Molecular Epidemiology of SARS-CoV-2 Omicron Sub-Lineages Isolated from Turkish Patients Infected with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
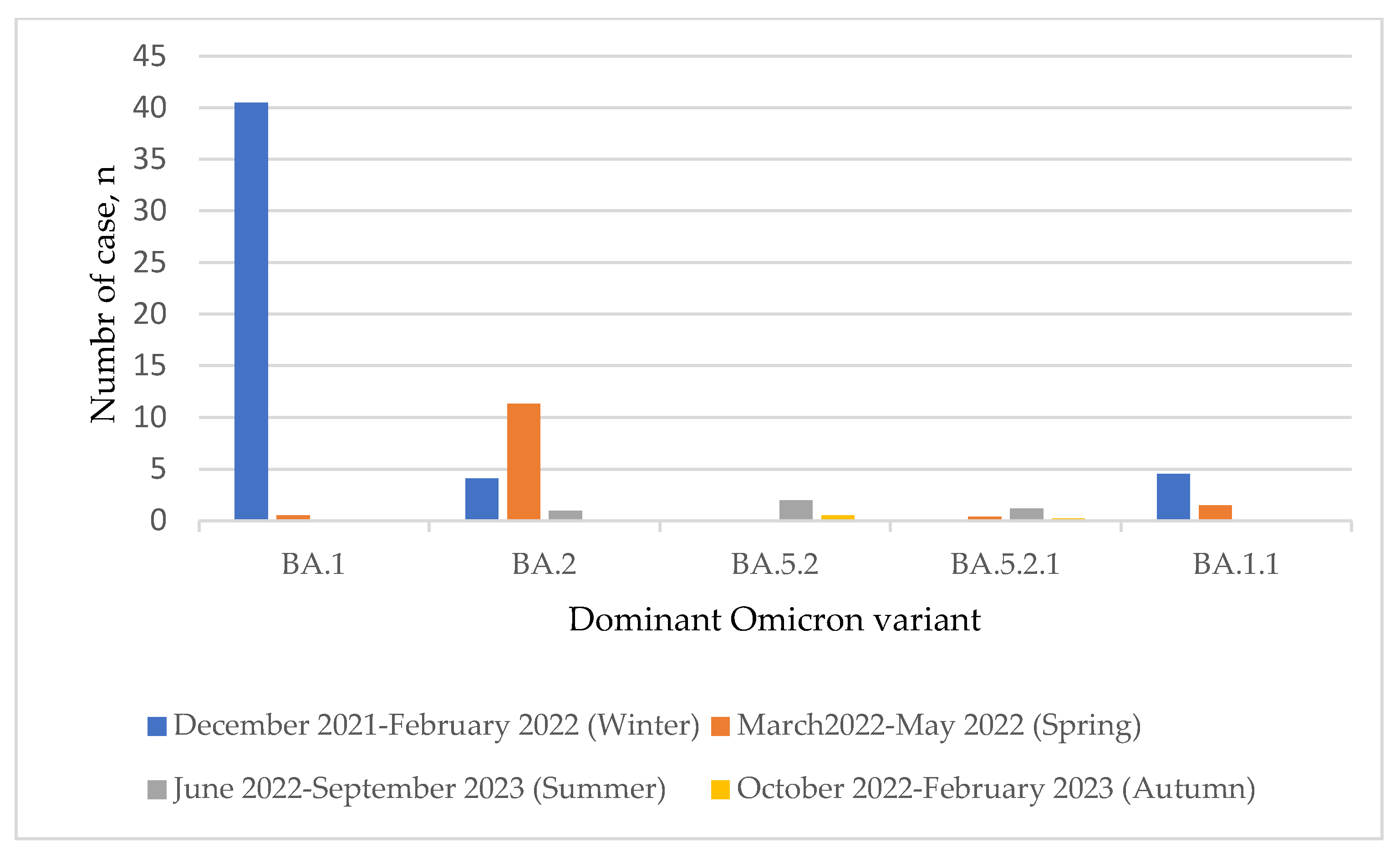
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Wang, D.; Abbas, J.; Duan, K.; Mubeen, R. Global financial crisis, smart lockdown strategies, and the COVID-19 spillover impacts: A global perspective implications from Southeast Asia. Front. Psychiatry 2021, 12, 643783. [Google Scholar] [CrossRef]
- Worlometer’s COVID-19 Data. Available online: https://www.worldometers.info/coronavirus/? (accessed on 13 March 2023).
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 7 March 2023).
- Nextrain. Genomic Epidemiology of SARS-CoV-2 with Subsampling Focused Globally over the Past 6 Months. Available online: https://nextstrain.org/ncov/gisaid/global/6m (accessed on 15 December 2022).
- Hassan, M.A.-K.; Aliyu, S. Delayed Access to COVID-19 Vaccines: A Perspective on Low-income Countries in Africa. Int. J. Health Serv. 2022, 52, 323–329. [Google Scholar] [CrossRef]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematic modeling study. Lancet 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Moeller, N.H.; Shi, K.; Demir, Ö.; Belica, C.; Banerjee, S.; Yin, L.; Durfee, C.; Amaro, R.E.; Aihara, H. Structure and dynamics of SARS-CoV-2 proofreading exoribonuclease ExoN. Proc. Natl. Acad. Sci. USA 2022, 119, e2106379119. [Google Scholar] [CrossRef]
- Shamsi, A.; Mohammad, T.; Anwar, S.; Amani, S.; Khan, M.S.; Husain, F.M.; Rehman, T.; Islam, A.; Hassan, I. Potential drug targets of SARS-CoV-2: From genomics to therapeutics. Int. J. Biol. Macromol. 2021, 177, 1–9. [Google Scholar] [CrossRef]
- Rajpal, V.R.; Sharma, S.; Sehgal, D.; Singh, A.; Kumar, A.; Vaishnavi, S.; Tiwari, M.; Bhalla, H.; Goel, S.; Raina, S.N. A comprehensive account of SARS-CoV-2 genome structure, incurred mutations, lineages and COVID-19 vaccination program. Future Virol. 2022, 17, 687–706. [Google Scholar] [CrossRef]
- Lv, Z.; Cano, K.E.; Jia, L.; Drag, M.; Huang, T.T.; Olsen, S.K. Targeting SARS-CoV-2 proteases for COVID-19 antiviral development. Front. Chem. 2022, 9, 819165. [Google Scholar] [CrossRef]
- Ghanbari, R.; Teimoori, A.; Sadeghi, A.; Mohamadkhani, A.; Rezasoltani, S.; Asadi, E.; Jouyban, A.; Sumner, S.C. Existing antiviral options against SARS-CoV-2 replication in COVID-19 patients. Future Virol. 2022, 15, 1747–1748. [Google Scholar] [CrossRef]
- Mishra, A.; Rathore, A.S. RNA-dependent RNA polymerase (RdRp) as a drug target for SARS-CoV-2. J. Biomol. Struct. Dyn. 2022, 40, 6039–6051. [Google Scholar] [CrossRef]
- Khater, S.; Kumar, P.; Dasgupta, N.; Das, G.; Ray, S.; Prakash, A. Combining SARS-CoV-2 proofreading exonuclease and RNA-dependent RNA polymerase inhibitors as a strategy to combat COVID-19: A high throughput in silico screening. Front. Microbiol. 2021, 12, 647693. [Google Scholar] [CrossRef]
- Rona, G.; Zeke, A.; Miwatani-Minter, B.; de Vries, M.; Kaur, R.; Schinlever, A.; Garcia, S.F.; Goldberg, H.V.; Wang, H.; Hinds, T.R.; et al. The NSP14/NSP10 RNA repair complex as a Pancirinavirus therapeutic target. Cell Death Differ. 2022, 29, 285–292. [Google Scholar] [CrossRef]
- The Food and Drug Administration (FDA) Coronavirus Drugs. Available online: https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs (accessed on 11 September 2022).
- The Food and Drug Administration (FDA) Approves the First Treatment for COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 22 October 2022).
- Tian, L.; Qiang, T.; Liang, C.; Ren, X.; Jia, M.; Zhang, J.; Li, J.; Wan, M.; YuWen, X.; Li, H.; et al. RNA-dependent RNA polymerase (RdRp) inhibitors: The current landscape and repurposing for the COVID-19 pandemic. Eur. J. Med. Chem. 2021, 213, 113201. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, C.Z.; Gorshkov, K.; Xu, M.; Lo, D.C.; Zheng, W. RNA-dependent RNA polymerase as a target for COVIS-19 drug discovery. SLAS Discov. 2020, 25, 1141–1151. [Google Scholar] [CrossRef]
- The Food and Drug Administration (FDA) Emergency Use Authorization. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs (accessed on 3 October 2022).
- Mali, K.R.; Eerike, M.; Raj, G.M.; Bisoi, D.; Priyadarshini, R.; Ravi, G.; Chaliserry, L.F.; Janti, S.S. Efficacy and safety of Molnupiravir in COVID-19 patients: A systematic review. Ir. J. Med. Sci. 2022, 1971, 1–14. [Google Scholar] [CrossRef]
- Sun, F.; Lin, Y.; Wang, X.; Gao, Y.; Ye, S. Pxovid in patients who are immunocompromised and hospitalized with SARS-CoV-2. Lancet 2022, 22, P1279. [Google Scholar] [CrossRef]
- Singh, M.; de Wit, E. Antiviral agents for the treatment of COVID-19: Progress and challenges. Cell Rep. Med. 2022, 3, 100549. [Google Scholar] [CrossRef]
- Arikan, A.; Sayan, M. Investigation of SARS-CoV-2 variants and their effects on SARS-CoV-2 monoclonal antibodies, convalescent and vaccine plasma by a novel web tool. Diagnostics 2022, 12, 2869. [Google Scholar] [CrossRef]
- Filippatos, C.; Ntanasis-Stathopoulos, I.; Sekeri, K.; Ntanasis-Stathopoulos, A.; Gavriatopoulou, M.; Psaltopoulou, T.; Dounias, G.; Sergentanis, T.N.; Terpos, E. Convalescent plasma therapy for COVID-19: A systematic review and meta-analysis of randomized controlled trials. Viruses 2023, 15, 765. [Google Scholar] [CrossRef]
- Tzou, P.L.; Tao, K.; Sahoo, M.K.; Pond, S.L.K.; Pinsky, B.A.; Shafer, R.W. Sierra SARS-CoV-2 sequence and antiviral resistance analysis program. J. Clin. Virol. 2022, 157, 105323. [Google Scholar] [CrossRef]
- The World Health Organisation (WHO) Weekly Epidemiological Update on COVID-19—21 December 2022. Available online: https://www.who.int/publications/m/item/covid-19-weekly-epidemiological-update---21-december-2022#:~:text=In%20the%20last%2028%20days,deaths%20have%20been%20reported%20globally (accessed on 21 December 2022).
- COVID-19 Weekly Epidemiological Update—8 February 2023. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---8-february-2023 (accessed on 8 February 2023).
- Kuroda, T.; Nobori, H.; Fukao, K.; Baba, K.; Matsumoto, K.; Yoshida, S.; Tanaka, Y.; Watari, R.; Oka, R.; Kasai, Y.; et al. Efficacy comparison of 3CL protease inhibitors ensitrelvir and nirmatrelvir/r against SARS-CoV-2 in vitro and in vivo. J. Anticrob. Chemother. 2023, 78, 946–952. [Google Scholar] [CrossRef]
- Kawashima, S.; Matsui, Y.; Adachi, T.; Morikawa, Y.; Inoue, K.; Takebayashi, S.; Nobori, H.; Rokushima, M.; Tachibana, Y.; Kato, T. Ensitrelvir is effective against SARS-CoV-2 3CL protease mutants circulating globally. Biochem. Biophys. Res. Commun. 2023, 645, 132–136. [Google Scholar] [CrossRef]
- Moghadasi, S.A.; Heilmann, E.; Khalil, A.M.; Nnabuife, C.; Kearns, F.L.; Ye, C.; Moraes, S.N.; Costacurta, F.; Esler, M.A.; Aihara, H.; et al. Transmissible SARS-CoV-2 variants with resistance to clinical protease inhibitors. bioRxiv, 2022; in press. [Google Scholar] [CrossRef]
- Lan, S.; Neilsen, G.; Slack, R.L.; Cantara, W.A.; Emanuelli Castaner, A.; Lorson, Z.C.; Lulkin, N.D.; Zhang, H.; Lee, J.; Cilento, M.E.; et al. Nirmatreivir resistance in SARS-CoV-2 Omicron BA.1 and WA.1 replicons and escape strategies. bioRxiv, 2022; in press. [Google Scholar] [CrossRef]
- Iketani, S.; Mohri, H.; Culbertson, B.; Hong, S.J.; Duan, Y.; Luck, M.I.; Annavajhala, M.K.; Guo, Y.; Sheng, Z.; Uhlemann, A.-C.; et al. Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir. Nature 2022, 613, 558–564. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Lavrijsen, M.; Lamers, M.M.; de Vries, A.C.; Rottier, R.J.; Bruno, M.J.; Peppelenbosch, M.P.; Haagmans, B.L.; Pan, Q. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022, 32, 322–324. [Google Scholar] [CrossRef]

| SARS-CoV-2 Omicron Sub-Lineage | GISAID Sequence n, % |
|---|---|
| B.1 | 354 (1.68) |
| BA.1 | 7293 (34.7) |
| BA.2 | 6463 (30.7) |
| BA.4 | 93 (0.44) |
| BA.5 | 4953 (23.5) |
| BE.1 | 285 (1.35) |
| BF.1 | 21 (0.10) |
| BF.28 | 41 (0.19) |
| BF.5 | 138 (0.65) |
| BF.6 | 20 (0.09) |
| BF.7 | 49 (0.23) |
| BM.4.1 | 27 (0.12) |
| BN.1 | 62 (0.29) |
| BN.3.1 | 25 (0.11) |
| BQ.1 | 834 (3.97) |
| CK.1 | 27 (0.12) |
| CL.1 | 34 (0.16) |
| XBB.1 | 70 (0.33) |
| Others * | 191 (0.91) |
| Unassigned ** | 9 (0.04) |
| TOTAL | 20.959 (100) |
| SARS-CoV-2 Omicron Sub-lineage | RdRp Inhibitor * a.a Substitutions | CoV-RDB, n (%) | 3CLpro Inhibitor ** a.a Substitutions | CoV-RDB, n (%) |
|---|---|---|---|---|
| B.1.1.529 | - | M49M/K/R/T | 1 (0.8) | |
| BA.1 | A449V, C799C/G/R/S | 2 (10.5) | G15S, L50L/F/I/V, A191A/E/G/T/V, F305F/L | 8 (6.1) |
| BA.1.1 | - | L50F, H172Q | 2 (1.5) | |
| BA.1.1.1 | - | R188S | 1 (0.8) | |
| BA.1.14 | - | G143G/C/R/S, A194A/P/S/T, T304T/I/N/S | 2 (1.5) | |
| BA.1.14.1 | - | F140F/L, N142N/D/H/Y, E166E/A/G/V | 2 (1.5) | |
| BA.1.17 | - | L167L/F, R188S | 1 (0.8) | |
| BA.1.17.2 | - | A191A/E/G/V | 1 (0.8) | |
| BA.1.5 | V557V/I/L, E802E/D | 2 (10.5) | G15S, L50L/F/I/V, F140F/L, T190I | 3 (2.3) |
| BA.1.9 | - | L50L/F/I/V, C160C/F/S/Y, A191A/P/S/T, D248E | 2 (1.5) | |
| BA.2 | R285R/C/G/S, A449A/D/G/V, D484Y, E796E/A/G/V, E802E/A/G/V | 10 (52.6) | G15G/C/R/S, T21I/N/S, T45T/I/N/S, D48D/H/N/Y, M49I/M/T, L50C/L/F/I/S/Y/V, Y54C, F140F/I/L/V, N142S, G143G/C/R/S, S144L, C160C/F/S/Y, M165M/K/R/T, E166K/Q, L167L/F, A173T/V, H172R, V186V/A/D/G, R188S, Q189K, T190I, A191A/E/G/P/S/T/V, A194A/P/S/T, Q192Q/K/L/P/R, P252L, T304T/I/N/S, F305F/L | 67 (51.2) |
| BA.2.10 | - | T190I | 1 (0.8) | |
| BA.2.12 | V792I | 1 (5.3) | - | |
| BA.2.17 | - | Q189K | 1 (0.8) | |
| BA.2.18 | - | T45T/I/N/S, L50L/F/I/V | 1 (0.8) | |
| BA.2.23 | - | Q189K | 1 (0.8) | |
| BA.2.27 | - | F140F/L, A173A/D/G/V, A191A/E/G/V | 1 (0.8) | |
| BA.2.3 | - | M49M/K/R/T, R188RS, Q189Q/E/K | 2 (1.5) | |
| BA.2.5 | - | M49M/I, S144T, Q189K, T304I, F305F/L | 3 (2.3) | |
| BA.2.58 | D484D/H/N/Y | 1 (5.3) | D48D/H/N/Y, L50F, Y54C | 3 (2.3) |
| BA.2.9 | - | T45T/I/N/S, F140F/L, M165M/K/R/T, H172H/Q, F305F/L | 5 (3.8) | |
| BA.2.93 | - | Y54Y/C/F/S | 1 (0.8) | |
| BA.4.6 | - | L50F | 1 (0.8) | |
| BA.5.1 | - | L50F | 1 (0.8) | |
| BA.5.2 | - | T21I, M49I | 9 (6.9) | |
| BA.5.2.1 | - | T21T/I/N/S, M49M/K/R/T, L50L/F/I/V, Y54C | 5 (3.8) | |
| BA.5.2.2 | - | R188R/S, Q189Q/E/K | 1 (0.8) | |
| BA.5.6 | - | T21I | 1 (0.8) | |
| BF.5 | A449V | 2 (10.53) | - | |
| BQ.1 | - | T304I | 1 (0.76) | |
| CK.1 | - | T21I | 1 (0.76) | |
| Unassigned | E802D | 1 (5.26) | M49I, Q192L | 2 (1.53) |
| Total | 19 (100) | 131 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayan, M.; Arikan, A.; Sanlidag, E. Molecular Epidemiology of SARS-CoV-2 Omicron Sub-Lineages Isolated from Turkish Patients Infected with COVID-19. Viruses 2023, 15, 1066. https://doi.org/10.3390/v15051066
Sayan M, Arikan A, Sanlidag E. Molecular Epidemiology of SARS-CoV-2 Omicron Sub-Lineages Isolated from Turkish Patients Infected with COVID-19. Viruses. 2023; 15(5):1066. https://doi.org/10.3390/v15051066
Chicago/Turabian StyleSayan, Murat, Ayse Arikan, and Erdal Sanlidag. 2023. "Molecular Epidemiology of SARS-CoV-2 Omicron Sub-Lineages Isolated from Turkish Patients Infected with COVID-19" Viruses 15, no. 5: 1066. https://doi.org/10.3390/v15051066
APA StyleSayan, M., Arikan, A., & Sanlidag, E. (2023). Molecular Epidemiology of SARS-CoV-2 Omicron Sub-Lineages Isolated from Turkish Patients Infected with COVID-19. Viruses, 15(5), 1066. https://doi.org/10.3390/v15051066






