Upper Respiratory Infection Drives Clinical Signs and Inflammatory Responses Following Heterologous Challenge of SARS-CoV-2 Variants of Concern in K18 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Next-Generation Sequencing
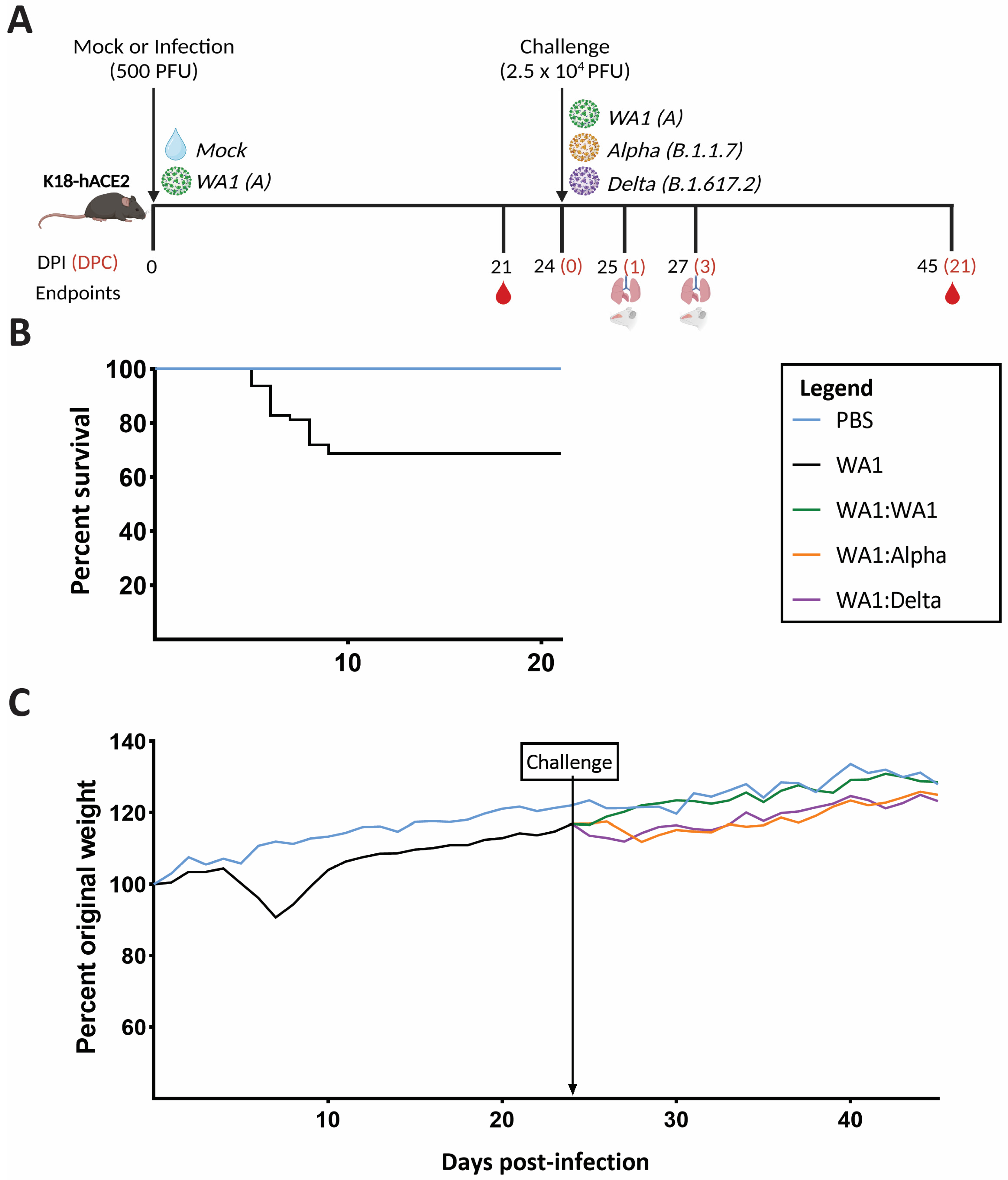
2.2. General Animal Information and Study Design
2.3. RNA-Seq
2.4. RT-qPCR
2.5. Plaque Reduction Neutralization Test
2.6. Bioinformatics
2.7. Statistics
3. Results
3.1. A Low Dose of SARS-CoV-2 Confers Greater Protection in Homologous- versus Heterologous-Challenged Mice
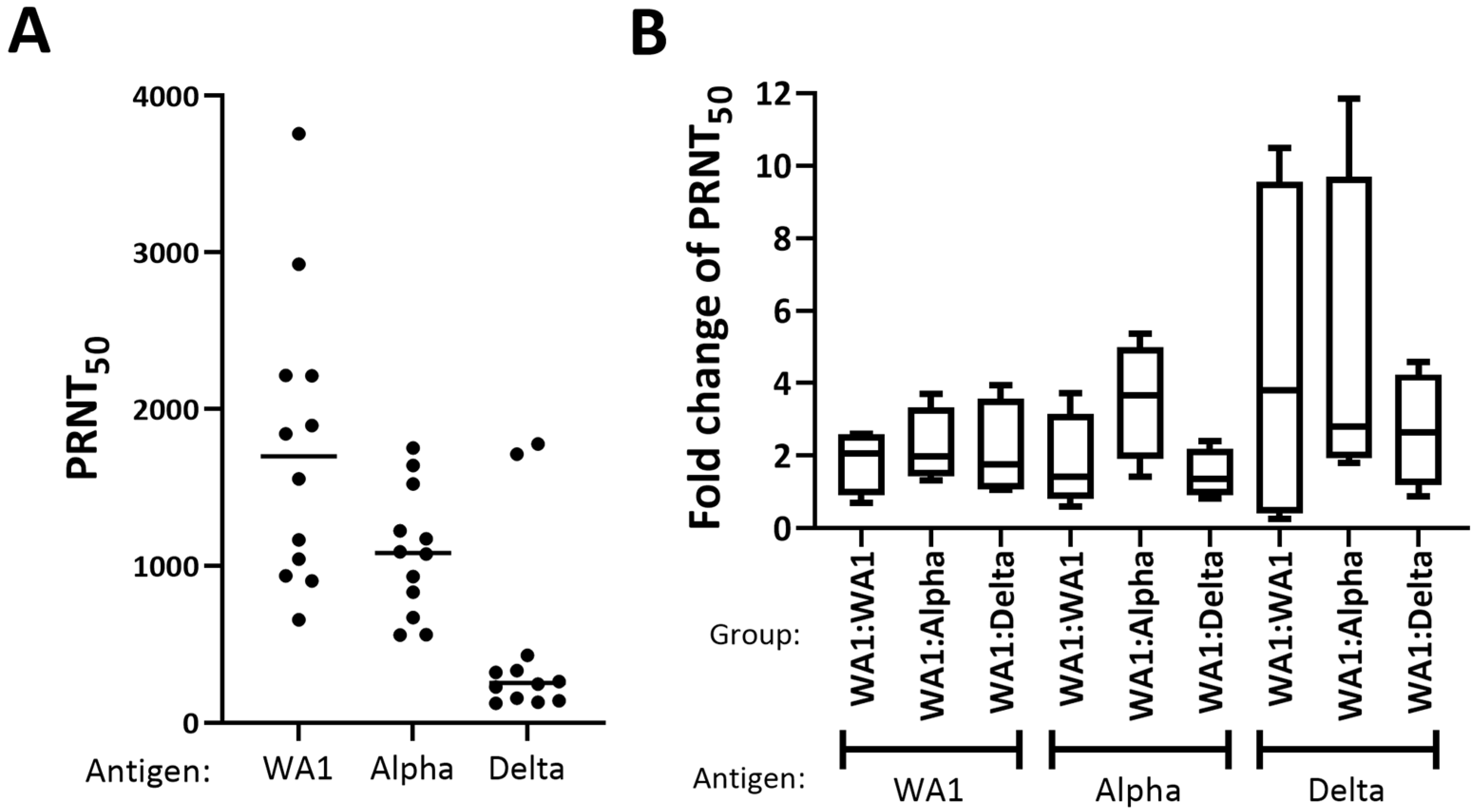
3.2. WA1, Alpha, and Delta Showed a Similar Boost in Neutralization Titer
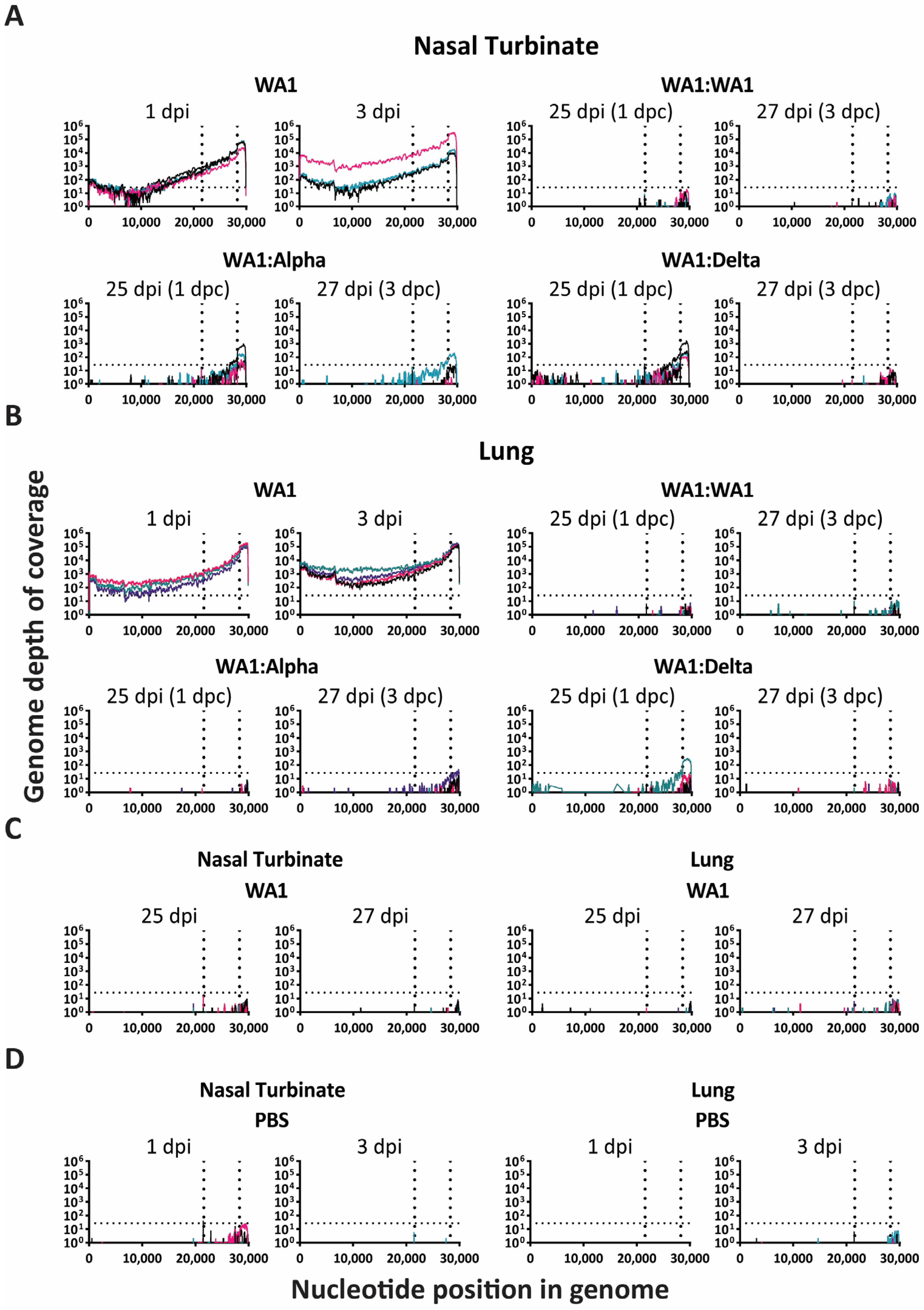
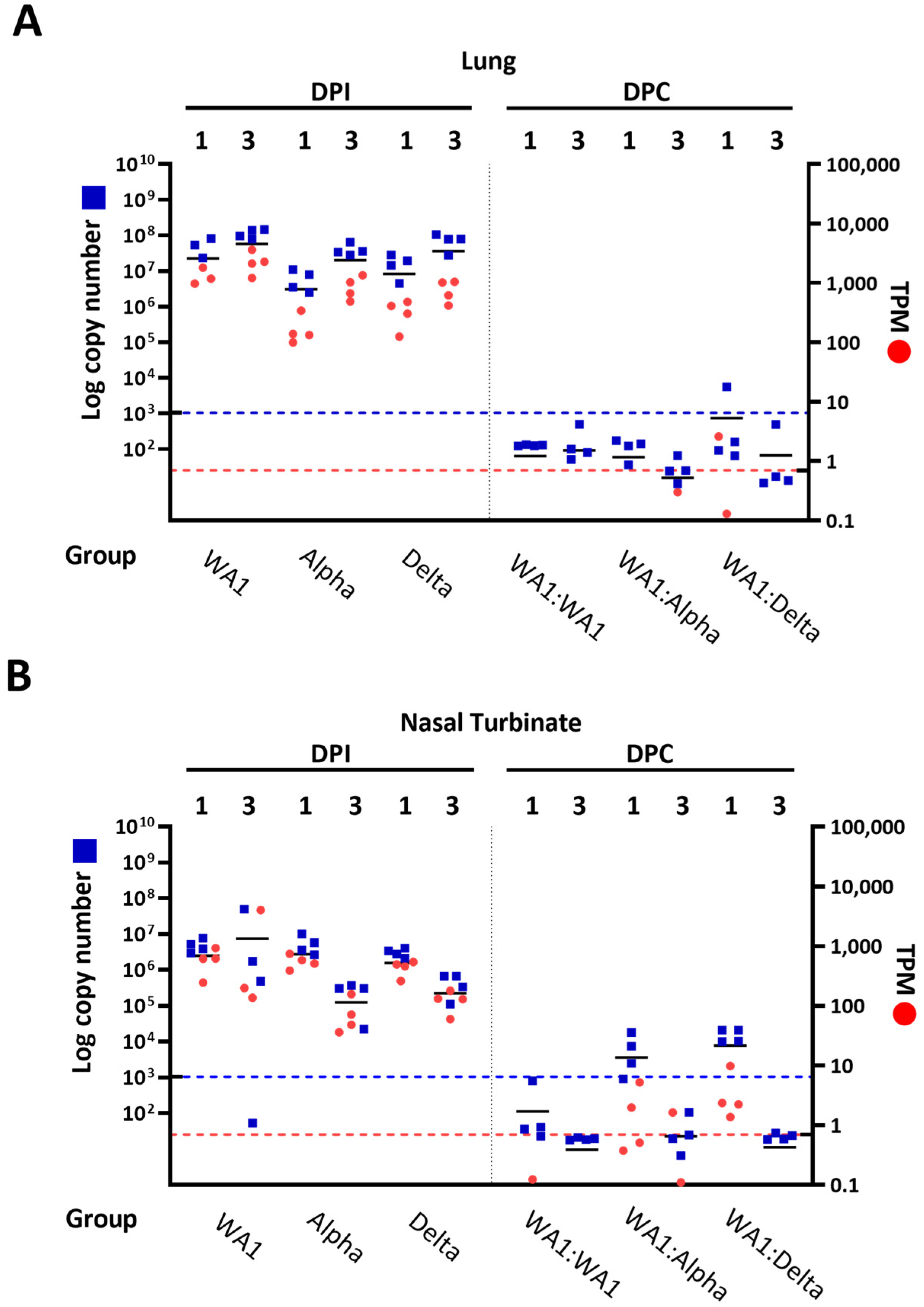
3.3. RNA-Seq Analysis of Viral Genomic RNA Showed Greater Replication in Nasal Turbinates Compared to Lungs of Infected: Challenged Mice
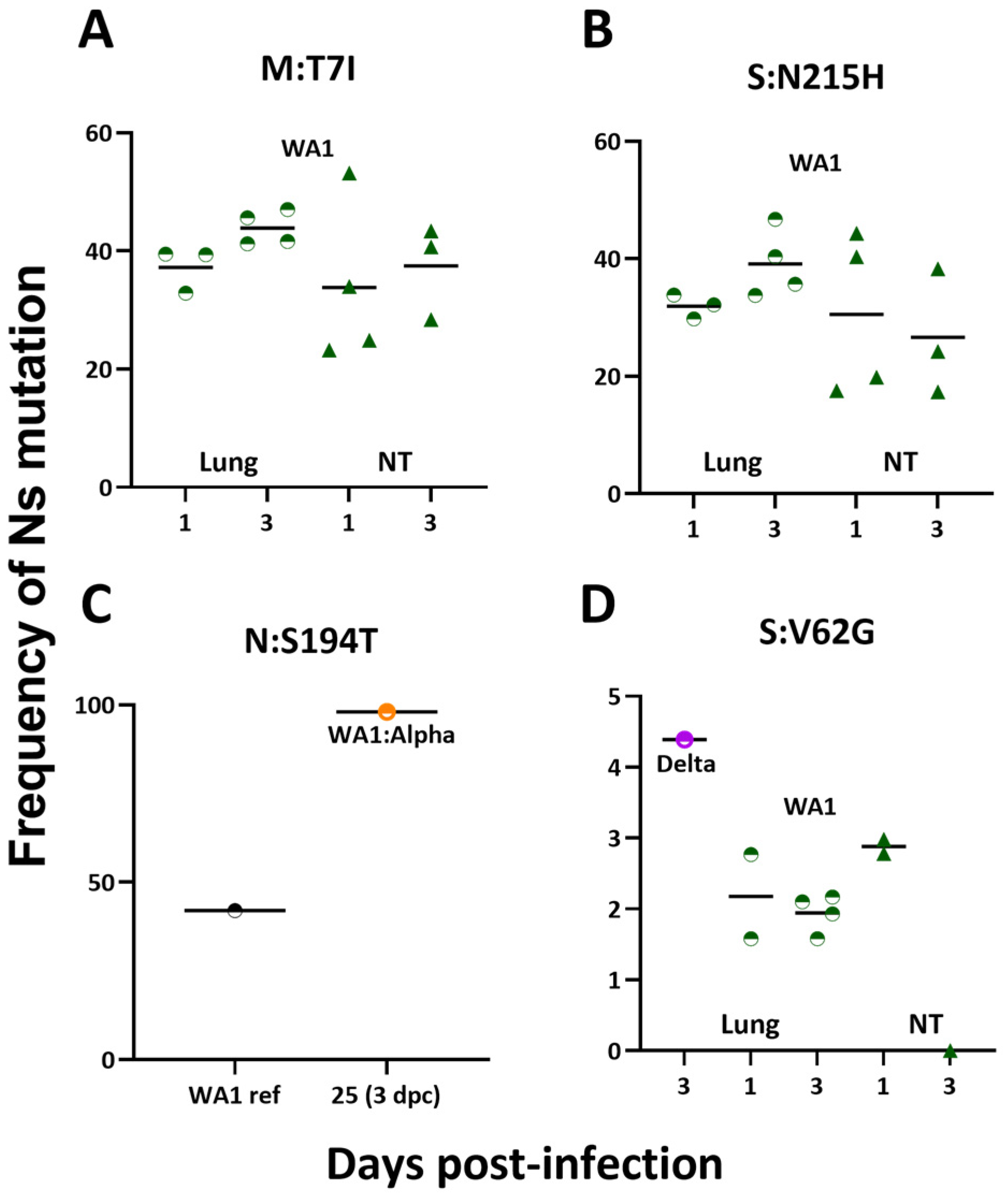
3.4. Nonsynonymous Mutations Emerged Early in Infection-Only Mice in Lungs and Nasal Turbinates
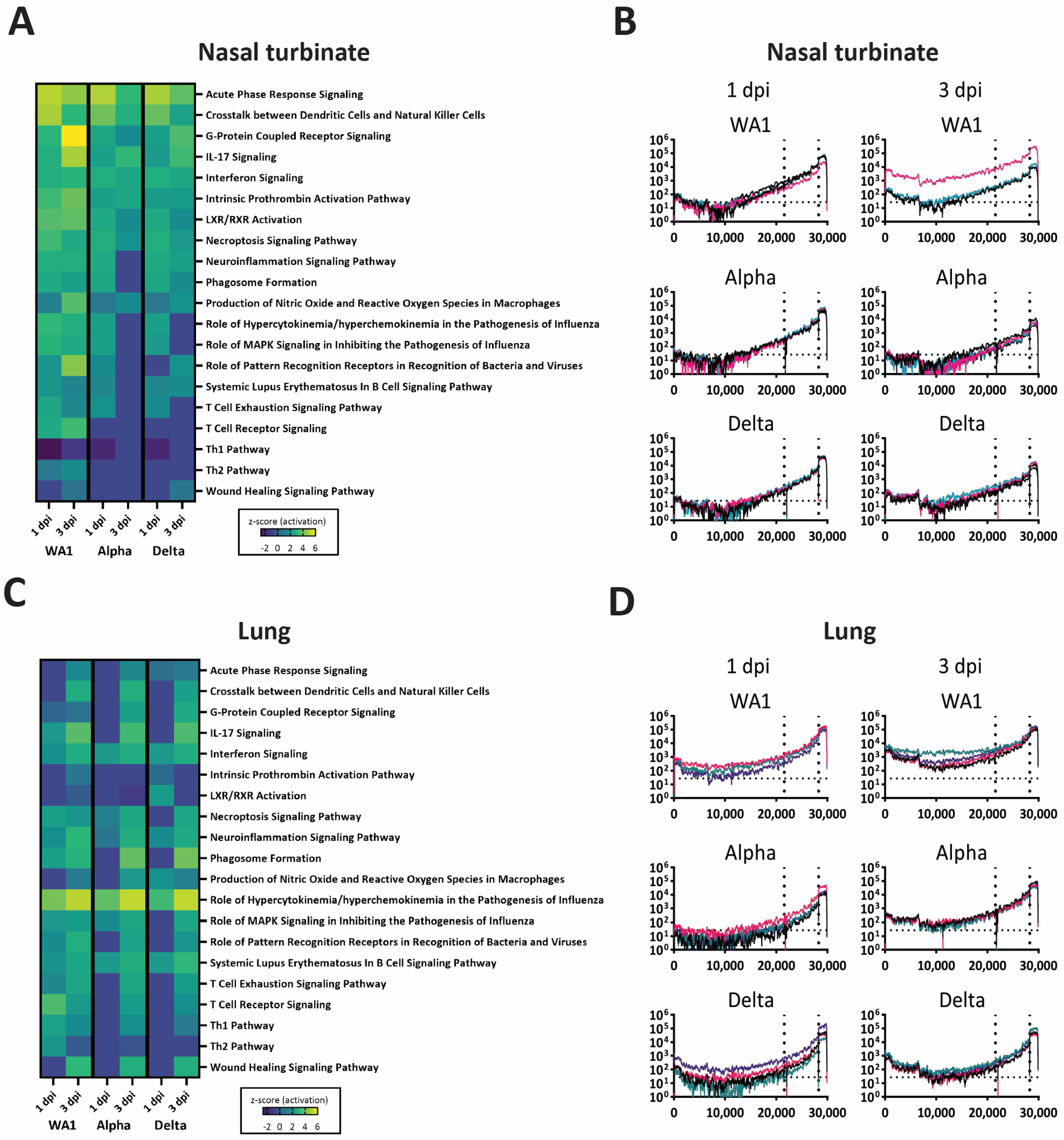
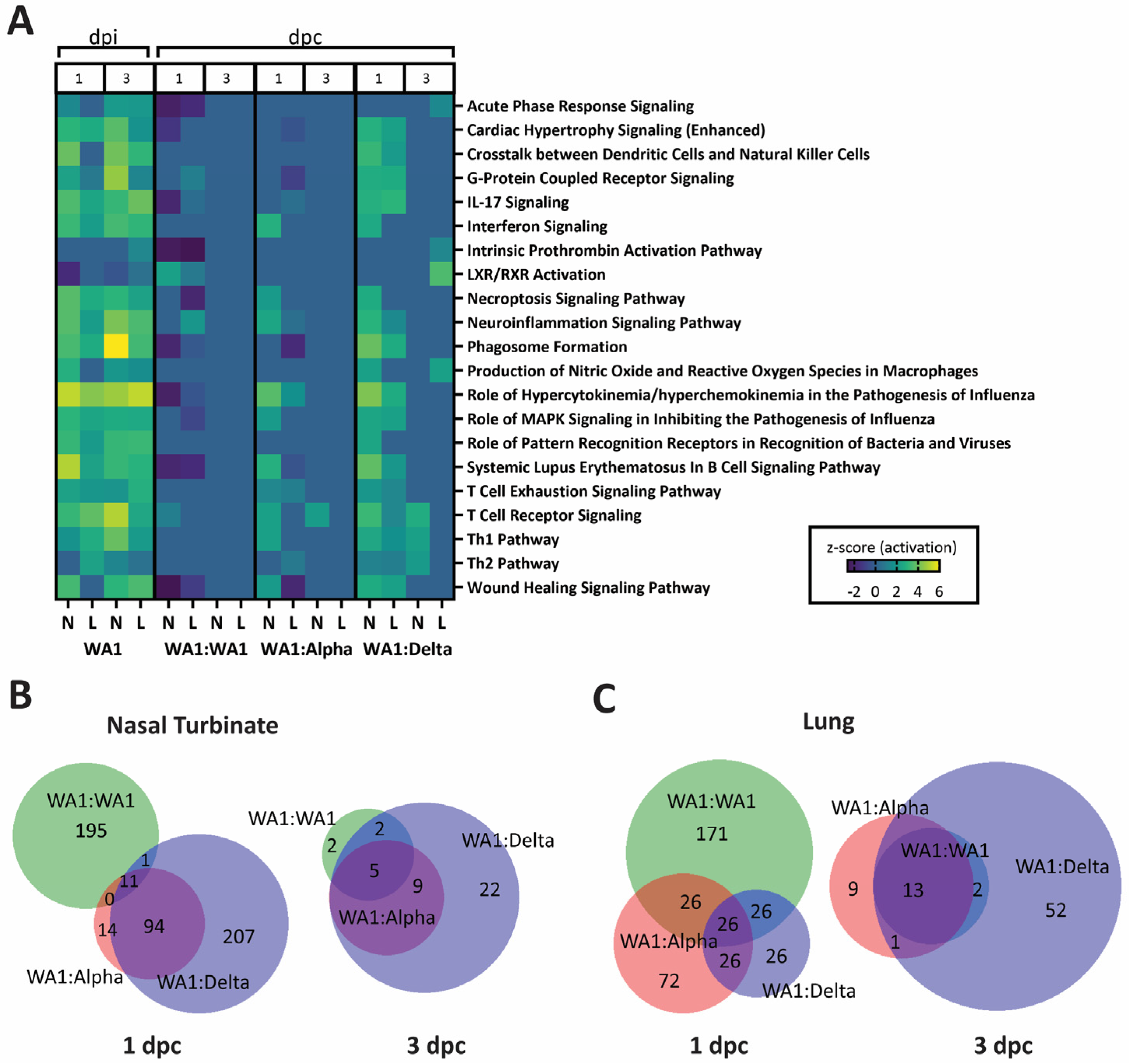
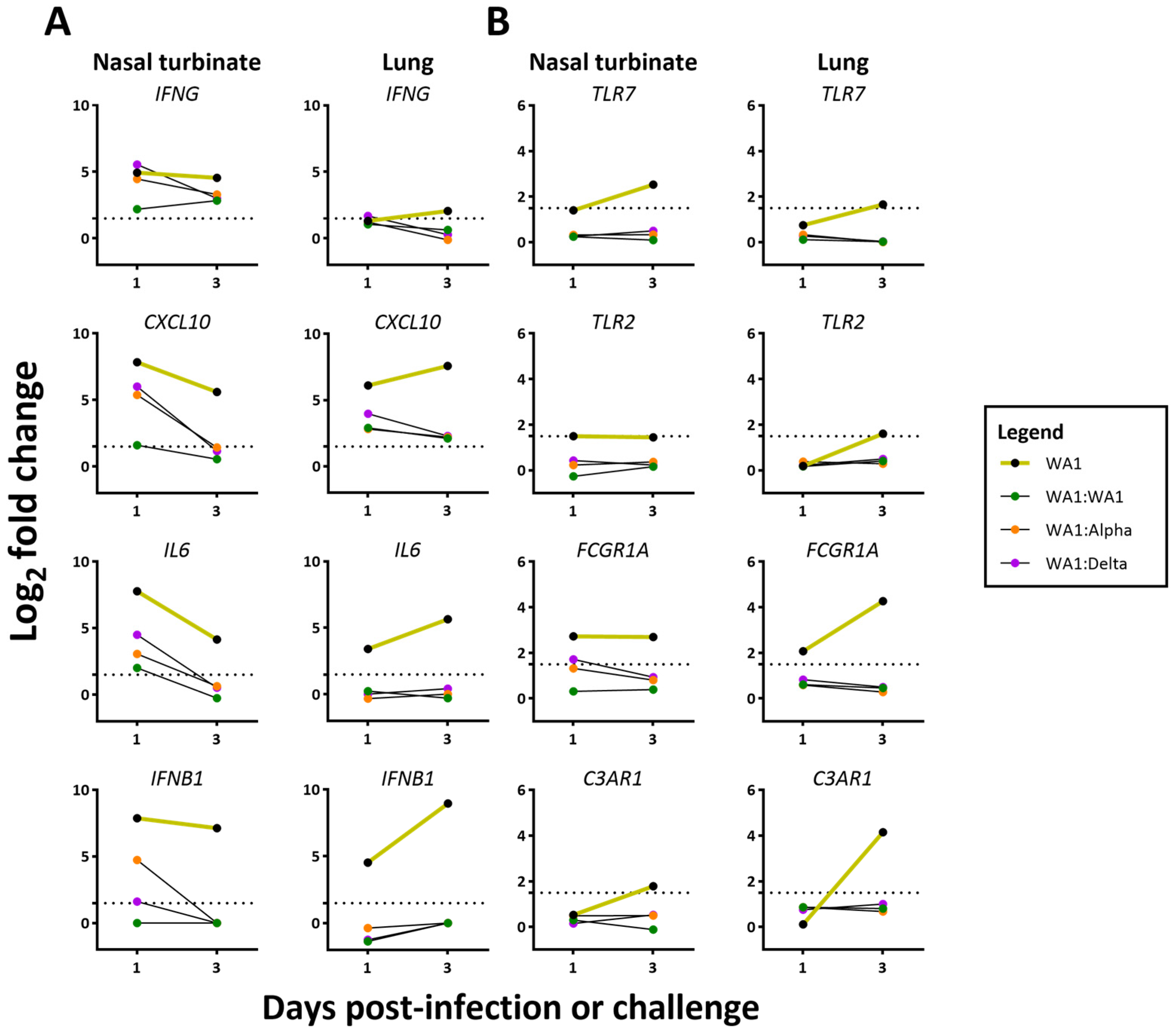
3.5. A Dampened Proinflammatory Response in the Lung, but Not the Nasal Turbinate, of Heterologous-Challenged Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhattacharya, M.; Chatterjee, S.; Sharma, A.R.; Agoramoorthy, G.; Chakraborty, C. D614G mutation and SARS-CoV-2: Impact on S-protein structure, function, infectivity, and immunity. Appl. Microbiol. Biotechnol. 2021, 105, 9035–9045. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Plante, K.S.; Plante, J.A.; Xie, X.; Zhang, X.; Ku, Z.; An, Z.; Scharton, D.; Schindewolf, C.; et al. The N501Y spike substitution enhances SARS-CoV-2 infection and transmission. Nature 2022, 602, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Horspool, A.M.; Russ, B.P.; Ye, C.; Lee, K.S.; Winters, M.T.; Bevere, J.R.; Miller, O.A.; Rader, N.A.; Cooper, M.; et al. Evaluating Antibody Mediated Protection against Alpha, Beta, and Delta SARS-CoV-2 Variants of Concern in K18-hACE2 Transgenic Mice. J. Virol. 2022, 96, e02184-21. [Google Scholar] [CrossRef]
- Chaudhari, A.M.; Joshi, M.; Kumar, D.; Patel, A.; Lokhande, K.B.; Krishnan, A.; Hanack, K.; Filipek, S.; Liepmann, D.; Renugopalakrishnan, V.; et al. Evaluation of immune evasion in SARS-CoV-2 Delta and Omicron variants. Comput. Struct. Biotechnol. 2022, 20, 4501–4516. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383.e9. [Google Scholar] [CrossRef]
- Shao, W.; Chen, X.; Zheng, C.; Liu, H.; Wang, G.; Zhang, B.; Li, Z.; Zhang, W. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern in real-world: A literature review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a Fourth Dose of CoVid-19 mRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, R.M.; Ashmawy, R.; Hamdy, N.A.; Elhadi, Y.A.M.; Reyad, O.A.; Elmalawany, D.; Almaghraby, A.; Shaaban, R.; Taha, S.H.N. Efficacy and Effectiveness of SARS-CoV-2 Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 350. [Google Scholar] [CrossRef]
- Yu, Y.; Esposito, D.; Kang, Z.; Lu, J.; Remaley, A.T.; De Giorgi, V.; Chen, L.N.; West, K.; Cao, L. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci. Rep. 2022, 12, 2628. [Google Scholar] [CrossRef]
- Lucas, C.; Vogels, C.B.F.; Yildirim, I.; Rothman, J.E.; Lu, P.; Monteiro, V.; Gehlhausen, J.R.; Campbell, M.; Silva, J.; Tabachnikova, A.; et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity. Nature 2021, 600, 523–529. [Google Scholar] [CrossRef]
- Collier, A.-r.Y.; Brown, C.M.; McMahan, K.A.; Yu, J.; Liu, J.; Jacob-Dolan, C.; Chandrashekar, A.; Tierney, D.; Ansel, J.L.; Rowe, M.; et al. Characterization of immune responses in fully vaccinated individuals after breakthrough infection with the SARS-CoV-2 delta variant. Sci. Transl. Med. 2022, 14, eabn6150. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, R.A.; Tsoleridis, T.; Jackson, H.J.; Cusin, L.; Duncan, J.D.; Chappell, J.G.; Tarr, A.W.; Nightingale, J.; Norrish, A.R.; Ikram, A.; et al. Two doses of the SARS-CoV-2 BNT162b2 vaccine enhance antibody responses to variants in individuals with prior SARS-CoV-2 infection. Sci. Transl. Med. 2021, 13, eabj0847. [Google Scholar] [CrossRef] [PubMed]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.-C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef]
- Sasaki, M.; Uemura, K.; Sato, A.; Toba, S.; Sanaki, T.; Maenaka, K.; Hall, W.W.; Orba, Y.; Sawa, H. SARS-CoV-2 variants with mutations at the S1/S2 cleavage site are generated in vitro during propagation in TMPRSS2-deficient cells. PLoS Pathog. 2021, 17, e1009233. [Google Scholar] [CrossRef]
- Taylor, M.K.; Williams, E.P.; Xue, Y.; Jenjaroenpun, P.; Wongsurawat, T.; Smith, A.P.; Smith, A.M.; Parvathareddy, J.; Kong, Y.; Vogel, P.; et al. Dissecting Phenotype from Genotype with Clinical Isolates of SARS-CoV-2 First Wave Variants. Viruses 2023, 15, 611. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Smith, A.P.; Williams, E.P.; Plunkett, T.R.; Selvaraj, M.; Lane, L.C.; Zalduondo, L.; Xue, Y.; Vogel, P.; Channappanavar, R.; Jonsson, C.B.; et al. Time-Dependent Increase in Susceptibility and Severity of Secondary Bacterial Infections During SARS-CoV-2. Front. Immunol. 2022, 13, 894534. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.; Fay, E.J.; Lee, Z.; Aron, S.; Hu, W.-S.; Langlois, R.A. Segment-Specific Kinetics of mRNA, cRNA, and vRNA Accumulation during Influenza Virus Infection. J. Virol. 2021, 95, e02102-20. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Rotondi, M. Interleukin-6, CXCL10 and Infiltrating Macrophages in COVID-19-Related Cytokine Storm: Not One for All But All for One! Front. Immunol. 2021, 12, 668507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, M.; Li, Y.; Yuen, H.H.; He, M.-L. The effects of SARS-CoV-2 infection on modulating innate immunity and strategies of combating inflammatory response for COVID-19 therapy. J. Biomed. Sci. 2022, 29, 27. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, S.G.; Sawicki, D.L.; Siddell, S.G. A contemporary view of coronavirus transcription. J. Virol. 2007, 81, 20–29. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Amanat, F.; Strohmeier, S.; Meade, P.S.; Dambrauskas, N.; Mühlemann, B.; Smith, D.J.; Vigdorovich, V.; Sather, D.N.; Coughlan, L.; Krammer, F. Vaccination with SARS-CoV-2 variants of concern protects mice from challenge with wild-type virus. PLoS Biol. 2021, 19, e3001384. [Google Scholar] [CrossRef]
- Bar-On, L.; Aftalion, M.; Makdasi, E.; Gur, D.; Alcalay, R.; Cohen, H.; Beth-Din, A.; Rosenfeld, R.; Achdout, H.; Bar-Haim, E.; et al. Prolonged Protective Immunity Induced by Mild SARS-CoV-2 Infection of K18-hACE2 Mice. Vaccines 2022, 10, 613. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Liu, J.; Martinot, A.J.; McMahan, K.; Mercado, N.B.; Peter, L.; Tostanoski, L.H.; Yu, J.; Maliga, Z.; Nekorchuk, M.; et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020, 369, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184. [Google Scholar] [CrossRef]
- Horiuchi, S.; Oishi, K.; Carrau, L.; Frere, J.; Møller, R.; Panis, M.; tenOever, B.R. Immune memory from SARS-CoV-2 infection in hamsters provides variant-independent protection but still allows virus transmission. Sci. Immunol. 2021, 6, eabm3131. [Google Scholar] [CrossRef]
- Lázaro-Frías, A.; Pérez, P.; Zamora, C.; Sánchez-Cordón, P.J.; Guzmán, M.; Luczkowiak, J.; Delgado, R.; Casasnovas, J.M.; Esteban, M.; García-Arriaza, J. Full efficacy and long-term immunogenicity induced by the SARS-CoV-2 vaccine candidate MVA-CoV2-S in mice. Npj Vaccines 2022, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Ying, B.; Whitener, B.; VanBlargan, L.A.; Hassan, A.O.; Shrihari, S.; Liang, C.Y.; Karl, C.E.; Mackin, S.; Chen, R.E.; Kafai, N.M.; et al. Protective activity of mRNA vaccines against ancestral and variant SARS-CoV-2 strains. Sci. Transl. Med. 2022, 14, eabm3302. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.R.; Field, C.J.; Septer, K.M.; Sim, D.G.; Jones, M.J.; Heinly, T.A.; Vanderford, T.H.; McGraw, E.A.; Sutton, T.C. Transmission and Protection against Reinfection in the Ferret Model with the SARS-CoV-2 USA-WA1/2020 Reference Isolate. J. Virol. 2021, 95, e0223220. [Google Scholar] [CrossRef] [PubMed]
- Lok, S.M. An NTD supersite of attack. Cell Host Microbe 2021, 29, 744–746. [Google Scholar] [CrossRef] [PubMed]
- Lorè, N.I.; De Lorenzo, R.; Rancoita, P.M.V.; Cugnata, F.; Agresti, A.; Benedetti, F.; Bianchi, M.E.; Bonini, C.; Capobianco, A.; Conte, C.; et al. CXCL10 levels at hospital admission predict COVID-19 outcome: Hierarchical assessment of 53 putative inflammatory biomarkers in an observational study. Mol. Med. 2021, 27, 129. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Zhang, X.; Wang, S.; Peng, Z.; Guo, J.; Jiang, H.; Liu, J.; Xie, Y.; Wang, J.; et al. Leptin correlates with monocytes activation and severe condition in COVID-19 patients. J. Leukoc. Biol. 2021, 110, 9–20. [Google Scholar] [CrossRef]
- Kwon, J.S.; Kim, J.Y.; Kim, M.C.; Park, S.Y.; Kim, B.N.; Bae, S.; Cha, H.H.; Jung, J.; Kim, M.J.; Lee, M.J.; et al. Factors of Severity in Patients with COVID-19: Cytokine/Chemokine Concentrations, Viral Load, and Antibody Responses. Am. J. Trop. Med. Hyg. 2020, 103, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.J.; Kelvin, A.A.; Leon, A.J.; Cameron, C.M.; Ran, L.; Xu, L.; Chu, Y.-K.; Danesh, A.; Fang, Y.; Li, Q.; et al. Lack of Innate Interferon Responses during SARS Coronavirus Infection in a Vaccination and Reinfection Ferret Model. PLoS ONE 2012, 7, e45842. [Google Scholar] [CrossRef]
- Bortolotti, D.; Gentili, V.; Rizzo, S.; Schiuma, G.; Beltrami, S.; Strazzabosco, G.; Fernandez, M.; Caccuri, F.; Caruso, A.; Rizzo, R. TLR3 and TLR7 RNA Sensor Activation during SARS-CoV-2 Infection. Microorganisms 2021, 9, 1820. [Google Scholar] [CrossRef]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Parry, B.; Ravid, S.; Clark, S.; Schrimpf, M.R.; et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 2022, 606, 576–584. [Google Scholar] [CrossRef]
- Yan, B.; Freiwald, T.; Chauss, D.; Wang, L.; West, E.; Mirabelli, C.; Zhang, C.J.; Nichols, E.-M.; Malik, N.; Gregory, R.; et al. SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci. Immunol. 2021, 6, eabg0833. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: A retrospective, total population cohort study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.H.; Michlmayr, D.; Gubbels, S.M.; Mølbak, K.; Ethelberg, S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population-level observational study. Lancet 2021, 397, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nichols, J.H.; Williams, E.P.; Parvathareddy, J.; Cao, X.; Kong, Y.; Fitzpatrick, E.; Webby, R.J.; Jonsson, C.B. Upper Respiratory Infection Drives Clinical Signs and Inflammatory Responses Following Heterologous Challenge of SARS-CoV-2 Variants of Concern in K18 Mice. Viruses 2023, 15, 946. https://doi.org/10.3390/v15040946
Nichols JH, Williams EP, Parvathareddy J, Cao X, Kong Y, Fitzpatrick E, Webby RJ, Jonsson CB. Upper Respiratory Infection Drives Clinical Signs and Inflammatory Responses Following Heterologous Challenge of SARS-CoV-2 Variants of Concern in K18 Mice. Viruses. 2023; 15(4):946. https://doi.org/10.3390/v15040946
Chicago/Turabian StyleNichols, Jacob H., Evan P. Williams, Jyothi Parvathareddy, Xueyuan Cao, Ying Kong, Elizabeth Fitzpatrick, Richard J. Webby, and Colleen B. Jonsson. 2023. "Upper Respiratory Infection Drives Clinical Signs and Inflammatory Responses Following Heterologous Challenge of SARS-CoV-2 Variants of Concern in K18 Mice" Viruses 15, no. 4: 946. https://doi.org/10.3390/v15040946
APA StyleNichols, J. H., Williams, E. P., Parvathareddy, J., Cao, X., Kong, Y., Fitzpatrick, E., Webby, R. J., & Jonsson, C. B. (2023). Upper Respiratory Infection Drives Clinical Signs and Inflammatory Responses Following Heterologous Challenge of SARS-CoV-2 Variants of Concern in K18 Mice. Viruses, 15(4), 946. https://doi.org/10.3390/v15040946






