L.; Wald, A. Overlapping reactivations of herpes simplex virus type 2 in the genital and perianal mucosa. J. Infect. Dis. 2010, 201, 499–504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wald, A.; Corey, L. Persistence in the population: Epidemiology, transmission. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. Available online: https://www.ncbi.nlm.nih.gov/books/NBK47447/ (accessed on 15 March 2022).
- Domercant, J.W.; Jean Louis, F.; Hulland, E.; Griswold, M.; Andre-Alboth, J.; Ye, T.; Marston, B.J. Seroprevalence of Herpes Simplex Virus type-2 (HSV-2) among pregnant women who participated in a national HIV surveillance activity in Haiti. BMC Infect. Dis. 2017, 17, 577. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Markowitz, L.E.; Gottlieb, S.L.; Berman, S.M. Seroprevalence of herpes simplex virus types 1 and 2 in pregnant women in the United States. Am. J. Obstet. Gynecol. 2007, 196, 43.e1–43.e6. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.; Klausner, J. The Growing Epidemic of Sexually Transmitted Infections in Adolescents: A Neglected Population. Curr. Opin. Pediatr. 2018, 30, 137–143. [Google Scholar] [CrossRef]
- Gutierrez, J.P.; Bertozzi, S.M.; Conde-Glez, C.J.; Sanchez-Aleman, M.A. Risk behaviors of 15–21 years old in Mexico lead to a high prevalence of sexually transmitted infections: Results of a survey in disadvantaged urban areas. BMC Public Health 2006, 6, 49. [Google Scholar] [CrossRef]
- Sánchez-Alemán, M.A.; Conde-Glez, C.J.; Gayet, C.; García-Cisneros, S.; Uribe-Salas, F. Sexual behavior and herpes simplex virus 2 infection in college students. Arch. Med. Res. 2005, 36, 574–580. [Google Scholar] [CrossRef]
- Shafii, T.; Burstein, G.R. The adolescent sexual health visit. Obstet. Gynecol. Clin. N. Am. 2009, 36, 99–117. [Google Scholar] [CrossRef]
- Schillinger, J.A.; McKinney, C.M.; Garg, R.; Gwynn, R.C.; White, K.; Lee, F.; Blank, S.; Thorpe, L.; Frieden, T. Seroprevalence of herpes simplex virus type 2 and characteristics associated with undiagnosed infection: New York City, 2004. Sex. Transm. Dis. 2008, 35, 599–606. [Google Scholar] [CrossRef]
- Garland, S.M.; Steben, M. Genital herpes. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 1098–1110. [Google Scholar] [CrossRef]
- Pinninti, S.G.; Kimberlin, D.W. Maternal and neonatal herpes simplex virus infections. Am. J. Perinatol. 2013, 30, 113–119. [Google Scholar] [CrossRef]
- Straface, G.; Selmin, A.; Zanardo, V.; De Santis, M.; Ercoli, A.; Scambia, G. Herpes simplex virus infection in pregnancy. Infect. Dis. Obstet. Gynecol. 2012, 2012, 385697. [Google Scholar] [CrossRef] [PubMed]
- Bahena-Román, M.; Sánchez-Alemán, M.A.; Contreras-Ochoa, C.O.; Lagunas-Martínez, A.; Olamendi-Portugal, M.; López-Estrada, G.; Delgado-Romero, K.; Guzmán-Olea, E.; Madrid-Marina, V.; Torres-Poveda, K. Prevalence of active infection by herpes simplex virus type 2 in patients with high-risk human papillomavirus infection: A cross-sectional study. J. Med. Virol. 2020, 92, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Ortiz, A.; Conde-Glez, C.J.; Vergara-Ortega, D.N.; García-Cisneros, S.; Olamendi-Portugal, M.L.; Sánchez-Alemán, M.A. Avidity of Antibodies against HSV-2 and Risk to Neonatal Transmission among Mexican Pregnant Women. Infect. Dis. Obstet. Gynecol. 2013, 2013, e140142. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alemán, M.A.; Uribe-Salas, F.J.; Lazcano-Ponce, E.C.; García-Cisneros, S.; Eguiza-Fano, S.; Conde-Glez, C.J. HSV-2 seroincidence among Mexican college students: The delay of sexual debut is not enough to avoid risky sexual behaviours and virus transmission. Sex. Transm. Infect. 2010, 86, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Ortega, D.N.; Sevilla-Reyes, E.E.; Herrera-Ortiz, A.; Torres-Ibarra, L.; Salmerón, J.; Lazcano-Ponce, E.; Sánchez-Alemán, M.A. Real time PCR to evaluate HSV-2 shedding from anal and genital samples among men who have sex with men, living with HIV. J. Med. Virol. 2018, 90, 745–752. [Google Scholar] [CrossRef]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef]
- Sanchez-Aleman, M.A.; Del Villar-Tapia, Y.G.; Gutierrez, J.P.; Garcia-Cisneros, S.; Olamendi-Portugal, M.L.; Herrera-Ortiz, A.; Velazquez-Meza, M.; Conde-Glez, C.J. Heterogeneity of Herpes Simplex Virus Type 2 Seroprevalence From a National Probability Survey in Mexico, 2012. Sex. Transm. Dis. 2018, 45, 111–117. [Google Scholar] [CrossRef]
- DGE. Anuario de Morbilidad 1984–2021. 2023. Available online: https://epidemiologia.salud.gob.mx/anuario/html/incidencia_enfermedad.html (accessed on 21 April 2023).
- Smith, J.S.; Robinson, N.J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J. Infect. Dis. 2002, 186 (Suppl. S1), S3–S28. [Google Scholar] [CrossRef]
- Shin, H.; Iwasaki, A. Generating protective immunity against genital herpes. Trends Immunol. 2013, 34, 487–494. [Google Scholar] [CrossRef]
- Rosenberg, M.; Pettifor, A.; Van Rie, A.; Thirumurthy, H.; Emch, M.; Miller, W.C.; Gómez-Olivé, F.X.; Twine, R.; Hughes, J.P.; Laeyendecker, O.; et al. The Relationship between Alcohol Outlets, HIV Risk Behavior, and HSV-2 Infection among South African Young Women: A Cross-Sectional Study. PLoS ONE 2015, 10, e0125510. [Google Scholar] [CrossRef]
- Gutierrez, J.P.; Conde-González, C.J.; Walker, D.M.; Bertozzi, S.M. Herpes Simplex Virus Type 2 among Mexican High School Adolescents: Prevalence and Association with Community Characteristics. Arch. Med. Res. 2007, 38, 774–782. [Google Scholar] [CrossRef]
- Kalinderi, K.; Delkos, D.; Kalinderis, M.; Athanasiadis, A.; Kalogiannidis, I. Urinary tract infection during pregnancy: Current concepts on a common multifaceted problem. J. Obstet. Gynaecol. 2018, 38, 448–453. [Google Scholar] [CrossRef]
- Birdthistle, I.; Floyd, S.; Nyagadza, A.; Mudziwapasi, N.; Gregson, S.; Glynn, J.R. Is education the link between orphanhood and HIV/HSV-2 risk among female adolescents in urban Zimbabwe? Soc. Sci. Med. 2009, 68, 1810–1818. [Google Scholar] [CrossRef]
- Stoner, M.C.D.; Edwards, J.K.; Miller, W.C.; Aiello, A.E.; Halpern, C.T.; Julien, A.; Rucinski, K.B.; Selin, A.; Twine, R.; Hughes, J.P. Does Partner Selection Mediate the Relationship Between School Attendance and HIV/Herpes Simplex Virus-2 Among Adolescent Girls and Young Women in South Africa: An Analysis of HIV Prevention Trials Network 068 Data. J. Acquir. Immune Defic. Syndr. 2018, 79, 20–27. [Google Scholar] [CrossRef]
- Stoner, M.C.D.; Edwards, J.K.; Miller, W.C.; Aiello, A.E.; Halpern, C.T.; Julien, A.; Selin, A.; Hughes, J.P.; Wang, J.; Gomez-Olive, F.X. Effect of Schooling on Age-Disparate Relationships and Number of Sexual Partners among Young Women in Rural South Africa Enrolled in HPTN 068. J. Acquir. Immune Defic. Syndr. 2017, 76, e107–e114. [Google Scholar] [CrossRef]
- Brown, Z.A.; Wald, A.; Morrow, R.A.; Selke, S.; Zeh, J.; Corey, L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 2003, 289, 203–209. [Google Scholar] [CrossRef]
- Picone, O. Genital herpes and pregnancy: Epidemiology, clinical manifestations, prevention and screening. Guidelines for clinical practice from the French College of Gynecologists and Obstetrician (CNGOF). Gynecol. Obstet. Fertil. Senol. Diciembre 2017, 45, 642–654. [Google Scholar] [CrossRef]
- Patel, R.; Kennedy, O.J.; Clarke, E.; Geretti, A.; Nilsen, A.; Lautenschlager, S.; Green, J.; Donders, G.; van der Meijden, W.; Gomberg, M.; et al. 2017 European guidelines for the management of genital herpes. Int. J. STD AIDS 2017, 28, 1366–1379. [Google Scholar] [CrossRef]
- Gardella, C.; Brown, Z.A.; Wald, A.; Morrow, R.A.; Selke, S.; Krantz, E.; Corey, L. Poor correlation between genital lesions and detection of herpes simplex virus in women in labor. Obstet. Gynecol. 2005, 106, 268–274. [Google Scholar] [CrossRef]
- Gardella, C.; Huang, M.L.; Wald, A.; Magaret, A.; Selke, S.; Morrow, R.; Corey, L. Rapid polymerase chain reaction assay to detect herpes simplex virus in the genital tract of women in labor. Obstet. Gynecol. 2010, 115, 1209–1216. [Google Scholar] [CrossRef]
- Management of Genital Herpes in Pregnancy: ACOG Practice Bulletin, Number 220. Obstet. Gynecol. 2020, 135, e193–e202. [CrossRef] [PubMed]
- Samies, N.L.; James, S.H. Prevention and treatment of neonatal herpes simplex virus infection. Antivir. Res. 2020, 176, 104721. [Google Scholar] [CrossRef] [PubMed]

| Gen | Primer | Annealing Temperature | Fragment |
|---|---|---|---|
| UL30 (HSV-2) | F1qVHS2 5′-TCA CCG ACA AGG TCA AAC TC-3′ R2qVHS2 5′-ACA CAA TAC TCG CCG ATC AC-3′ | 63 °C | 151 pb |
| Human β-globina | F2qBETA 5′-GGG CTG TCA TCA CTT AGA CCT CAC-3′ R2qBETA 5′-CCG CTG TCA GAA GCA AAT GTA AGC AAT AG-3’ | 60 °C | 140 pb |
| Sociodemographic Characteristics | n | % | |
|---|---|---|---|
| Age | Young | 264 | 53.2 |
| Adolescents | 232 | 46.8 | |
| Educative level | High school/University | 181 | 36.5 |
| Middle school | 247 | 49.8 | |
| No studies/Elementary | 68 | 13.7 | |
| Smoke | Yes/Before | 130 | 26.2 |
| Never | 366 | 73.8 | |
| Alcohol consumption | Frequently | 90 | 18.1 |
| Occasionally | 216 | 43.5 | |
| Never | 190 | 38.3 | |
| Illegal drug use | Ever | 130 | 26.2 |
| Neither | 366 | 73.8 | |
| CLINICAL | |||
| Trimester | 3° | 145 | 29.2 |
| 2° | 212 | 42.7 | |
| 1° | 139 | 28.0 | |
| Clinical signs and symptoms | Yes | 287 | 57.9 |
| No | 209 | 42.1 | |
| Genital lesions | Yes | 94 | 19.0 |
| No | 402 | 81.0 | |
| Abortions/stillbirths | Yes | 53 | 10.7 |
| No | 443 | 89.3 | |
| Antibiotic use | Yes | 131 | 26.4 |
| No | 365 | 73.6 | |
| SEXUAL BEHAVIOR | |||
| STI risk perception | Not probably | 252 | 50.8 |
| Probably/very probably | 244 | 49.2 | |
| Lifetime sexual partners | ≥3 | 157 | 31.7 |
| 2 | 121 | 24.4 | |
| 1 | 218 | 44.0 | |
| Frequency of sex | 1–7 times/week | 446 | 89.9 |
| 1–3 times/month | 50 | 10.1 | |
| Anal sex | Yes | 74 | 14.9 |
| No | 422 | 85.1 | |
| Oral sex | Yes | 190 | 38.3 |
| No | 306 | 61.7 | |
| Hormonal contraceptives | Yes | 64 | 12.9 |
| No | 432 | 87.1 | |
| Frequency condom use | Never | 215 | 43.3 |
| Yes, at least sometimes | 281 | 56.7 | |
| Sociodemographic Characteristics | n | % HSV-2 | ORc (IC95%) | ORa (IC95%) | |
|---|---|---|---|---|---|
| Age | Young | 264 | 12.1 | 3.1 (1.47–6.38) | 3.4 (1.59–7.23) |
| Adolescents | 232 | 4.3 | 1 | 1 | |
| Educative level | High school/University | 181 | 9.4 | 1.5 (0.49–4.49) | 2.0 (0.63–6.45) |
| Middle school | 247 | 8.5 | 1.7 (0.54–5.12) | 1.79 (0.58–5.58) | |
| No studies/Elementary | 68 | 5.9 | 1 | 1 | |
| Smoke | Yes/Before | 130 | 12.3 | 1.8 (0.95–3.54) | 1.2 (0.55–2.54) |
| Never | 366 | 7.1 | 1 | 1 | |
| Alcohol consumption | Frequently | 90 | 15.6 | 2.7 (1.21–6.18) | 2.9 (1.27–6.99) |
| Occasionally | 216 | 7.4 | 1.2 (0.55–2.57) | 1.2 (0.52–2.57) | |
| Never | 190 | 6.3 | 1 | 1 | |
| Illegal drug use | Ever | 130 | 10.0 | 1.3 (0.65–2.57) | 0.9 (0.41–2.02) |
| Neither | 366 | 7.9 | 1 | 1 | |
| CLINICAL | |||||
| Trimester | 3° | 145 | 11.0 | 1.2 (0.56–2.60) | 1.2 (0.53–2.71) |
| 2° | 212 | 6.1 | 0.6 (0.28–1.41) | 0.6 (0.27–1.39) | |
| 1° | 139 | 9.4 | 1 | 1 | |
| Clinical signs and symptoms | Yes | 287 | 8.7 | 1.1 (0.57–2.05) | 0.9 (0.45–1.79) |
| No | 209 | 8.1 | 1 | 1 | |
| Genital lesions | Yes | 94 | 12.8 | 1.8 (0.89–3.69) | 1.7 (0.78–3.59) |
| No | 402 | 7.5 | 1 | 1 | |
| Abortions/stillbirths | Yes | 53 | 7.6 | 0.9 (0.29–2.54) | 0.6 (0.19–1.76) |
| No | 443 | 8.6 | 1 | 1 | |
| Antibiotic use | Yes | 131 | 12.9 | 2.0 (1.06–3.89) | 1.9 (0.95–3.79) |
| No | 365 | 6.9 | 1 | 1 | |
| SEXUAL BEHAVIOR | |||||
| STI risk perception | Not probably | 252 | 9.9 | 1.5 (0.77–2.97) | 1.3 (0.64–2.56) |
| Probably/ very probably | 244 | 6.9 | 1 | 1 | |
| Lifetime sexual partners | ≥3 | 157 | 9.6 | 1.1 (0.54–2.25) | 0.7 (0.30–1.54) |
| 2 | 121 | 6.6 | 0.7 (0.31–1.75) | 0.6 (0.30–1.54) | |
| 1 | 218 | 8.7 | 1 | ||
| Frequency of sex | 1–7 times/week | 446 | 9.2 | 4.9 (0.67–36.86) | 5.84 (0.76–44.7) |
| 1–3 times/month | 50 | 2.0 | 1 | 1 | |
| Anal sex | Yes | 74 | 10.8 | 1.4 (0.61–3.12) | 1.0 (0.42–2.43) |
| No | 422 | 8.1 | 1 | 1 | |
| Oral sex | Yes | 190 | 9.5 | 1.2 (0.65–2.33) | 0.8 (0.39–1.58) |
| No | 306 | 7.8 | 1 | 1 | |
| Hormonal contraceptives | Yes | 64 | 4.7 | 0.5 (0.15–1.65) | 0.5 (0.15–1.90) |
| No | 432 | 9.0 | 1 | 1 | |
| Frequency condom use | Never | 215 | 9.8 | 1.3 (0.71–2.52) | 1.36 (0.70–2.64) |
| Yes, at least sometimes | 281 | 7.5 | 1 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñiz-Salgado, J.C.; la Cruz, G.J.-D.; Vergara-Ortega, D.N.; García-Cisneros, S.; Olamendi-Portugal, M.; Sánchez-Alemán, M.Á.; Herrera-Ortiz, A. Seroprevalence and Vaginal Shedding of Herpes Simplex Virus Type 2 in Pregnant Adolescents and Young Women from Morelos, Mexico. Viruses 2023, 15, 1122. https://doi.org/10.3390/v15051122
Muñiz-Salgado JC, la Cruz GJ-D, Vergara-Ortega DN, García-Cisneros S, Olamendi-Portugal M, Sánchez-Alemán MÁ, Herrera-Ortiz A. Seroprevalence and Vaginal Shedding of Herpes Simplex Virus Type 2 in Pregnant Adolescents and Young Women from Morelos, Mexico. Viruses. 2023; 15(5):1122. https://doi.org/10.3390/v15051122
Chicago/Turabian StyleMuñiz-Salgado, Julio Cesar, Gabriela Juárez-De la Cruz, Dayana Nicté Vergara-Ortega, Santa García-Cisneros, María Olamendi-Portugal, Miguel Ángel Sánchez-Alemán, and Antonia Herrera-Ortiz. 2023. "Seroprevalence and Vaginal Shedding of Herpes Simplex Virus Type 2 in Pregnant Adolescents and Young Women from Morelos, Mexico" Viruses 15, no. 5: 1122. https://doi.org/10.3390/v15051122
APA StyleMuñiz-Salgado, J. C., la Cruz, G. J.-D., Vergara-Ortega, D. N., García-Cisneros, S., Olamendi-Portugal, M., Sánchez-Alemán, M. Á., & Herrera-Ortiz, A. (2023). Seroprevalence and Vaginal Shedding of Herpes Simplex Virus Type 2 in Pregnant Adolescents and Young Women from Morelos, Mexico. Viruses, 15(5), 1122. https://doi.org/10.3390/v15051122








