Impact of Borna Disease Virus Infection on the Transcriptome of Differentiated Neuronal Cells and Its Modulation by Antiviral Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Real-Time RT-PCR
2.3. RNA-Seq Analysis
2.4. siRNA Transfection
2.5. Western Blot
2.6. Statistics
3. Results
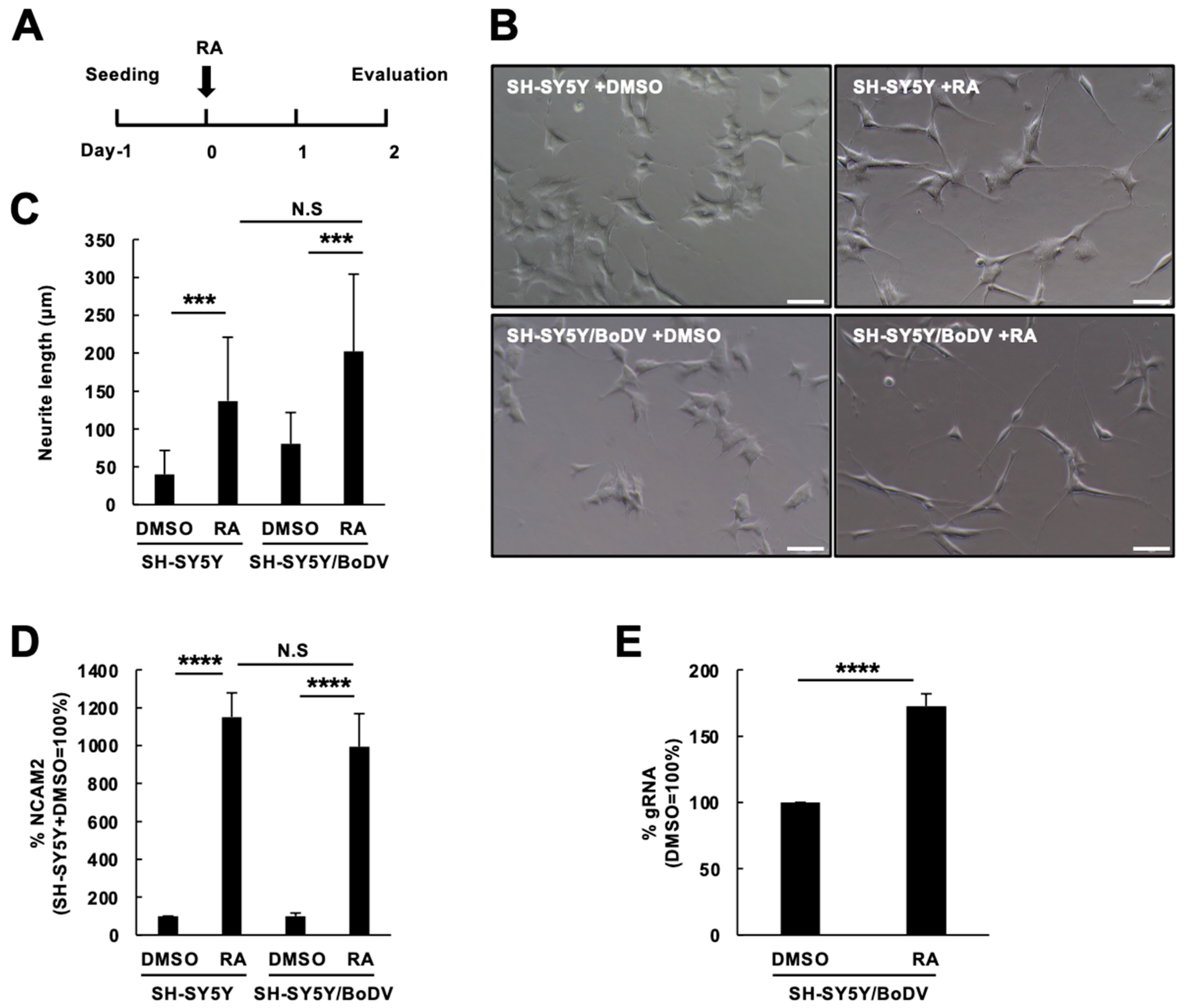
3.1. Impact of BoDV-1 Infection on Neuronal Differentiation in SH-SY5Y Cells
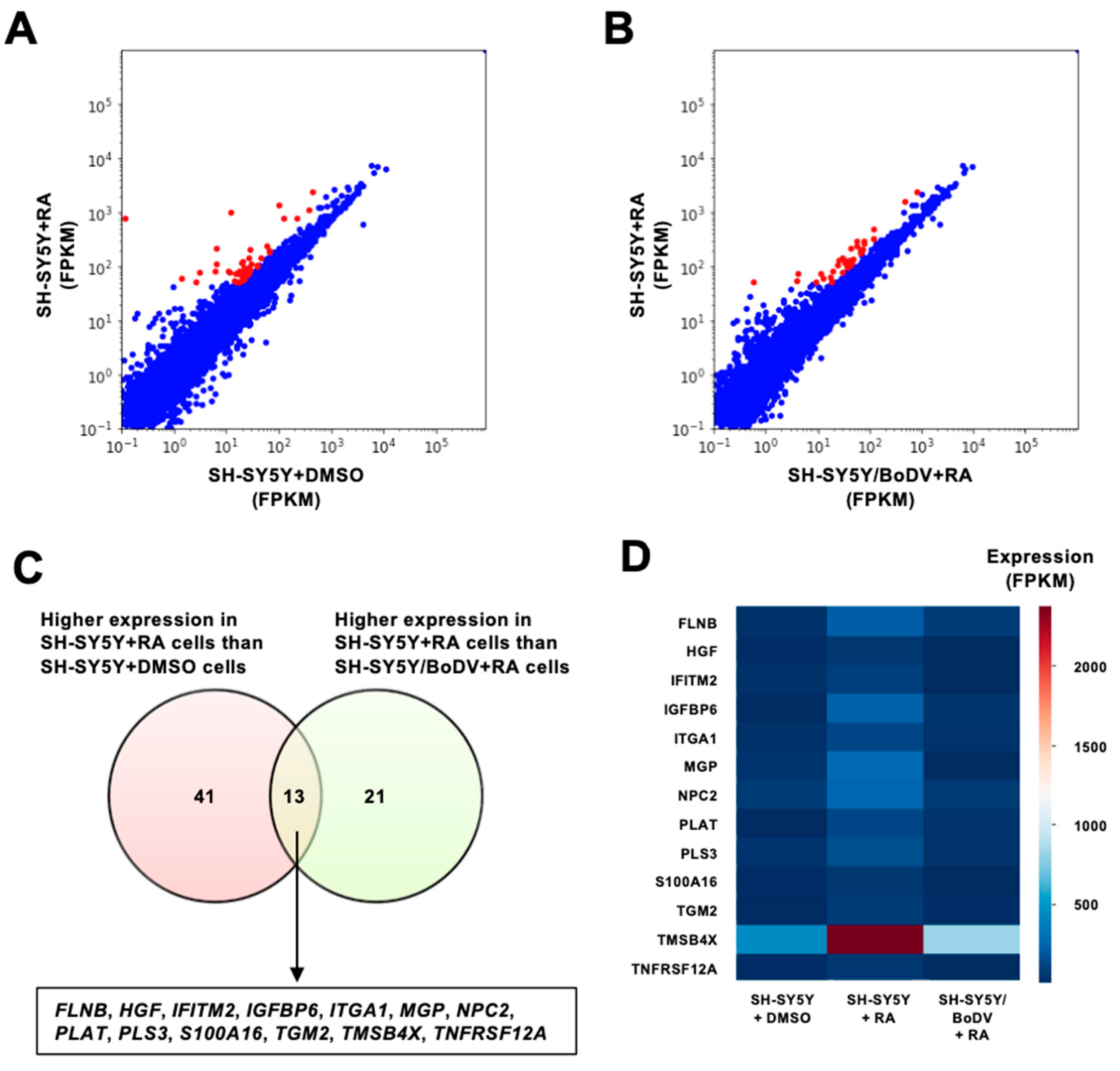
3.2. Impairment in the Induction of Differentiation-Related Genes by BoDV-1 Infection
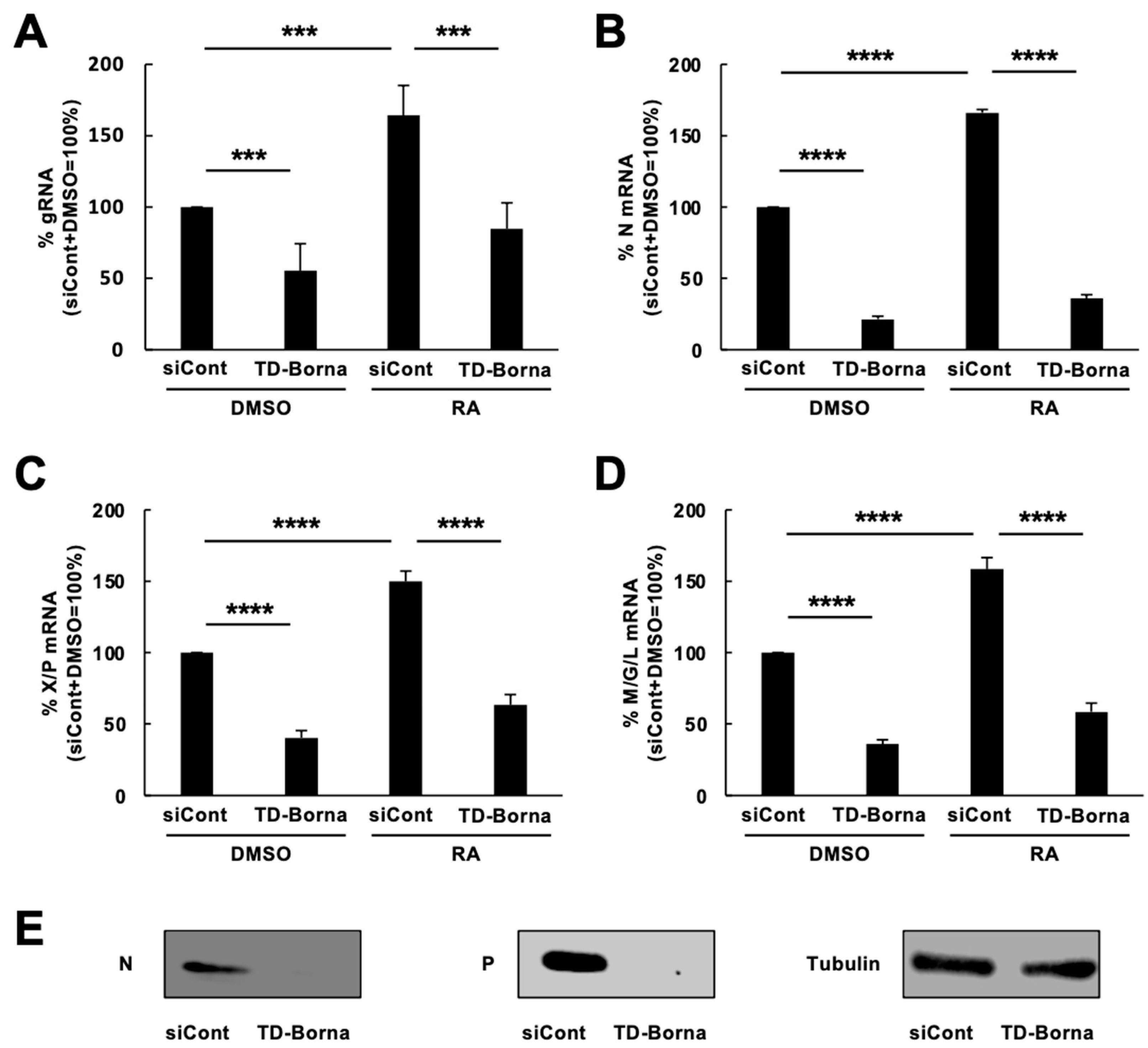
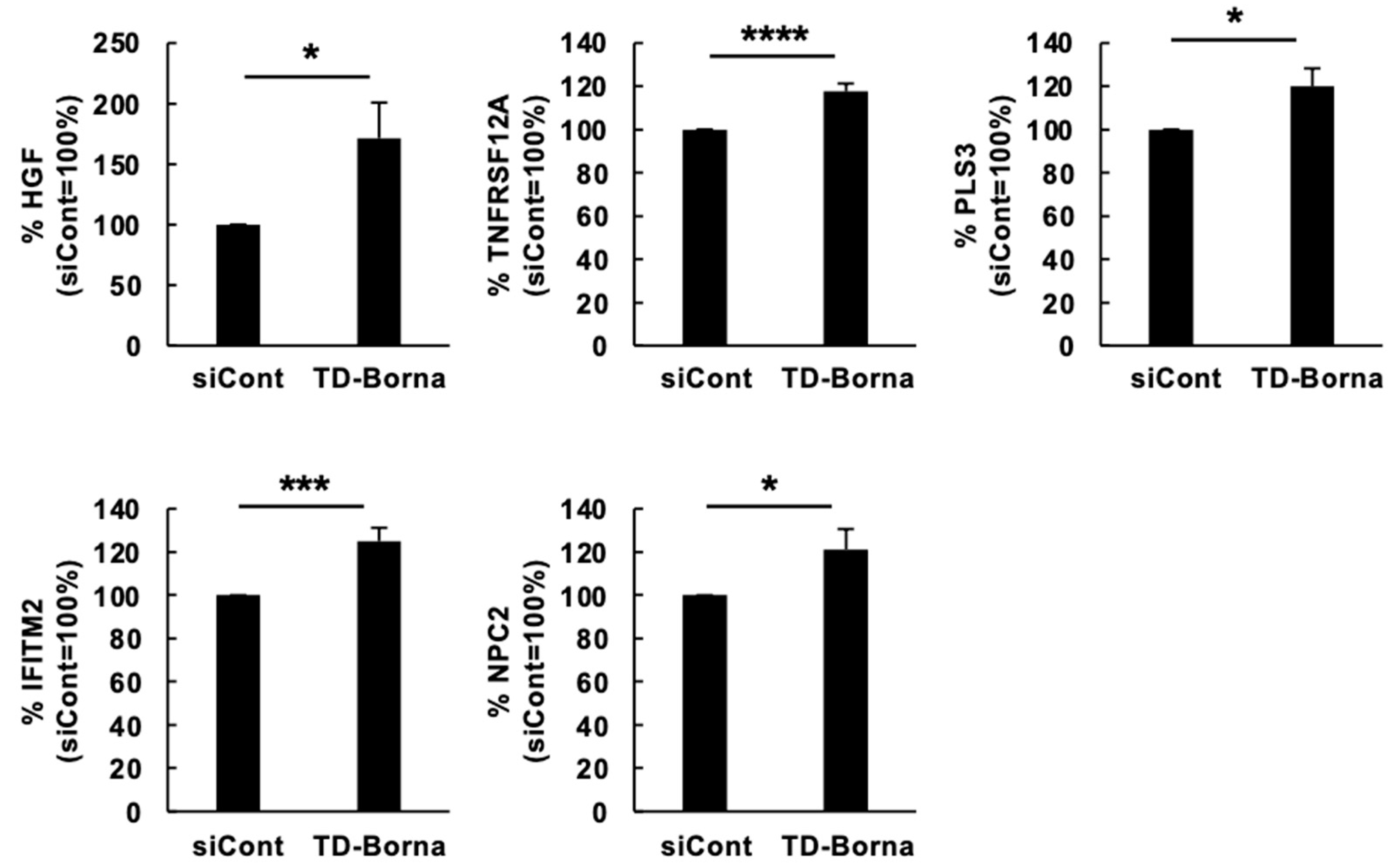
3.3. Effect of a siRNA Cocktail, TD-Borna, on BoDV-1 Infection and the Expression of Differentiation-Related Genes Impaired by BoDV-1 Infection
3.4. Effect of TD-Borna on Cell Viability during Neuronal Differentiation in BoDV-1-Infected SH-SY5Y Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amarasinghe, G.K.; Ayllón, M.A.; Bào, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the order Mononegavirales: Update 2019. Arch. Virol. 2019, 164, 1967–1980. [Google Scholar] [CrossRef] [PubMed]
- Rubbenstroth, D.; Briese, T.; Dürrwald, R.; Horie, M.; Hyndman, T.H.; Kuhn, J.H.; Nowotny, N.; Payne, S.; Stenglein, M.D.; Tomonaga, K.; et al. ICTV Virus Taxonomy Profile: Bornaviridae. J. Gen. Virol. 2021, 102, 001613. [Google Scholar] [CrossRef] [PubMed]
- Staeheli, P.; Sauder, C.; Hausmann, J.; Ehrensperger, F.; Schwemmle, M. Epidemiology of Borna disease virus. J. Gen. Virol. 2000, 81, 2123–2135. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, K.; Kobayashi, T.; Ikuta, K. Molecular and cellular biology of Borna disease virus infection. Microbes Infect. 2002, 4, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Tomonaga, K. Nucleocytoplasmic shuttling of viral proteins in borna disease virus infection. Viruses 2013, 5, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Tappe, D.; Höper, D.; Herden, C.; Boldt, A.; Mawrin, C.; Niederstraßer, O.; Müller, T.; Jenckel, M.; van der Grinten, E.; et al. A Variegated Squirrel Bornavirus Associated with Fatal Human Encephalitis. N. Engl. J. Med. 2015, 373, 154–162. [Google Scholar] [CrossRef]
- Schlottau, K.; Forth, L.; Angstwurm, K.; Höper, D.; Zecher, D.; Liesche, F.; Hoffmann, B.; Kegel, V.; Seehofer, D.; Platen, S.; et al. Fatal Encephalitic Borna Disease Virus 1 in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2018, 379, 1377–1379. [Google Scholar] [CrossRef]
- Korn, K.; Coras, R.; Bobinger, T.; Herzog, S.M.; Lücking, H.; Stöhr, R.; Huttner, H.B.; Hartmann, A.; Ensser, A. Fatal Encephalitis Associated with Borna Disease Virus 1. N. Engl. J. Med. 2018, 379, 1375–1377. [Google Scholar] [CrossRef]
- Hornig, M.; Solbrig, M.; Horscroft, N.; Weissenböck, H.; Lipkin, W.I. Borna disease virus infection of adult and neonatal rats: Models for neuropsychiatric disease. Curr. Top. Microbiol. Immunol. 2001, 253, 157–177. [Google Scholar] [CrossRef]
- Pletnikov, M.V.; Moran, T.H.; Carbone, K.M. Borna disease virus infection of the neonatal rat: Developmental brain injury model of autism spectrum disorders. Front. Biosci. 2002, 7, d593–d607. [Google Scholar] [CrossRef]
- Pletnikov, M.V.; Jones, M.L.; Rubin, S.A.; Moran, T.H.; Carbone, K.M. Rat model of autism spectrum disorders. Genetic background effects on Borna disease virus-induced developmental brain damage. Ann. N. Y. Acad. Sci. 2001, 939, 318–319. [Google Scholar] [CrossRef] [PubMed]
- Pletnikov, M.V.; Rubin, S.A.; Vasudevan, K.; Moran, T.H.; Carbone, K.M. Developmental brain injury associated with abnormal play behavior in neonatally Borna disease virus-infected Lewis rats: A model of autism. Behav. Brain Res. 1999, 100, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Billich, C.; Sauder, C.; Frank, R.; Herzog, S.; Bechter, K.; Takahashi, K.; Peters, H.; Staeheli, P.; Schwemmle, M. High-avidity human serum antibodies recognizing linear epitopes of Borna disease virus proteins. Biol. Psychiatry 2002, 51, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Briese, T.; Licinio, J.; Khabbaz, R.F.; Altshuler, L.L.; Potkin, S.G.; Schwemmle, M.; Siemetzki, U.; Mintz, J.; Honkavuori, K.; et al. Absence of evidence for bornavirus infection in schizophrenia, bipolar disorder and major depressive disorder. Mol. Psychiatry 2012, 17, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Arias, I.; Sorlozano, A.; Villegas, E.; de Dios Luna, J.; McKenney, K.; Cervilla, J.; Gutierrez, B.; Gutierrez, J. Infectious agents associated with schizophrenia: A meta-analysis. Schizophr. Res. 2012, 136, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Lei, Y.; Liu, X.; Zhou, X.; Liu, Y.; Wang, M.; Yang, L.; Zhang, L.; Fan, S.; et al. Meta-analysis of infectious agents and depression. Sci. Rep. 2014, 4, 4530. [Google Scholar] [CrossRef] [PubMed]
- Azami, M.; Jalilian, F.A.; Khorshidi, A.; Mohammadi, Y.; Tardeh, Z. The association between Borna Disease Virus and schizophrenia: A systematic review and meta-analysis. Asian J. Psychiatr. 2018, 34, 67–73. [Google Scholar] [CrossRef]
- Tokunaga, T.; Yamamoto, Y.; Sakai, M.; Tomonaga, K.; Honda, T. Antiviral activity of favipiravir (T-705) against mammalian and avian bornaviruses. Antiviral Res. 2017, 143, 237–245. [Google Scholar] [CrossRef]
- Mizutani, T.; Inagaki, H.; Araki, K.; Kariwa, H.; Arikawa, J.; Takashima, I. Inhibition of Borna disease virus replication by ribavirin in persistently infected cells. Arch. Virol. 1998, 143, 2039–2044. [Google Scholar] [CrossRef]
- Musser, J.M.B.; Heatley, J.J.; Koinis, A.V.; Suchodolski, P.F.; Guo, J.; Escandon, P.; Tizard, I.R. Ribavirin Inhibits Parrot Bornavirus 4 Replication in Cell Culture. PLoS ONE 2015, 10, e0134080. [Google Scholar] [CrossRef]
- Reuter, A.; Horie, M.; Höper, D.; Ohnemus, A.; Narr, A.; Rinder, M.; Beer, M.; Staeheli, P.; Rubbenstroth, D. Synergistic antiviral activity of Ribavirin and interferon-α against parrot bornaviruses in avian cells. J. Gen. Virol. 2016, 97, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Teng, D.; Obika, S.; Ueda, K.; Honda, T. A Small Interfering RNA Cocktail Targeting the Nucleoprotein and Large Protein Genes Suppresses Borna Disease Virus Infection. Front. Microbiol. 2019, 10, 2781. [Google Scholar] [CrossRef] [PubMed]
- Hallensleben, W.; Staeheli, P. Inhibition of Borna disease virus multiplication by interferon: Cell line differences in susceptibility. Arch. Virol. 1999, 144, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Pletnikov, M.V.; Rubin, S.A.; Moran, T.H.; Carbone, K.M. Exploring the cerebellum with a new tool: Neonatal Borna disease virus (BDV) infection of the rat’s brain. Cerebellum 2003, 2, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Bautista, J.R.; Rubin, S.A.; Moran, T.H.; Schwartz, G.J.; Carbone, K.M. Developmental injury to the cerebellum following perinatal Borna disease virus infection. Brain Res. Dev. Brain Res. 1995, 90, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Eisenman, L.M.; Brothers, R.; Tran, M.H.; Kean, R.B.; Dickson, G.M.; Dietzschold, B.; Hooper, D.C. Neonatal Borna disease virus infection in the rat causes a loss of Purkinje cells in the cerebellum. J. Neurovirol. 1999, 5, 181–189. [Google Scholar] [CrossRef]
- Kamitani, W.; Ono, E.; Yoshino, S.; Kobayashi, T.; Taharaguchi, S.; Lee, B.-J.; Yamashita, M.; Kobayashi, T.; Okamoto, M.; Taniyama, H.; et al. Glial expression of Borna disease virus phosphoprotein induces behavioral and neurological abnormalities in transgenic mice. Proc. Natl. Acad. Sci. USA 2003, 100, 8969–8974. [Google Scholar] [CrossRef]
- Honda, T.; Fujino, K.; Okuzaki, D.; Ohtaki, N.; Matsumoto, Y.; Horie, M.; Daito, T.; Itoh, M.; Tomonaga, K. Upregulation of insulin-like growth factor binding protein 3 in astrocytes of transgenic mice that express Borna disease virus phosphoprotein. J. Virol. 2011, 85, 4567–4571. [Google Scholar] [CrossRef]
- Sauder, C.; Hallensleben, W.; Pagenstecher, A.; Schneckenburger, S.; Biro, L.; Pertlik, D.; Hausmann, J.; Suter, M.; Staeheli, P. Chemokine gene expression in astrocytes of Borna disease virus-infected rats and mice in the absence of inflammation. J. Virol. 2000, 74, 9267–9280. [Google Scholar] [CrossRef]
- Jie, J.; Xu, X.; Xia, J.; Tu, Z.; Guo, Y.; Li, C.; Zhang, X.; Wang, H.; Song, W.; Xie, P. Memory Impairment Induced by Borna Disease Virus 1 Infection is Associated with Reduced H3K9 Acetylation. Cell. Physiol. Biochem. 2018, 49, 381–394. [Google Scholar] [CrossRef]
- Zocher, M.; Czub, S.; Schulte-Mönting, J.; de La Torre, J.C.; Sauder, C. Alterations in neurotrophin and neurotrophin receptor gene expression patterns in the rat central nervous system following perinatal Borna disease virus infection. J. Neurovirol. 2000, 6, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Sauder, C.; Wolfer, D.P.; Lipp, H.P.; Staeheli, P.; Hausmann, J. Learning deficits in mice with persistent Borna disease virus infection of the CNS associated with elevated chemokine expression. Behav. Brain Res. 2001, 120, 189–201. [Google Scholar] [CrossRef]
- Rauch, J.; Steffen, J.F.; Muntau, B.; Gisbrecht, J.; Pörtner, K.; Herden, C.; Niller, H.H.; Bauswein, M.; Rubbenstroth, D.; Mehlhoop, U.; et al. Human Borna disease virus 1 encephalitis shows marked pro-inflammatory biomarker and tissue immunoactivation during the course of disease. Emerg. Microbes Infect. 2022, 11, 1843–1856. [Google Scholar] [CrossRef]
- Nakamura, Y.; Takahashi, H.; Shoya, Y.; Nakaya, T.; Watanabe, M.; Tomonaga, K.; Iwahashi, K.; Ameno, K.; Momiyama, N.; Taniyama, H.; et al. Isolation of Borna Disease Virus from Human Brain Tissue. J. Virol. 2000, 74, 4601–4611. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-San Martín, C.; Li, T.; Bouquet, J.; Streithorst, J.; Yu, G.; Paranjpe, A.; Chiu, C.Y. Differentiation enhances Zika virus infection of neuronal brain cells. Sci. Rep. 2018, 8, 14543. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Horie, M.; Daito, T.; Honda, T.; Ikuta, K.; Tomonaga, K. Heat shock cognate protein 70 controls Borna disease virus replication via interaction with the viral non-structural protein X. Microbes Infect. 2009, 11, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Honda, T.; Matsumoto, Y.; Tomonaga, K. Heat stress is a potent stimulus for enhancing rescue efficiency of recombinant Borna disease virus. Microbiol. Immunol. 2014, 58, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Korecka, J.A.; van Kesteren, R.E.; Blaas, E.; Spitzer, S.O.; Kamstra, J.H.; Smit, A.B.; Swaab, D.F.; Verhaagen, J.; Bossers, K. Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PLoS ONE 2013, 8, e63862. [Google Scholar] [CrossRef]
- Solbrig, M.V.; Adrian, R.; Baratta, J.; Lauterborn, J.C.; Koob, G.F. Kappa opioid control of seizures produced by a virus in an animal model. Brain 2006, 129, 642–654. [Google Scholar] [CrossRef]
- Solbrig, M.V.; Hermanowicz, N. Cannabinoid rescue of striatal progenitor cells in chronic Borna disease viral encephalitis in rats. J. Neurovirol. 2008, 14, 252–260. [Google Scholar] [CrossRef]
- Brnic, D.; Stevanovic, V.; Cochet, M.; Agier, C.; Richardson, J.; Montero-Menei, C.N.; Milhavet, O.; Eloit, M.; Coulpier, M. Borna disease virus infects human neural progenitor cells and impairs neurogenesis. J. Virol. 2012, 86, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Scordel, C.; Huttin, A.; Cochet-Bernoin, M.; Szelechowski, M.; Poulet, A.; Richardson, J.; Benchoua, A.; Gonzalez-Dunia, D.; Eloit, M.; Coulpier, M. Borna disease virus phosphoprotein impairs the developmental program controlling neurogenesis and reduces human GABAergic neurogenesis. PLoS Pathog. 2015, 11, e1004859. [Google Scholar] [CrossRef] [PubMed]
- Hans, A.; Syan, S.; Crosio, C.; Sassone-Corsi, P.; Brahic, M.; Gonzalez-Dunia, D. Borna Disease Virus Persistent Infection Activates Mitogen-activated Protein Kinase and Blocks Neuronal Differentiation of PC12 Cells. J. Biol. Chem. 2001, 276, 7258–7265. [Google Scholar] [CrossRef] [PubMed]
- Honda, T. Bornavirus infection in human diseases and its molecular neuropathology. Clin. Exp. Neuroimmunol. 2022, 13, 7–16. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Hayashi, Y.; Omori, H.; Honda, T.; Daito, T.; Horie, M.; Ikuta, K.; Fujino, K.; Nakamura, S.; Schneider, U.; et al. Bornavirus closely associates and segregates with host chromosomes to ensure persistent intranuclear infection. Cell Host Microbe 2012, 11, 492–503. [Google Scholar] [CrossRef]
- Hirai, Y.; Hirano, Y.; Matsuda, A.; Hiraoka, Y.; Honda, T.; Tomonaga, K. Borna Disease Virus Assembles Porous Cage-like Viral Factories in the Nucleus. J. Biol. Chem. 2016, 291, 25789–25798. [Google Scholar] [CrossRef]
- Suberbielle, E.; Stella, A.; Pont, F.; Monnet, C.; Mouton, E.; Lamouroux, L.; Monsarrat, B.; Gonzalez-Dunia, D. Proteomic analysis reveals selective impediment of neuronal remodeling upon Borna disease virus infection. J. Virol. 2008, 82, 12265–12279. [Google Scholar] [CrossRef]
- Bonnaud, E.M.; Szelechowski, M.; Bétourné, A.; Foret, C.; Thouard, A.; Gonzalez-Dunia, D.; Malnou, C.E. Borna Disease Virus Phosphoprotein Modulates Epigenetic Signaling in Neurons To Control Viral Replication. J. Virol. 2015, 89, 5996–6008. [Google Scholar] [CrossRef]
- Iguchi, Y.; Ishihara, S.; Uchida, Y.; Tajima, K.; Mizutani, T.; Kawabata, K.; Haga, H. Filamin B Enhances the Invasiveness of Cancer Cells into 3D Collagen Matrices. Cell Struct. Funct. 2015, 40, 61–67. [Google Scholar] [CrossRef]
- Brun, C.; Demeaux, A.; Guaddachi, F.; Jean-Louis, F.; Oddos, T.; Bagot, M.; Bensussan, A.; Jauliac, S.; Michel, L. T-plastin expression downstream to the calcineurin/NFAT pathway is involved in keratinocyte migration. PLoS ONE 2014, 9, e104700. [Google Scholar] [CrossRef]
- Bétourné, A.; Szelechowski, M.; Thouard, A.; Abrial, E.; Jean, A.; Zaidi, F.; Foret, C.; Bonnaud, E.M.; Charlier, C.M.; Suberbielle, E.; et al. Hippocampal expression of a virus-derived protein impairs memory in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 588–610. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.; Jariwala, U.; Woo, J.; Zhan, Y.; Burke, K.A.; Zhu, L.; Anderson, W.F.; Zhao, Y. Involvement of Niemann-Pick type C2 protein in hematopoiesis regulation. Stem Cells 2006, 24, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Daniel-Carmi, V.; Makovitzki-Avraham, E.; Reuven, E.-M.; Goldstein, I.; Zilkha, N.; Rotter, V.; Tzehoval, E.; Eisenbach, L. The human 1-8D gene (IFITM2) is a novel p53 independent pro-apoptotic gene. Int. J. Cancer 2009, 125, 2810–2819. [Google Scholar] [CrossRef]
- Ma, Y.; Lai, W.; Zhao, M.; Yue, C.; Shi, F.; Li, R.; Hu, Z. Plastin 3 down-regulation augments the sensitivity of MDA-MB-231 cells to paclitaxel via the p38 MAPK signalling pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 685–695. [Google Scholar] [CrossRef]
- Schwemmle, M. Borna Disease Virus P-protein Is Phosphorylated by Protein Kinase Cepsilon and Casein Kinase II. J. Biol. Chem. 1997, 272, 21818–21823. [Google Scholar] [CrossRef]
- Nakayama, M.; Ishidoh, K.; Kayagaki, N.; Kojima, Y.; Yamaguchi, N.; Nakano, H.; Kominami, E.; Okumura, K.; Yagita, H. Multiple Pathways of TWEAK-Induced Cell Death. J. Immunol. 2002, 168, 734–743. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, D.; Ueda, K.; Honda, T. Impact of Borna Disease Virus Infection on the Transcriptome of Differentiated Neuronal Cells and Its Modulation by Antiviral Treatment. Viruses 2023, 15, 942. https://doi.org/10.3390/v15040942
Teng D, Ueda K, Honda T. Impact of Borna Disease Virus Infection on the Transcriptome of Differentiated Neuronal Cells and Its Modulation by Antiviral Treatment. Viruses. 2023; 15(4):942. https://doi.org/10.3390/v15040942
Chicago/Turabian StyleTeng, Da, Keiji Ueda, and Tomoyuki Honda. 2023. "Impact of Borna Disease Virus Infection on the Transcriptome of Differentiated Neuronal Cells and Its Modulation by Antiviral Treatment" Viruses 15, no. 4: 942. https://doi.org/10.3390/v15040942
APA StyleTeng, D., Ueda, K., & Honda, T. (2023). Impact of Borna Disease Virus Infection on the Transcriptome of Differentiated Neuronal Cells and Its Modulation by Antiviral Treatment. Viruses, 15(4), 942. https://doi.org/10.3390/v15040942




