Usefulness of Non-Skin Samples in the PCR Diagnosis of Mpox (Monkeypox)
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Recommends New Name for Monkeypox Disease. Available online: https://www.who.int/europe/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease (accessed on 15 December 2022).
- European Centre for Disease Prevention and Control (ECDPC). Monkeypox Multi-Country Outbreak. May 2022. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Monkeypox-multi-country-outbreak.pdf (accessed on 14 September 2022).
- Ministry of Health. Situation Report in Spain and Other Non-Endemic Countries. Available online: http://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/alertaMonkeypox/ (accessed on 5 January 2023).
- Li, Y.; Zhao, W.; Wilkins, K.; Hughes, C.; Damon, I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J. Virol. Meth. 2010, 169, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Happi, C.; Adetifa, I.M.O.; Mbala, P.; Njouom, R.; Nakouné, E.; Happi, A.; Ndodo, N.; Ayansola, O.T.; Mboowa, G.; Bedford, T.; et al. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. PLoS Biol. 2022, 20, e3001769. [Google Scholar] [CrossRef] [PubMed]
- Karagoz, A.; Tombuloglu, H.; Alsaeed, M.; Tombuloglu, G.; AlRubaish, A.A.; Mahmoud, A.; Smajlović, S.; Ćordić, S.; Rabaan, A.; Alsuhaimi, E. Monkeypox (mpox) virus: Classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis. J. Infect. Public Health 2023, 16, 531–541. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDPC). Factsheet for Health Professionals on Monkeypox October 2022. Available online: https://www.ecdc.europa.eu/en/all-topics-z/monkeypox/factsheet-health-professionals (accessed on 10 January 2023).
- World Health Organization. Monkeypox. Available online: https://www.who.int/health-topics/monkeypox#tab=tab_1 (accessed on 21 January 2023).
- Moore, M.; Zahra, F. Monkeypox [Updated 22 May 2022]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK574519/ (accessed on 17 October 2022).
- McCollum, A.M.; Damon, I.K. Human monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Shcherbakov, D.N.; Maksyutov, R.A.; Gavrilova, E.V. Species-specific identification of variola, monkeypox, cowpox, and vaccinia viruses by multiplex real-time PCR assay. J. Virol. Meth. 2011, 175, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Gul, I.; Liu, C.; Yuan, X.; Du, Z.; Zhai, S.; Lei, Z.; Chen, Q.; Raheem, M.A.; He, Q.; Hu, Q.; et al. Current and perspective sensing methods for Monkeypox virus. Bioengineering 2022, 9, 571. [Google Scholar] [CrossRef] [PubMed]
- Maksyutov, R.A.; Gavrilova, E.V.; Shchelkunov, S.N. Species-specific differentiation of variola, monkeypox, and varicella-zoster viruses by multiplex real-time PCR assay. J. Virol. Meth. 2016, 236, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wilkins, K.; McCollum, A.M.; Osadebe, L.; Kabamba, J.; Nguete, B.; Likafi, T.; Balilo, M.; Lushima, R.S.; Malekani, J.; et al. Evaluation of the GeneXpert for human monkeypox diagnosis. Am. J. Trop. Med. Hyg. 2017, 96, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Nalca, A.; Rimoin, A.W.; Bavari, S.; Whitehouse, C.A. Reemergence of monkeypox: Prevalence, diagnostics, and countermeasures. Clin. Infect. Dis. 2005, 41, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Gavrilova, E.V.; Babkin, I.V. Multiplex PCR detection and species differentiation of orthopoxviruses pathogenic to humans. Mol. Cell. Probes. 2005, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peiro-Mestres, A.; Fuertes, I.; Camprubí-Ferrer, D.; Marcos, M.A.; Vilella, A.; Navarro, M.; Rodriguez-Elena, L.; Riera, J.; Català, A.; Martínez, M.; et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022, 27, 2200503. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.K.; Madona, C.P.; Taylor, J.F.; Fourali, L.H.; Sehmi, J.K.; Stone, M.J.; Pond, M.; Cliff, P.; Pope, C. Performance evaluation of the Viasure PCR assay for the diagnosis of monkeypox: A multicentre study. J. Clin. Virol. 2022, 158, 105350. [Google Scholar] [CrossRef] [PubMed]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.; Rampling, T.; Beadsworth, M.; Duncan, C.J.A.; et al. NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 3099, 1–10. [Google Scholar] [CrossRef]
- Antinori, A.; Mazzotta, V.; Vita, S.; Carletti, F.; Tacconi, D.; Lapini, L.E.; D’Abramo, A.; Cicalini, S.; Lapa, D.; Pittalis, S.; et al. INMI Monkeypox Group. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Noe, S.; Zange, S.; Seilmaier, M.; Antwerpen, M.H.; Fenzl, T.; Schneider, J.; Spinner, C.; Bugert, J.; Wendtner, C.; Wölfel, R. Clinical and virological features of first human monkeypox cases in Germany. Infection 2023, 51, 265–270. [Google Scholar] [CrossRef] [PubMed]
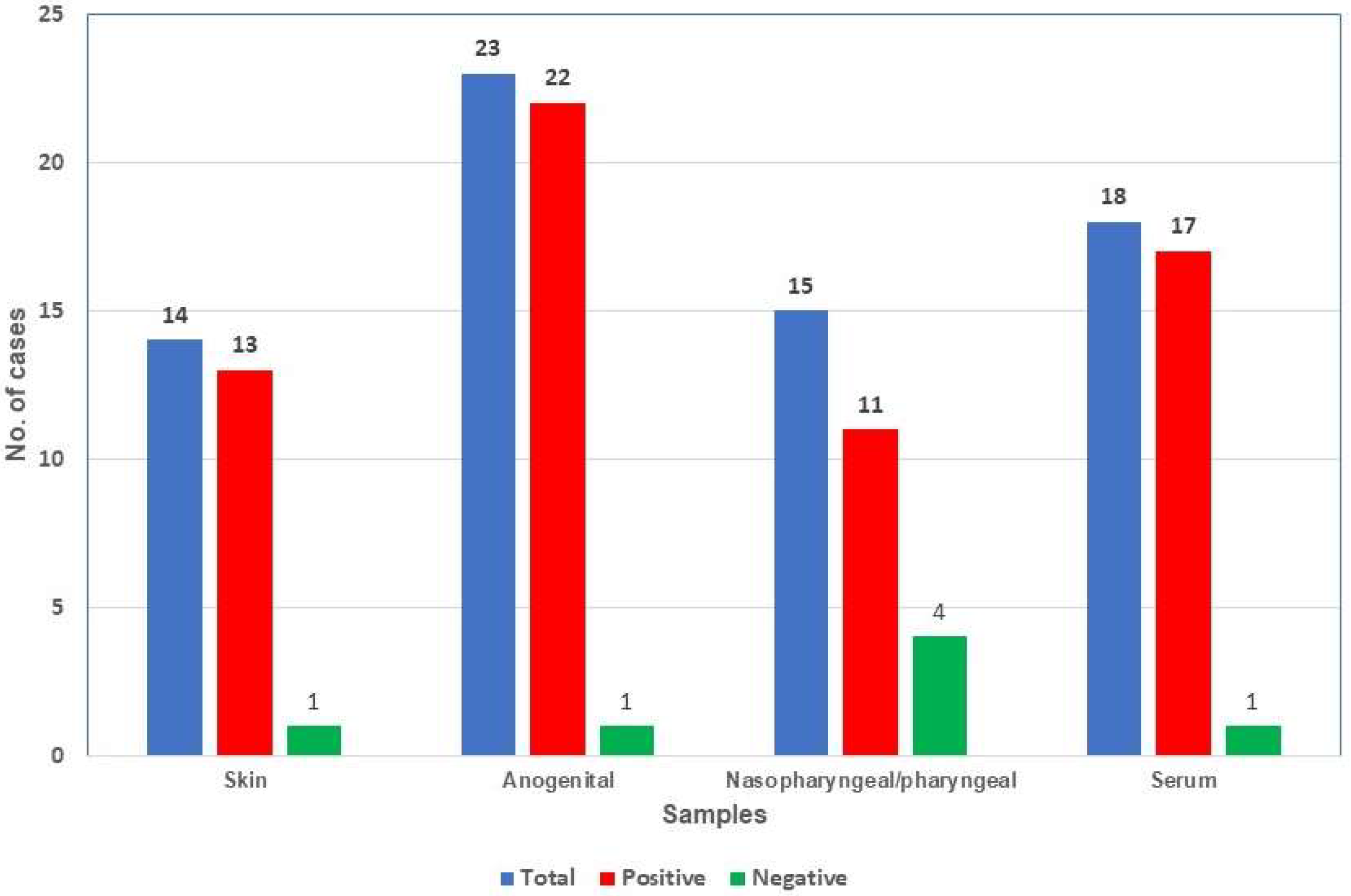
| Samples | No. | Days after the Onset of Symptoms (Mean) | Positive (%) | Mean Ct |
|---|---|---|---|---|
| Skin | 14 | 2–9 (4.9) | 13 (92.9) | 26.0 |
| Anogenital | 23 | 1–7 (3.7) | 22 (95.7) | 23.7 |
| Nasopharyngeal/Pharyngeal | 15 | 2–10 (4.8) | 11 (78.6) | 31.3 |
| Serum | 18 | 1–9 (5.1) | 17 (94.4) | 34.6 |
| Total | 70 | 1–10 (4.5) | 63 (90.0) | 28.4 |
| Case | Skin 1 | Skin 2 | Genital 1 | Genital 2 | Anal | NP | Pharyngeal | Serum | Days after the Onset of Symptoms |
|---|---|---|---|---|---|---|---|---|---|
| 3 | POS | NEG | POS | 3 | |||||
| 4 | POS | POS | 4 | ||||||
| 5 | POS | POS | POS | 6 | |||||
| 7 | POS | POS | POS | POS | 3 | ||||
| 8 | POS | POS | 6 | ||||||
| 9 | POS | POS | POS | NEG | 2 | ||||
| 10 | POS | POS | POS | 2 | |||||
| 13 | POS | POS | POS | 6 | |||||
| 14 | POS | POS * | 3 and 5 * | ||||||
| 21 | POS | POS | 7 | ||||||
| 22 | POS | NEG | POS | 4 | |||||
| 23 | POS | NEG ** | POS | 9 and 10 ** | |||||
| 24 | POS | POS | POS | 4 | |||||
| 26 | POS | POS | POS | POS | 4 | ||||
| 29 | POS | POS | POS | 7 | |||||
| 30 | POS | POS | NEG | NEG | 3 | ||||
| 31 | POS | POS | POS | 1 | |||||
| 36 | POS | POS | POS | 3 | |||||
| 43 | POS | POS *** | POS | 4 and 5 *** | |||||
| 46 | POS | POS | POS | 7 | |||||
| 47 | NEG | POS | POS | POS | 7 |
| Samples | No. | Negative (%) | Days after the Onset of Symptoms (Mean) |
|---|---|---|---|
| Skin | 18 | 18 (100) | 1–19 (5.4) |
| Anogenital | 8 | 8 (100) | 2–14 (9.6) |
| Nasopharyngeal | 3 | 3 (100) | 1–4 (2.0) |
| Serum | 7 | 7 (100) | 1–14 (8.6) |
| Total | 36 | 36 (100) | 1–19 (6.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algarate, S.; Bueno, J.; Crusells, M.J.; Ara, M.; Alonso, H.; Alvarado, E.; Ducons, M.; Arnal, S.; Benito, R. Usefulness of Non-Skin Samples in the PCR Diagnosis of Mpox (Monkeypox). Viruses 2023, 15, 1107. https://doi.org/10.3390/v15051107
Algarate S, Bueno J, Crusells MJ, Ara M, Alonso H, Alvarado E, Ducons M, Arnal S, Benito R. Usefulness of Non-Skin Samples in the PCR Diagnosis of Mpox (Monkeypox). Viruses. 2023; 15(5):1107. https://doi.org/10.3390/v15051107
Chicago/Turabian StyleAlgarate, Sonia, Jessica Bueno, María J. Crusells, Mariano Ara, Henar Alonso, Elena Alvarado, María Ducons, Sara Arnal, and Rafael Benito. 2023. "Usefulness of Non-Skin Samples in the PCR Diagnosis of Mpox (Monkeypox)" Viruses 15, no. 5: 1107. https://doi.org/10.3390/v15051107
APA StyleAlgarate, S., Bueno, J., Crusells, M. J., Ara, M., Alonso, H., Alvarado, E., Ducons, M., Arnal, S., & Benito, R. (2023). Usefulness of Non-Skin Samples in the PCR Diagnosis of Mpox (Monkeypox). Viruses, 15(5), 1107. https://doi.org/10.3390/v15051107






