Agua Salud Alphavirus Infection, Dissemination and Transmission in Aedes aegypti Mosquitoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquitoes
2.2. Virus Stocks and Titration
2.3. ASALV Transmission Experiments
2.3.1. Adult Infections through Feeding and Intrathoracic Injections
2.3.2. ASALV Dissemination
2.3.3. ASALV Horizontal Transmission
2.3.4. Salivation Assay
2.3.5. ASALV Vertical Transmission
2.4. ASALV Quantification
2.5. Polymerase Chain Reaction (PCR)
2.6. Small RNA Sequencing
2.7. Data Analyses
3. Results
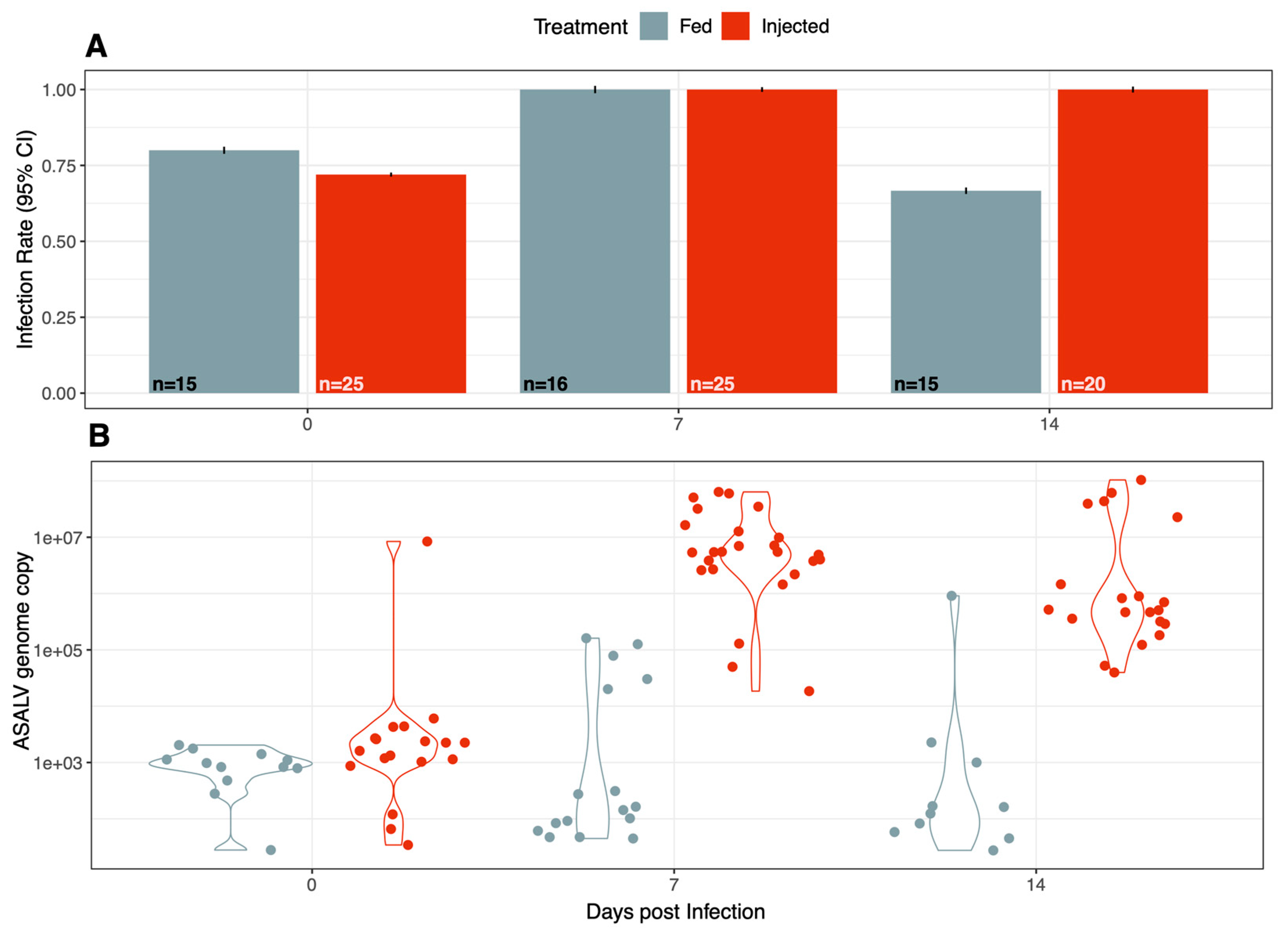
3.1. ASALV Infects and Replicates in Ae. aegypti
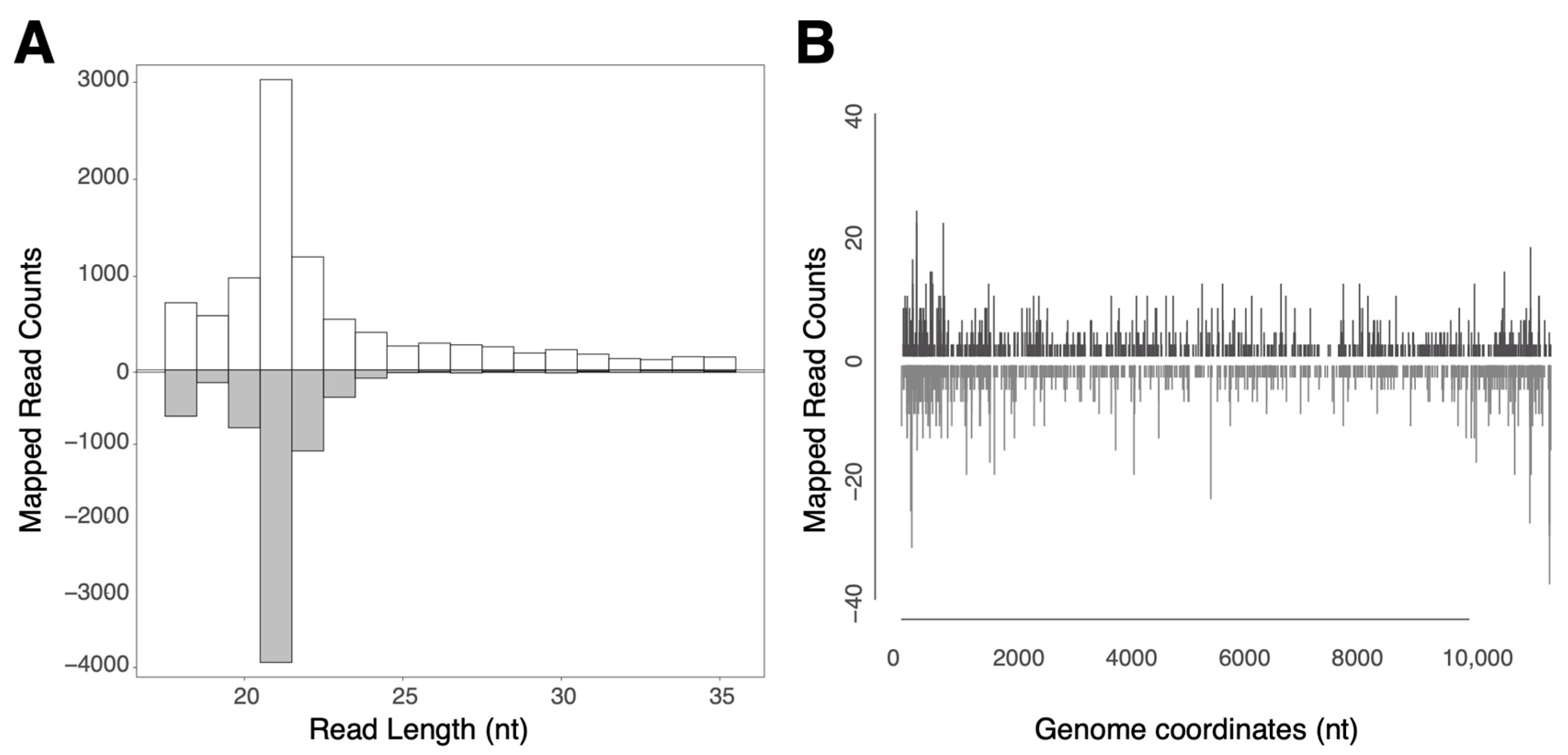
3.2. ASALV Interacts with the RNAi Response in the Midgut
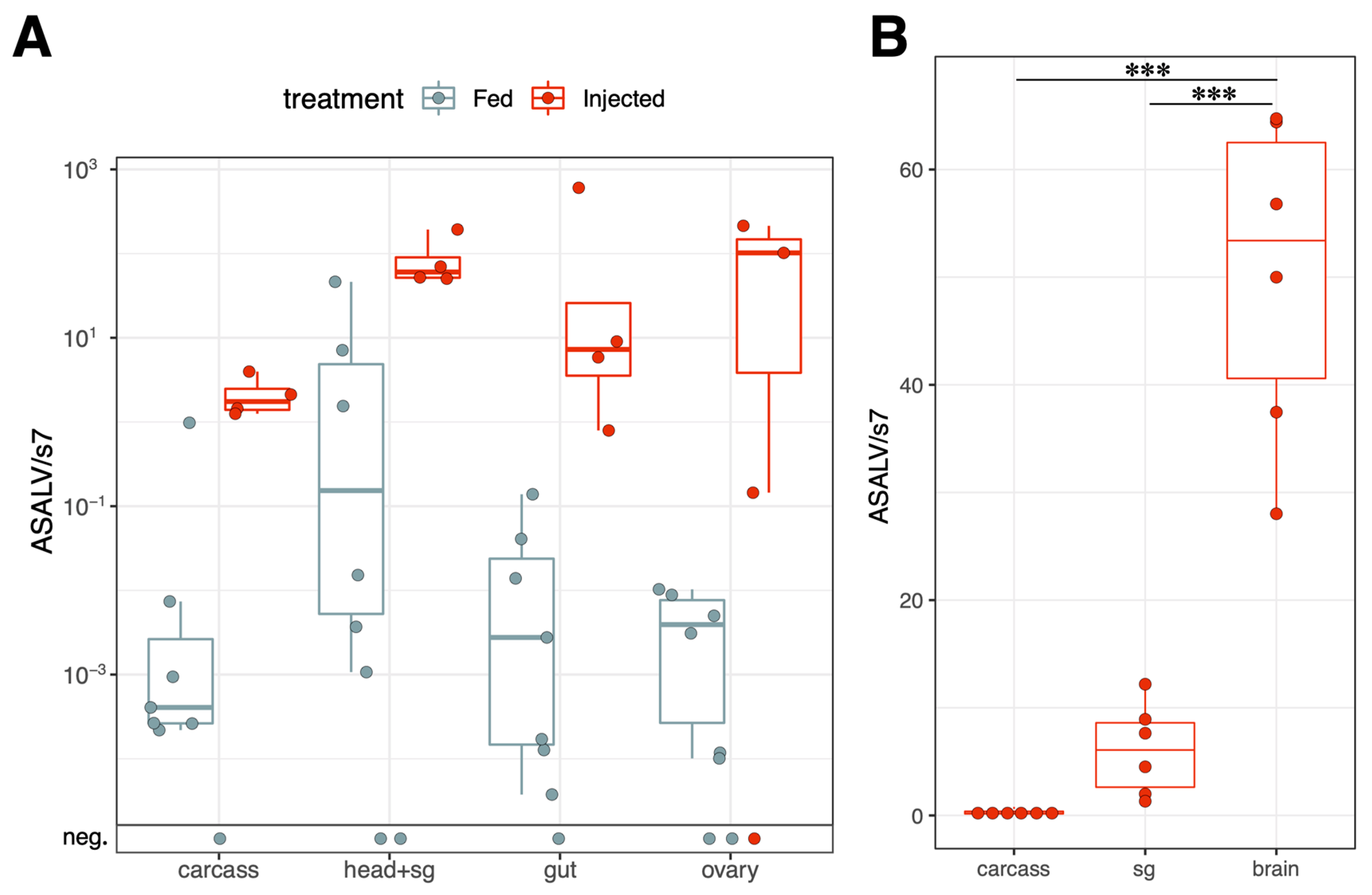
3.3. ASALV Disseminates to Different Tissues and Replicates Mostly in Heads
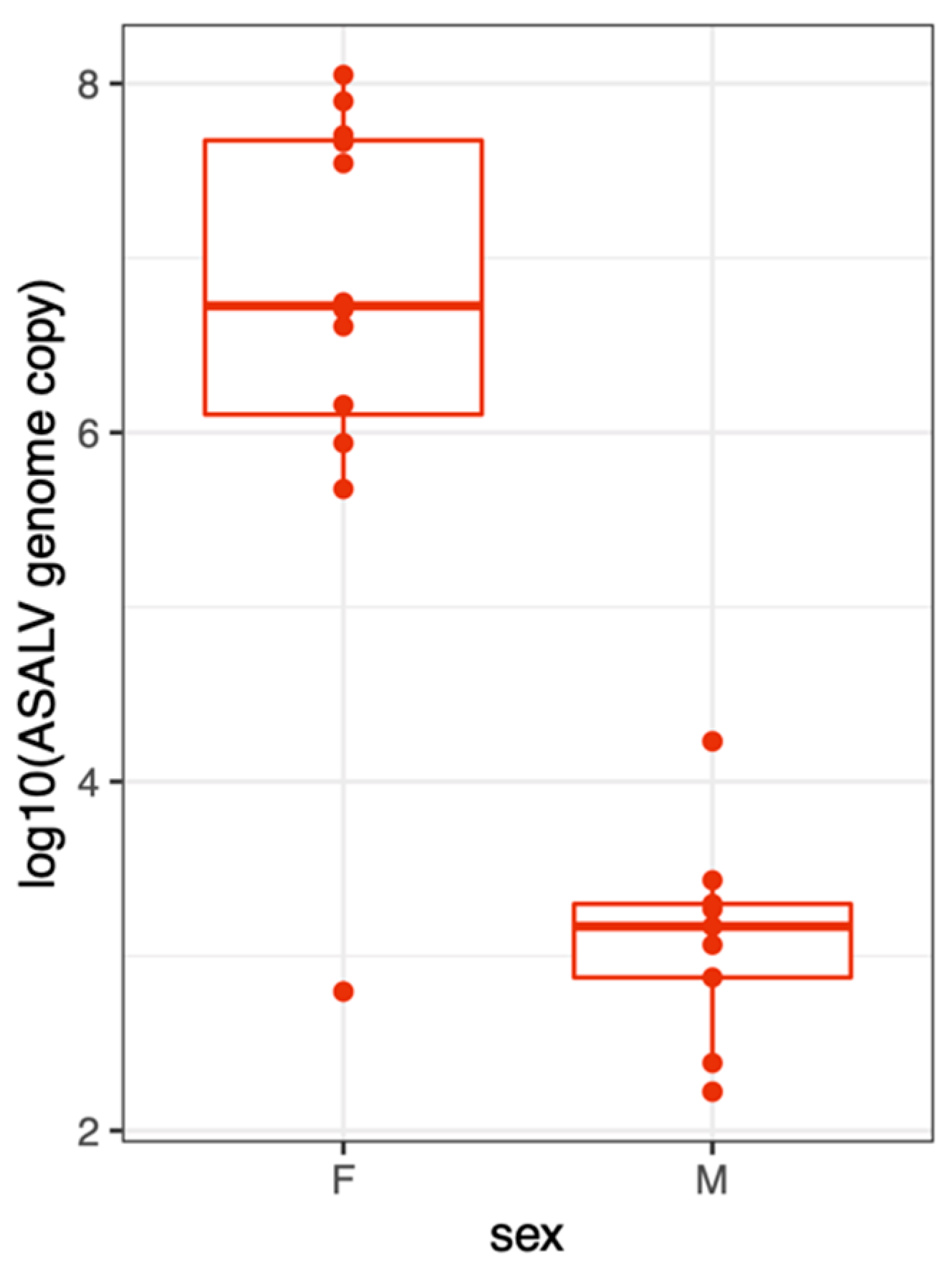
3.4. ASALV Transmission Routes in Ae. aegypti
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roundy, C.M.; Azar, S.R.; Rossi, S.L.; Weaver, S.C.; Vasilakis, N. Insect-Specific Viruses: A Historical Overview and Recent Developments. Adv. Virus Res. 2017, 98, 119–146. [Google Scholar] [CrossRef]
- Nouri, S.; Matsumura, E.E.; Kuo, Y.W.; Falk, B.W. Insect-Specific Viruses: From Discovery to Potential Translational Applications. Curr. Opin. Virol. 2018, 33, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Agboli, E.; Leggewie, M.; Altinli, M.; Schnettler, E. Mosquito-Specific Viruses—Transmission and Interaction. Viruses 2019, 11, 873. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Seymour, R.L.; Kaelber, J.T.; Kim, D.Y.; Leal, G.; Sherman, M.B.; Frolov, I.; Chiu, W.; Weaver, S.C.; Nasar, F. Novel Insect-Specific Eilat Virus-Based Chimeric Vaccine Candidates Provide Durable, Mono- and Multivalent, Single-Dose Protection against Lethal Alphavirus Challenge. J. Virol. 2018, 92, e01274-17. [Google Scholar] [CrossRef] [PubMed]
- Hobson-Peters, J.; Harrison, J.J.; Watterson, D.; Hazlewood, J.E.; Vet, L.J.; Newton, N.D.; Warrilow, D.; Colmant, A.M.G.; Taylor, C.; Huang, B.; et al. A Recombinant Platform for Flavivirus Vaccines and Diagnostics Using Chimeras of a New Insect-Specific Virus. Sci. Transl. Med. 2019, 11, eaax7888. [Google Scholar] [CrossRef]
- Bolling, B.G.; Olea-Popelka, F.J.; Eisen, L.; Moore, C.G.; Blair, C.D. Transmission Dynamics of an Insect-Specific Flavivirus in a Naturally Infected Culex Pipiens Laboratory Colony and Effects of Co-Infection on Vector Competence for West Nile Virus. Virology 2012, 427, 90–97. [Google Scholar] [CrossRef]
- Kent, R.J.; Crabtree, M.B.; Miller, B.R. Transmission of West Nile Virus by Culex Quinquefasciatus Say Infected with Culex Flavivirus Izabal. PLoS Negl. Trop. Dis. 2010, 4, e671. [Google Scholar] [CrossRef]
- Hobson-Peters, J.; Yam, A.W.Y.; Lu, J.W.F.; Setoh, Y.X.; May, F.J.; Kurucz, N.; Walsh, S.; Prow, N.A.; Davis, S.S.; Weir, R.; et al. A New Insect-Specific Flavivirus from Northern Australia Suppresses Replication of West Nile Virus and Murray Valley Encephalitis Virus in Co-Infected Mosquito Cells. PLoS ONE 2013, 8, e56534. [Google Scholar] [CrossRef]
- Schultz, M.J.; Frydman, H.M.; Connor, J.H. Dual Insect Specific Virus Infection Limits Arbovirus Replication in Aedes Mosquito Cells. Virology 2018, 518, 406–413. [Google Scholar] [CrossRef]
- Romo, H.; Kenney, J.L.; Blitvich, B.J.; Brault, A.C. Restriction of Zika Virus Infection and Transmission in Aedes aegypti Mediated by an Insect-Specific Flavivirus. Emerg. Microbes Infect. 2018, 7, 181. [Google Scholar] [CrossRef]
- Hall-Mendelin, S.; McLean, B.J.; Bielefeldt-Ohmann, H.; Hobson-Peters, J.; Hall, R.A.; Van Den Hurk, A.F. The Insect-Specific Palm Creek Virus Modulates West Nile Virus Infection in and Transmission by Australian Mosquitoes. Parasit. Vectors 2016, 9, 414. [Google Scholar] [CrossRef]
- Goenaga, S.; Kenney, J.L.; Duggal, N.K.; Delorey, M.; Ebel, G.D.; Zhang, B.; Levis, S.C.; Enria, D.A.; Brault, A.C. Potential for Co-Infection of a Mosquito-Specific Flavivirus, Nhumirim Virus, to Block West Nile Virus Transmission in Mosquitoes. Viruses 2015, 7, 5801–5812. [Google Scholar] [CrossRef]
- Altinli, M.; Schnettler, E.; Sicard, M. Symbiotic Interactions Between Mosquitoes and Mosquito Viruses. Front. Cell. Infect. Microbiol. 2021, 11, 694020. [Google Scholar] [CrossRef]
- Blitvich, B.J.; Firth, A.E. Insect-Specific Flaviviruses: A Systematic Review of Their Discovery, Host Range, Mode of Transmission, Superinfection Exclusion Potential and Genomic Organization. Viruses 2015, 7, 1927–1959. [Google Scholar] [CrossRef]
- Nasar, F.; Palacios, G.; Gorchakov, R.V.; Guzman, H.; Travassos Da Rosa, A.P.; Savji, N.; Popov, V.L.; Sherman, M.B.; Lipkin, W.I.; Tesh, R.B.; et al. Eilat Virus, a Unique Alphavirus with Host Range Restricted to Insects by RNA Replication. Proc. Natl. Acad. Sci. USA 2012, 109, 14622–14627. [Google Scholar] [CrossRef]
- Hermanns, K.; Zirkel, F.; Kopp, A.; Marklewitz, M.; Rwego, I.B.; Estrada, A.; Gillespie, T.R.; Drosten, C.; Junglen, S. Discovery of a Novel Alphavirus Related to Eilat Virus. J. Gen. Virol. 2017, 98, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Orba, Y.; Hang’ombe, B.M.; Mweene, A.S.; Wada, Y.; Anindita, P.D.; Phongphaew, W.; Qiu, Y.; Kajihara, M.; Mori-Kajihara, A.; et al. Discovery of Mwinilunga Alphavirus: A Novel Alphavirus in Culex Mosquitoes in Zambia. Virus Res. 2018, 250, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Batovska, J.; Buchmann, J.P.; Holmes, E.C.; Lynch, S.E. Coding-Complete Genome Sequence of Yada Yada Virus, a Novel Alphavirus Detected in Australian Mosquitoes. Microbiol. Resour. Announc. 2020, 9, e01476-19. [Google Scholar] [CrossRef]
- Hermanns, K.; Marklewitz, M.; Zirkel, F.; Overheul, G.J.; Page, R.A.; Loaiza, J.R.; Drosten, C.; van Rij, R.P.; Junglen, S. Agua Salud Alphavirus Defines a Novel Lineage of Insect-Specific Alphaviruses Discovered in the New World. J. Gen. Virol. 2020, 101, 96–104. [Google Scholar] [CrossRef]
- Nasar, F.; Haddow, A.D.; Tesh, R.B.; Weaver, S.C. Eilat Virus Displays a Narrow Mosquito Vector Range. Parasit. Vectors 2014, 7, 595. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.E.; Urakova, N.; Werling, K.L.; Metz, H.C.; Montanari, K.; Rasgon, J.L. Culex Tarsalis Is a Competent Host of the Insect-Specific Alphavirus Eilat Virus (EILV). bioRxiv 2022. [Google Scholar] [CrossRef]
- Nasar, F.; Erasmus, J.H.; Haddow, A.D.; Tesh, R.B.; Weaver, S.C. Eilat Virus Induces Both Homologous and Heterologous Interference. Virology 2015, 484, 51–58. [Google Scholar] [CrossRef]
- Altinli, M.; Leggewie, M.; Badusche, M.; Gyanwali, R.; Scherer, C.; Schulze, J.; Sreenu, V.B.; Fegebank, M.; Zibrat, B.; Fuss, J.; et al. Antiviral RNAi Response against the Insect-Specific Agua Salud Alphavirus. mSphere 2022, 7, e01003-21. [Google Scholar] [CrossRef] [PubMed]
- Donald, C.L.; Kohl, A.; Schnettler, E. New Insights into Control of Arbovirus Replication and Spread by Insect RNA Interference Pathways. Insects 2012, 3, 511–531. [Google Scholar] [CrossRef] [PubMed]
- Leggewie, M.; Schnettler, E. RNAi-Mediated Antiviral Immunity in Insects and Their Possible Application. Curr. Opin. Virol. 2018, 32, 108–114. [Google Scholar] [CrossRef]
- Liu, J.; Swevers, L.; Kolliopoulou, A.; Smagghe, G. Arboviruses and the Challenge to Establish Systemic and Persistent Infections in Competent Mosquito Vectors: The Interaction With the RNAi Mechanism. Front. Physiol. 2019, 10, 890. [Google Scholar] [CrossRef]
- Airs, P.M.; Bartholomay, L.C. Insects RNA Interference for Mosquito and Mosquito-Borne Disease Control. Insects 2017, 8, 4. [Google Scholar] [CrossRef]
- Hess, A.M.; Prasad, A.N.; Ptitsyn, A.; Ebel, G.D.; Olson, K.E.; Barbacioru, C.; Monighetti, C.; Campbell, C.L. Small RNA Profiling of Dengue Virus-Mosquito Interactions Implicates the PIWI RNA Pathway in Anti-Viral Defense. BMC Microbiol. 2011, 11, 45. [Google Scholar] [CrossRef]
- Scherer, C.; Knowles, J.; Sreenu, V.B.; Fredericks, A.C.; Fuss, J.; Maringer, K.; Fernandez-Sesma, A.; Merits, A.; Varjak, M.; Kohl, A.; et al. An Aedes aegypti-Derived Ago2 Knockout Cell Line to Investigate Arbovirus Infections. Viruses 2021, 13, 1066. [Google Scholar] [CrossRef]
- Miesen, P.; Joosten, J.; van Rij, R.P. PIWIs Go Viral: Arbovirus-Derived PiRNAs in Vector Mosquitoes. PLoS Pathog. 2016, 12, e1006017. [Google Scholar] [CrossRef] [PubMed]
- Varjak, M.; Leggewie, M.; Schnettler, E. The Antiviral PiRNA Response in Mosquitoes? J. Gen. Virol. 2018, 99, 1551–1562. [Google Scholar] [CrossRef]
- Rodhain, F.; Chungue, E.; Failloux, A.B.; Vazeille-Falcoz, M.; Mousson, L. Variation in Oral Susceptibility to Dengue Type 2 Virus of Populations of Aedes aegypti from the Islands of Tahiti and Moorea, French Polynesia. Am. J. Trop. Med. Hyg. 1999, 60, 292–299. [Google Scholar] [CrossRef]
- Hierholzer, J.C.; Killington, R.A. Virus Isolation and Quantitation. In Virology Methods Manual; Academic Press: Cambridge, MA, USA, 1996; pp. 25–46. [Google Scholar] [CrossRef]
- Varjak, M.; Maringer, K.; Watson, M.; Sreenu, V.B.; Fredericks, A.C.; Pondeville, E.; Donald, C.L.; Sterk, J.; Kean, J.; Vazeille, M.; et al. Aedes aegypti Piwi4 Is a Noncanonical PIWI Protein Involved in Antiviral Responses. mSphere 2017, 2, e00144-17. [Google Scholar] [CrossRef] [PubMed]
- Rückert, C.; Prasad, A.N.; Garcia-Luna, S.M.; Robison, A.; Grubaugh, N.D.; Weger-Lucarelli, J.; Ebel, G.D. Small RNA Responses of Culex Mosquitoes and Cell Lines during Acute and Persistent Virus Infection. Insect Biochem. Mol. Biol. 2019, 109, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Heinig-Hartberger, M.; Hellhammer, F.; Zöller, D.D.J.A.; Dornbusch, S.; Bergmann, S.; Vocadlova, K.; Junglen, S.; Stern, M.; Lee, K.-Z.; Becker, S.C. Culex Y Virus: A Native Virus of Culex Species Characterized In Vivo. Viruses 2023, 15, 235. [Google Scholar] [CrossRef]
- Franz, A.W.E.; Kantor, A.M.; Passarelli, A.L.; Clem, R.J. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; De Souza Caroci, A.; Ribolla, P.E.M.; De Bianchi, A.G.; Bijovsky, A.T. Functional Morphology of Adult Female Culex Quinquefasciatus Midgut during Blood Digestion. Tissue Cell 2002, 34, 210–219. [Google Scholar] [CrossRef]
- Li, J.; Dong, Y.; Sun, Y.; Lai, Z.; Zhao, Y.; Liu, P.; Gao, Y.; Chen, X.; Gu, J. A Novel Densovirus Isolated from the Asian Tiger Mosquito Displays Varied Pathogenicity Depending on Its Host Species. Front. Microbiol. 2019, 10, 1549. [Google Scholar] [CrossRef]
- Newman, C.M.; Anderson, T.K.; Goldberg, T.L. Decreased Flight Activity in Culex Pipiens (Diptera: Culicidae) Naturally Infected With Culex Flavivirus. J. Med. Entomol. 2016, 53, 233–236. [Google Scholar] [CrossRef]
- Lima-Camara, T.N.; Bruno, R.V.; Luz, P.M.; Castro, M.G.; Lourenço-de-Oliveira, R.; Sorgine, M.H.F.; Peixoto, A.A. Dengue Infection Increases the Locomotor Activity of Aedes aegypti Females. PLoS ONE 2011, 6, e17690. [Google Scholar] [CrossRef]
- Öhlund, P.; Lundén, H.; Blomström, A.L. Insect-Specific Virus Evolution and Potential Effects on Vector Competence. Virus Genes 2019, 55, 127. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.G.; Eisen, L.; Moore, C.G.; Blair, C.D. Insect-Specific Flaviviruses from Culex Mosquitoes in Colorado, with Evidence of Vertical Transmission. Am. J. Trop. Med. Hyg. 2011, 85, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Saiyasombat, R.; Bolling, B.G.; Brault, A.C.; Bartholomay, L.C.; Blitvich, B.J. Evidence of Efficient Transovarial Transmission of Culex Flavivirus by Culex Pipiens (Diptera: Culicidae). J. Med. Entomol. 2011, 48, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Lutomiah, J.J.L.; Mwandawiro, C.; Magambo, J.; Sang, R.C. Infection and Vertical Transmission of Kamiti River Virus in Laboratory Bred Aedes aegypti Mosquitoes. J. Insect Sci. Online 2007, 7, 55. [Google Scholar] [CrossRef]
- Logan, R.A.E.; Quek, S.; Muthoni, J.N.; von Eicken, A.; Brettell, L.E.; Anderson, E.R.; Villena, M.E.N.; Hegde, S.; Patterson, G.T.; Heinz, E.; et al. Vertical and Horizontal Transmission of Cell Fusing Agent Virus in Aedes aegypti. Appl. Environ. Microbiol. 2022, 88, e01062-22. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagtap, S.V.; Brink, J.; Frank, S.C.; Badusche, M.; Leggewie, M.; Sreenu, V.B.; Fuss, J.; Schnettler, E.; Altinli, M. Agua Salud Alphavirus Infection, Dissemination and Transmission in Aedes aegypti Mosquitoes. Viruses 2023, 15, 1113. https://doi.org/10.3390/v15051113
Jagtap SV, Brink J, Frank SC, Badusche M, Leggewie M, Sreenu VB, Fuss J, Schnettler E, Altinli M. Agua Salud Alphavirus Infection, Dissemination and Transmission in Aedes aegypti Mosquitoes. Viruses. 2023; 15(5):1113. https://doi.org/10.3390/v15051113
Chicago/Turabian StyleJagtap, Swati V., Jorn Brink, Svea C. Frank, Marlis Badusche, Mayke Leggewie, Vattipally B. Sreenu, Janina Fuss, Esther Schnettler, and Mine Altinli. 2023. "Agua Salud Alphavirus Infection, Dissemination and Transmission in Aedes aegypti Mosquitoes" Viruses 15, no. 5: 1113. https://doi.org/10.3390/v15051113
APA StyleJagtap, S. V., Brink, J., Frank, S. C., Badusche, M., Leggewie, M., Sreenu, V. B., Fuss, J., Schnettler, E., & Altinli, M. (2023). Agua Salud Alphavirus Infection, Dissemination and Transmission in Aedes aegypti Mosquitoes. Viruses, 15(5), 1113. https://doi.org/10.3390/v15051113






