Functional Profiling and Evolutionary Analysis of a Marine Microalgal Virus Pangenome
Abstract
1. Introduction
2. Materials and Methods
2.1. Pangenome Analysis of the Genus Coccolithovirus and Comparison to Chloroviruses and Prasinoviruses
2.2. Expanding the Coccolithovirus Annotation Profile
2.3. Taxonomy Distribution of EhV-86 Protein Homologs
2.4. Transcriptome Analysis
3. Results and Discussion
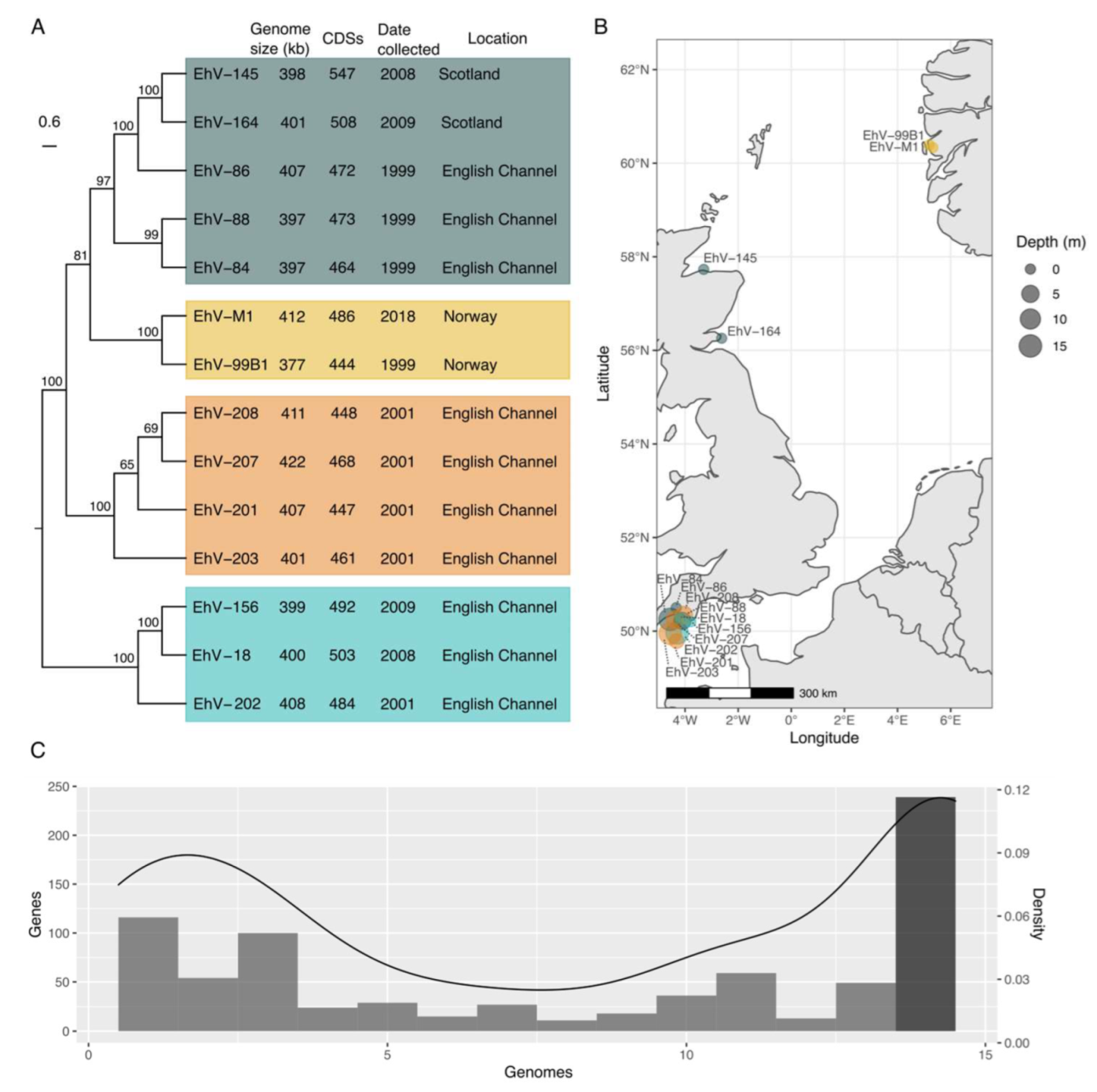
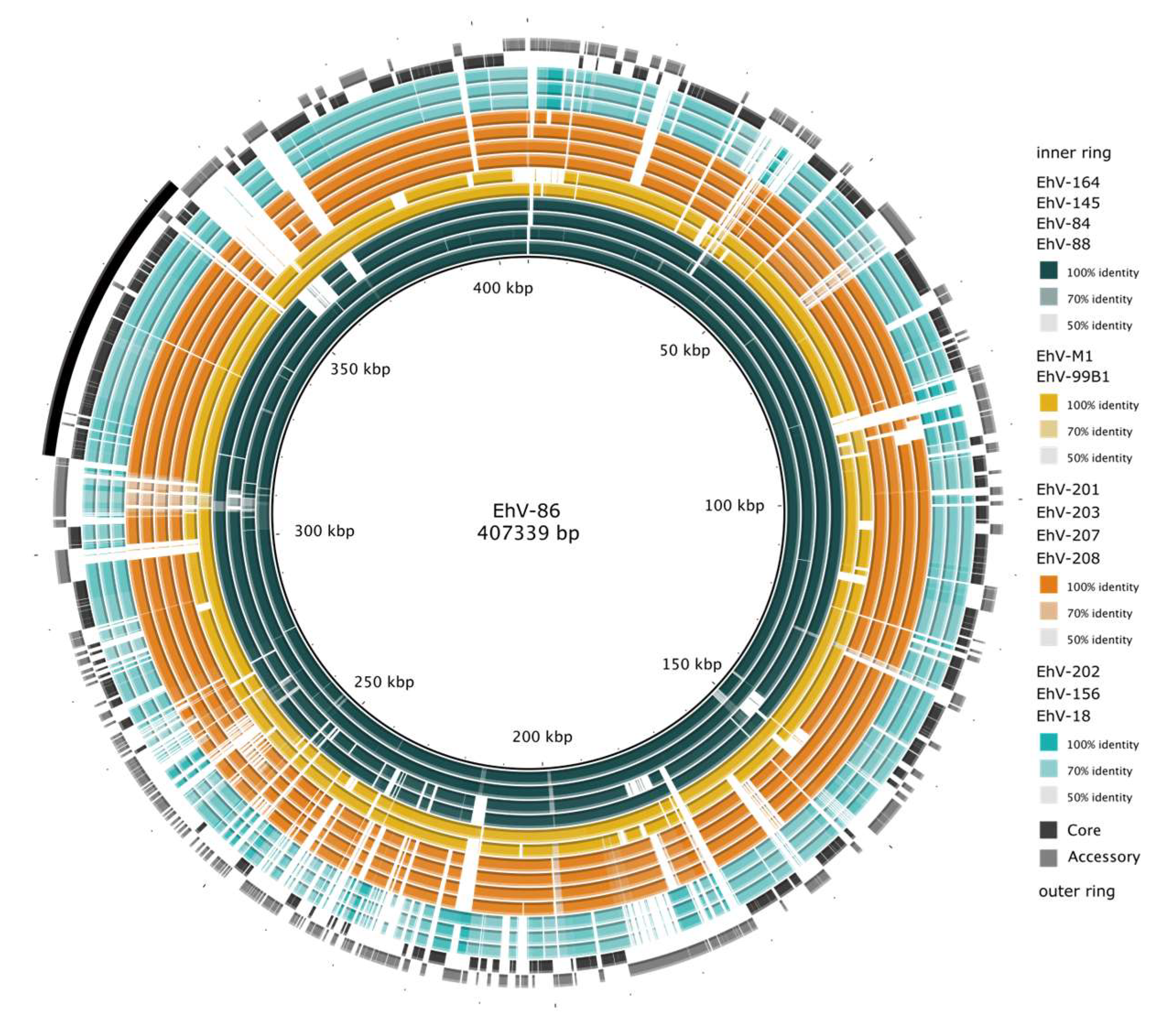
3.1. The Coccolithovirus Pangenome
3.2. Expanding the Coccolithovirus Functional Profile
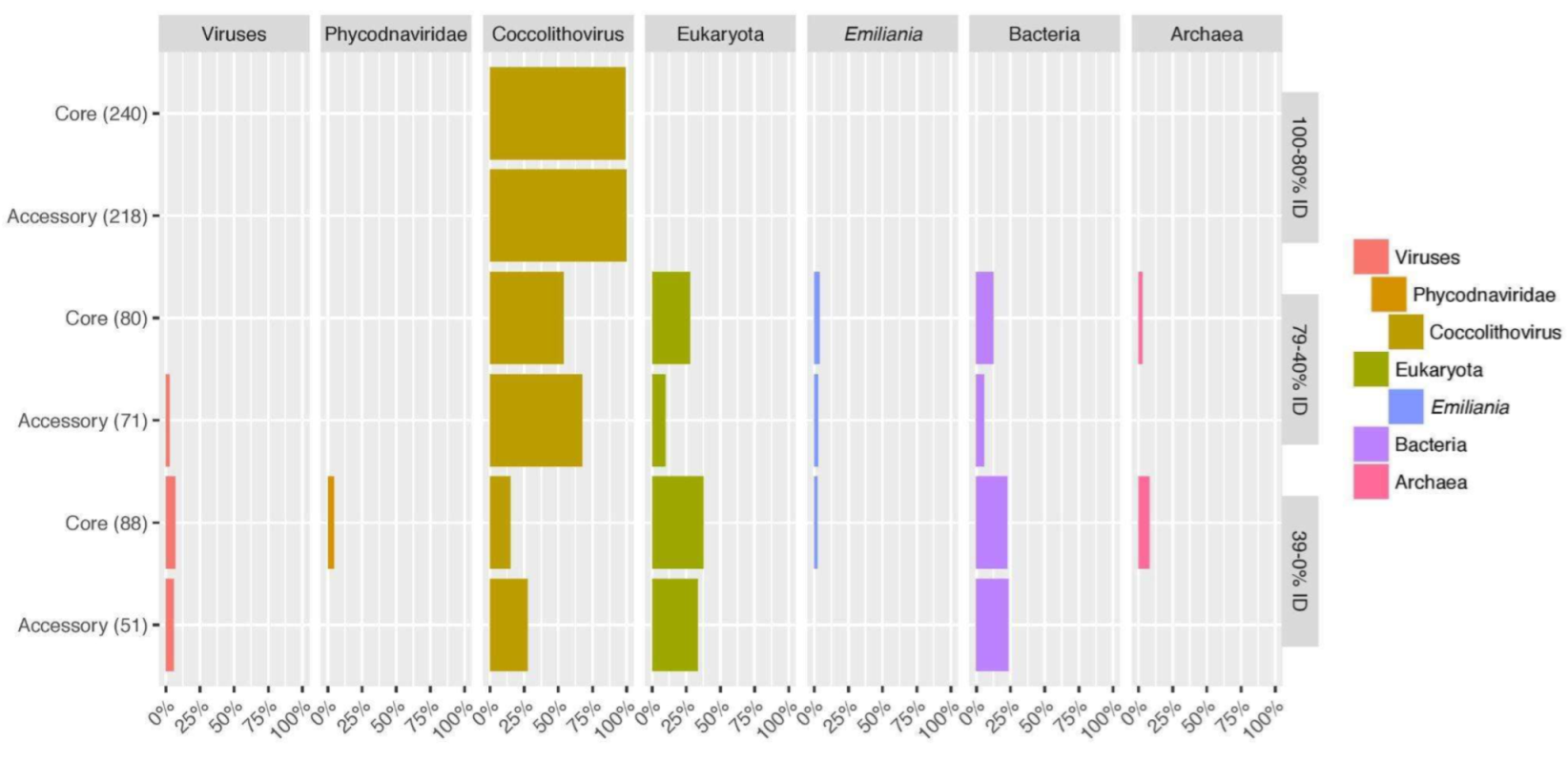
3.3. Taxonomy Distribution of EhV-86 Protein Homologues
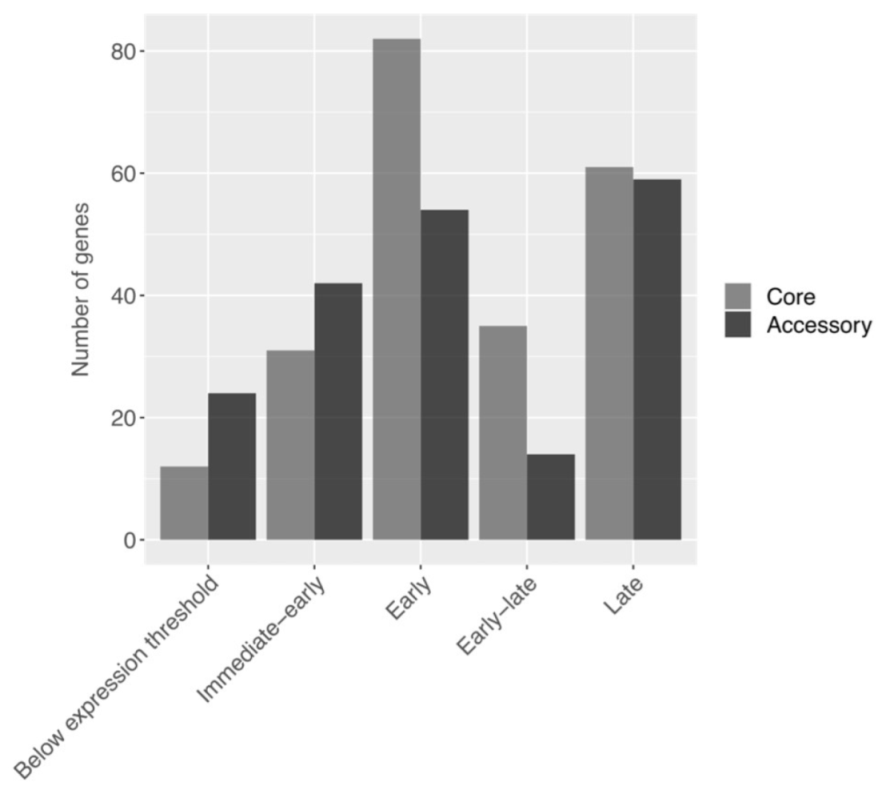
3.4. Pangenome Expression Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parikka, K.J.; Le Romancer, M.; Wauters, N.; Jacquet, S. Deciphering the Virus-to-Prokaryote Ratio (VPR): Insights into Virus–Host Relationships in a Variety of Ecosystems. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1081–1100. [Google Scholar] [CrossRef]
- Roux, S.; Brum, J.R.; Dutilh, B.E.; Sunagawa, S.; Duhaime, M.B.; Loy, A.; Poulos, B.T.; Solonenko, N.; Lara, E.; Poulain, J.; et al. Ecogenomics and Potential Biogeochemical Impacts of Globally Abundant Ocean Viruses. Nature 2016, 537, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Bratbak, G.; Egge, J.; Heldal, M. Viral Mortality of the Marine Alga Emiliania Huxleyi (Haptophyceae) and Termination of Algal Blooms. Mar. Ecol. Prog. Ser. 1993, 93, 39–48. [Google Scholar] [CrossRef]
- Brussaard, C.P.D.; Kempers, R.S.; Kop, A.J.; Reigman, R.; Heldal, M. Virus-like Particles in a Summer Bloom of Emiliania Huxleyi in the North Sea. Aquat. Microb. Ecol. 1996, 10, 105–113. [Google Scholar] [CrossRef]
- Wilson, W.H.; Tarran, G.; Zubkov, M.V. Virus Dynamics in a Coccolithophore-Dominated Bloom in the North Sea. Deep-Sea Res. II 2002, 49, 2951–2963. [Google Scholar] [CrossRef]
- Suttle, C.A. Viruses in the Sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef]
- Monier, A.; Pagarete, A.; de Vargas, C.; Allen, M.J.; Read, B.; Claverie, J.-M.; Ogata, H. Horizontal Gene Transfer of an Entire Metabolic Pathway between a Eukaryotic Alga and Its DNA Virus. Genome Res. 2009, 19, 1441–1449. [Google Scholar] [CrossRef]
- Crummett, L.T.; Puxty, R.J.; Weihe, C.; Marston, M.F.; Martiny, J.B.H. The Genomic Content and Context of Auxiliary Metabolic Genes in Marine Cyanomyoviruses. Virology 2016, 499, 219–229. [Google Scholar] [CrossRef]
- Van Etten, J.L.; Dunigan, D.D.; Nagasaki, K.; Schroeder, D.C.; Grimsley, N.; Brussaard, C.P.D.; Nissimov, J.I. Phycodnaviruses (Phycodnaviridae). In Encyclopedia of Virology, 4th ed.; Bamford, D.H., Zuckerman, M., Eds.; Academic Press: Oxford, UK, 2021; pp. 687–695. ISBN 978-0-12-814516-6. [Google Scholar]
- Wilson, W.H.; Van Etten, J.L.; Allen, M.J. The Phycodnaviridae: The Story of How Tiny Giants Rule the World. In Lesser Known Large dsDNA Viruses; Current Topics in Microbiology and Immunology; Van Etten, J.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–42. ISBN 978-3-540-68618-7. [Google Scholar]
- Mackinder, L.C.M.; Worthy, C.A.; Biggi, G.; Hall, M.; Ryan, K.P.; Varsani, A.; Harper, G.M.; Wilson, W.H.; Brownlee, C.; Schroeder, D.C.Y. A Unicellular Algal Virus, Emiliania Huxleyi Virus 86, Exploits an Animal-like Infection Strategy. J. Gen. Virol. 2009, 90, 2306–2316. [Google Scholar] [CrossRef]
- Nissimov, J.I.; Pagarete, A.; Ma, F.; Cody, S.; Dunigan, D.D.; Kimmance, S.A.; Allen, M.J. Coccolithoviruses: A Review of Cross-Kingdom Genomic Thievery and Metabolic Thuggery. Viruses 2017, 9, 52. [Google Scholar] [CrossRef]
- Wilson, W.H.; Schroeder, D.C.; Allen, M.J.; Holden, M.T.G.; Parkhill, J.; Barrell, B.G.; Churcher, C.; Hamlin, N.; Mungall, K.; Norbertczak, H.; et al. Complete Genome Sequence and Lytic Phase Transcription Profile of a Coccolithovirus. Science 2005, 309, 1090–1092. [Google Scholar] [CrossRef]
- Rosenwasser, S.; Mausz, M.A.; Schatz, D.; Sheyn, U.; Malitsky, S.; Aharoni, A.; Weinstock, E.; Tzfadia, O.; Ben-Dor, S.; Feldmesser, E.; et al. Rewiring Host Lipid Metabolism by Large Viruses Determines the Fate of Emiliania Huxleyi, a Bloom-Forming Alga in the Ocean. Plant Cell 2014, 26, 2689–2707. [Google Scholar] [CrossRef]
- Nissimov, J.I.; Talmy, D.; Haramaty, L.; Fredricks, H.F.; Zelzion, E.; Knowles, B.; Eren, A.M.; Vandzura, R.; Laber, C.P.; Schieler, B.M.; et al. Biochemical Diversity of Glycosphingolipid Biosynthesis as a Driver of Coccolithovirus Competitive Ecology. Environ. Microbiol. 2019, 21, 2182–2197. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.; Sheyn, U.; Sebé-Pedrós, A.; Ben-Dor, S.; Schatz, D.; Tanay, A.; Rosenwasser, S.; Vardi, A. A Single-Cell View on Alga-Virus Interactions Reveals Sequential Transcriptional Programs and Infection States. Sci. Adv. 2020, 6, eaba4137. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Baumdicker, F.; Neher, R.A. PanX: Pan-Genome Analysis and Exploration. Nucleic Acids Res. 2018, 46, e5. [Google Scholar] [CrossRef]
- Fromm, A.; Schatz, D.; Ben-Dor, S.; Feldmesser, E.; Vardi, A. Complete Genome Sequence of Emiliania Huxleyi Virus Strain M1, Isolated from an Induced E. Huxleyi Bloom in Bergen, Norway. Microbiol. Resour. Announc. 2022, 11, e0007122. [Google Scholar] [CrossRef] [PubMed]
- Snipen, L.; Liland, K.H. Micropan: An R-Package for Microbial Pan-Genomics. BMC Bioinform. 2015, 16, 79. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Aramaki, T.; Blanc-Mathieu, R.; Endo, H.; Ohkubo, K.; Kanehisa, M.; Goto, S.; Ogata, H. KofamKOALA: KEGG Ortholog Assignment Based on Profile HMM and Adaptive Score Threshold. Bioinformatics 2020, 36, 2251–2252. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam Protein Families Database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Zhang, Z.; Wood, W.I. A Profile Hidden Markov Model for Signal Peptides Generated by HMMER. Bioinformatics 2003, 19, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 Web Portal for Protein Modeling, Prediction and Analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. EggNOG-Mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef] [PubMed]
- Steinegger, M.; Meier, M.; Mirdita, M.; Vöhringer, H.; Haunsberger, S.J.; Söding, J. HH-Suite3 for Fast Remote Homology Detection and Deep Protein Annotation. BMC Bioinform. 2019, 20, 473. [Google Scholar] [CrossRef]
- Mirdita, M.; von den Driesch, L.; Galiez, C.; Martin, M.J.; Söding, J.; Steinegger, M. Uniclust Databases of Clustered and Deeply Annotated Protein Sequences and Alignments. Nucleic Acids Res. 2017, 45, D170–D176. [Google Scholar] [CrossRef]
- Mirzakhanyan, Y.; Gershon, P.D. Structure-Based Deep Mining Reveals First-Time Annotations for 46 Percent of the Dark Annotation Space of the 9,671-Member Superproteome of the Nucleocytoplasmic Large DNA Viruses. J. Virol. 2020, 94, e00854-20. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Lobb, B. AlphaFold Predicted Structures for EhV-86. Dryad, Dataset. 2023. [CrossRef]
- van Kempen, M.; Kim, S.S.; Tumescheit, C.; Mirdita, M.; Gilchrist, C.L.; Söding, J.; Steinegger, M. Foldseek: Fast and accurate protein structure search. bioRxiv 2022. [Google Scholar] [CrossRef]
- Rowe, J.M.; Fabre, M.-F.; Gobena, D.; Wilson, W.H.; Wilhelm, S.W. Application of the Major Capsid Protein as a Marker of the Phylogenetic Diversity of Emiliania Huxleyi Viruses. FEMS Microbiol. Ecol. 2011, 76, 373–380. [Google Scholar] [CrossRef]
- Nissimov, J.I.; Worthy, C.A.; Rooks, P.; Napier, J.A.; Kimmance, S.A.; Henn, M.R.; Ogata, H.; Allen, M.J. Draft Genome Sequence of the Coccolithovirus EhV-84. Stand Genom. Sci. 2011, 5, 1–11. [Google Scholar] [CrossRef]
- Rodrigues, R.A.L.; Queiroz, V.F.; Ghosh, J.; Dunigan, D.D.; Van Etten, J.L. Functional Genomic Analyses Reveal an Open Pan-Genome for the Chloroviruses and a Potential for Genetic Innovation in New Isolates. J. Virol. 2022, 96, e0136721. [Google Scholar] [CrossRef] [PubMed]
- Jeanniard, A.; Dunigan, D.D.; Gurnon, J.R.; Agarkova, I.V.; Kang, M.; Vitek, J.; Duncan, G.; McClung, O.W.; Larsen, M.; Claverie, J.-M.; et al. Towards Defining the Chloroviruses: A Genomic Journey through a Genus of Large DNA Viruses. BMC Genom. 2013, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Weynberg, K.D.; Allen, M.J.; Wilson, W.H. Marine Prasinoviruses and Their Tiny Plankton Hosts: A Review. Viruses 2017, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, W.; Li, X.; Pan, Y.; Yan, S.; Wang, Y. The Genome of a Prasinoviruses-Related Freshwater Virus Reveals Unusual Diversity of Phycodnaviruses. BMC Genom. 2018, 19, 49. [Google Scholar] [CrossRef]
- Van Etten, J.L.; Agarkova, I.V.; Dunigan, D.D. Chloroviruses. Viruses 2019, 12, 20. [Google Scholar] [CrossRef]
- Darienko, T.; Rad-Menéndez, C.; Campbell, C.; Pröschold, T. Are There Any True Marine Chlorella Species? Molecular Phylogenetic Assessment and Ecology of Marine Chlorella-like Organisms, Including a Description of Droopiella gen. nov. Syst. Biodivers. 2019, 17, 811–829. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, J.; Liu, T.; Yu, Y.; Pan, Y.; Yan, S.; Wang, Y. Four Novel Algal Virus Genomes Discovered from Yellowstone Lake Metagenomes. Sci. Rep. 2015, 5, 15131. [Google Scholar] [CrossRef]
- Alikhan, N.-F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple Prokaryote Genome Comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef]
- Lobb, B.; Tremblay, B.J.-M.; Moreno-Hagelsieb, G.; Doxey, A.C. An Assessment of Genome Annotation Coverage across the Bacterial Tree of Life. Microb. Genom. 2020, 6, e000341. [Google Scholar] [CrossRef]
- Huntemann, M.; Ivanova, N.N.; Mavromatis, K.; Tripp, H.J.; Paez-Espino, D.; Palaniappan, K.; Szeto, E.; Pillay, M.; Chen, I.-M.A.; Pati, A.; et al. The Standard Operating Procedure of the DOE-JGI Microbial Genome Annotation Pipeline (MGAP v.4). Stand Genomic Sci. 2015, 10, 86. [Google Scholar] [CrossRef]
- Lu, Z.; Kutish, G.F.; Sussman, M.D.; Rock, D.L. An African Swine Fever Virus Gene with a Similarity to Eukaryotic RNA Polymerase Subunit 6. Nucleic Acids Res. 1993, 21, 2940. [Google Scholar] [CrossRef] [PubMed]
- Hubbs, A.E.; Wright, C.F. The A2L Intermediate Gene Product Is Required for in Vitro Transcription from a Vaccinia Virus Late Promoter. J. Virol. 1996, 70, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Deneke, J.; Ziegelin, G.; Lurz, R.; Lanka, E. The Protelomerase of Temperate Escherichia Coli Phage N15 Has Cleaving-Joining Activity. Proc. Natl. Acad. Sci. USA 2000, 97, 7721–7726. [Google Scholar] [CrossRef]
- Deneke, J.; Ziegelin, G.; Lurz, R.; Lanka, E. Phage N15 Telomere Resolution. Target requirements for recognition and processing by the protelomerase. J. Biol. Chem. 2002, 277, 10410–10419. [Google Scholar] [CrossRef]
- Mir, T.; Huang, S.H.; Kobryn, K. The Telomere Resolvase of the Lyme Disease Spirochete, Borrelia Burgdorferi, Promotes DNA Single-Strand Annealing and Strand Exchange. Nucleic Acids Res. 2013, 41, 10438–10448. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Cozart, M.R.; Hart, M.A.; Kobryn, K. The Borrelia Burgdorferi Telomere Resolvase, ResT, Possesses ATP-Dependent DNA Unwinding Activity. Nucleic Acids Res. 2017, 45, 1319–1329. [Google Scholar] [CrossRef]
- Mardanov, A.V.; Ravin, N.V. Conversion of Linear DNA with Hairpin Telomeres into a Circular Molecule in the Course of Phage N15 Lytic Replication. J. Mol. Biol. 2009, 391, 261–268. [Google Scholar] [CrossRef]
- Pushkarev, A.; Inoue, K.; Larom, S.; Flores-Uribe, J.; Singh, M.; Konno, M.; Tomida, S.; Ito, S.; Nakamura, R.; Tsunoda, S.P.; et al. A Distinct Abundant Group of Microbial Rhodopsins Discovered Using Functional Metagenomics. Nature 2018, 558, 595–599. [Google Scholar] [CrossRef]
- Philosof, A.; Béjà, O. Bacterial, Archaeal and Viral-like Rhodopsins from the Red Sea. Environ. Microbiol. Rep. 2013, 5, 475–482. [Google Scholar] [CrossRef]
- Yutin, N.; Koonin, E.V. Proteorhodopsin Genes in Giant Viruses. Biol. Direct. 2012, 7, 34. [Google Scholar] [CrossRef]
- Hososhima, S.; Mizutori, R.; Abe-Yoshizumi, R.; Rozenberg, A.; Shigemura, S.; Pushkarev, A.; Konno, M.; Katayama, K.; Inoue, K.; Tsunoda, S.P.; et al. Proton-Transporting Heliorhodopsins from Marine Giant Viruses. Elife 2022, 11, e78416. [Google Scholar] [CrossRef] [PubMed]
- Knowles, B.; Bonachela, J.A.; Behrenfeld, M.J.; Bondoc, K.G.; Cael, B.B.; Carlson, C.A.; Cieslik, N.; Diaz, B.; Fuchs, H.L.; Graff, J.R.; et al. Temperate Infection in a Virus–Host System Previously Known for Virulent Dynamics. Nat. Commun. 2020, 11, 4626. [Google Scholar] [CrossRef]
- Vardi, A.; Van Mooy, B.A.S.; Fredricks, H.F.; Popendorf, K.J.; Ossolinski, J.E.; Haramaty, L.; Bidle, K.D. Viral Glycosphingolipids Induce Lytic Infection and Cell Death in Marine Phytoplankton. Science 2009, 326, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Ziv, C.; Malitsky, S.; Othman, A.; Ben-Dor, S.; Wei, Y.; Zheng, S.; Aharoni, A.; Hornemann, T.; Vardi, A. Viral Serine Palmitoyltransferase Induces Metabolic Switch in Sphingolipid Biosynthesis and Is Required for Infection of a Marine Alga. Proc. Natl. Acad. Sci. USA 2016, 113, E1907–E1916. [Google Scholar] [CrossRef] [PubMed]
- Nissimov, J.I.; Jones, M.; Napier, J.A.; Munn, C.B.; Kimmance, S.A.; Allen, M.J. Functional Inferences of Environmental Coccolithovirus Biodiversity. Virol. Sin. 2013, 28, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Ploubidou, A.; Way, M. Viral Transport and the Cytoskeleton. Curr. Opin. Cell Biol. 2001, 13, 97–105. [Google Scholar] [CrossRef]
- Nissimov, J.I.; Napier, J.A.; Allen, M.J.; Kimmance, S.A. Intragenus Competition between Coccolithoviruses: An Insight on How a Select Few Can Come to Dominate Many. Environ. Microbiol. 2016, 18, 133–145. [Google Scholar] [CrossRef]
- Van Etten, J.L.; Agarkova, I.; Dunigan, D.D.; Tonetti, M.; De Castro, C.; Duncan, G.A. Chloroviruses Have a Sweet Tooth. Viruses 2017, 9, 88. [Google Scholar] [CrossRef]
- Speciale, I.; Notaro, A.; Abergel, C.; Lanzetta, R.; Lowary, T.L.; Molinaro, A.; Tonetti, M.; Van Etten, J.L.; De Castro, C. The Astounding World of Glycans from Giant Viruses. Chem. Rev. 2022, 122, 15717–15766. [Google Scholar] [CrossRef]
- Brockhurst, M.A.; Harrison, E.; Hall, J.P.J.; Richards, T.; McNally, A.; MacLean, C. The Ecology and Evolution of Pangenomes. Curr. Biol. 2019, 29, R1094–R1103. [Google Scholar] [CrossRef]
- Allen, M.J.; Forster, T.; Schroeder, D.C.; Hall, M.; Roy, D.; Ghazal, P.; Wilson, W.H. Locus-Specific Gene Expression Pattern Suggests a Unique Propagation Strategy for a Giant Algal Virus. J. Virol. 2006, 80, 7699–7705. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Oosterhof, M.; Sandaa, R.-A.; Larsen, A.; Pagarete, A. Emerging Interaction Patterns in the Emiliania Huxleyi-EhV System. Viruses 2017, 9, 61. [Google Scholar] [CrossRef] [PubMed]

| Coccolithovirus Pangenome | Common Domain/Protein Family/Structural Matches for Non-Annotated Proteins | Number of Sources with Similar Hits | Sources with Similar Hits | |
|---|---|---|---|---|
| ehv116 | accessory (12 strains) | Fatty acid hydroxylase | 8 | InterPro, Pfam, PDB, Phyre2, EggNOG, Uniclust, Ku et al. [16], Foldseek |
| ehv138 | accessory (12 strains) | Recombination endonuclease VII domain-containing protein | 8 | InterPro, PFAM, PDB, Phyre2, EggNOG, panX, Ku et al. [16], Foldseek |
| ehv088 | accessory (12 strains) | Fatty acid hydroxylase | 7 | InterPro, Pfam, PDB, Phyre2, EggNOG, Uniclust, Foldseek |
| ehv411 | core | Glycosyltransferase (GlcNAc) | 7 | InterPro, Pfam, PDB, EggNOG, Uniclust, Ku et al. [16], Foldseek |
| ehv452 | core | HMG-box domain-containing protein (Uniclust annotated as signal recognition particle-docking protein FtsY) | 7 | InterPro, Pfam, PDB, Phyre2, EggNOG* (Chromatin structure and dynamics: DNA binding, bending), panX, Foldseek |
| ehv001 | accessory (5 strains) | Recombination endonuclease VII domain-containing protein | 6 | InterPro, Pfam, PDB, Phyre2, Uniclust, Foldseek |
| ehv091 | accessory (12 strains) | Zinc finger, C3HC4 RING-type | 6 | InterPro, Pfam, PDB, Phyre2, Uniclust, Foldseek |
| ehv403 | core | Poxvirus VLTF3 | 6 | InterPro, Pfam, EggNOG, Uniclust, Ku et al. [16], Foldseek |
| ehv463 | accessory (9 strains) | NFACT protein RNA-binding domain-containing protein (can show up in fibronectin-/fibrinogen-binding proteins by similarity) | 6 | Pfam, PDB, Uniclust, panX, Ku et al. [16], Foldseek |
| ehv355 | core | Phytanoyl-CoA dioxygenase domain-containing protein (Uniclust annotated as ricin B-type lectin domain-containing protein) | 6 | InterPro, Pfam, PDB, Phyre2, EggNOG* (acid phosphatase activity), Foldseek |
| ehv458 | core | RNA polymerase Rpb6 | 6 | InterPro, PDB*(RNA polymerase Rpb8), Phyre2, Uniclust* (DNA-directed RNA polymerase I II), panX, Foldseek |
| ehv058 | core | Helicase C-like | 5 | InterPro, Pfam, PDB, EggNOG, Foldseek |
| ehv177 | accessory (10 strains) | Zinc finger, RING-type | 5 | InterPro, PDB, Phyre2, Uniclust, Foldseek |
| ehv387 | core | Telomere resolvase | 5 | InterPro, Pfam, PDB, Ku et al. [16], Foldseek |
| ehv367 | core | SEC-C motif-containing protein | 5 | InterPro, Pfam, PDB, Phyre2* (low alignment coverage), Foldseek |
| ehv055 | core | Heliorhodopsin | 5 | InterPro, Pfam, Phyre2, PDB* (rhodopsin), Foldseek |
| ehv078 | core | Heliorhodopsin | 5 | InterPro, Pfam, Phyre2, PDB* (rhodopsin), Foldseek |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobb, B.; Shapter, A.; Doxey, A.C.; Nissimov, J.I. Functional Profiling and Evolutionary Analysis of a Marine Microalgal Virus Pangenome. Viruses 2023, 15, 1116. https://doi.org/10.3390/v15051116
Lobb B, Shapter A, Doxey AC, Nissimov JI. Functional Profiling and Evolutionary Analysis of a Marine Microalgal Virus Pangenome. Viruses. 2023; 15(5):1116. https://doi.org/10.3390/v15051116
Chicago/Turabian StyleLobb, Briallen, Anson Shapter, Andrew C. Doxey, and Jozef I. Nissimov. 2023. "Functional Profiling and Evolutionary Analysis of a Marine Microalgal Virus Pangenome" Viruses 15, no. 5: 1116. https://doi.org/10.3390/v15051116
APA StyleLobb, B., Shapter, A., Doxey, A. C., & Nissimov, J. I. (2023). Functional Profiling and Evolutionary Analysis of a Marine Microalgal Virus Pangenome. Viruses, 15(5), 1116. https://doi.org/10.3390/v15051116







