Pathologic Mechanisms of the Newcastle Disease Virus
Abstract
1. Introduction
2. Clinical Signs and Damage to Organs
3. Cytokine Secretion during NDV Replication
4. Receptors for NDV PAMP
5. Mechanisms for NDV to Facilitate Its Replication
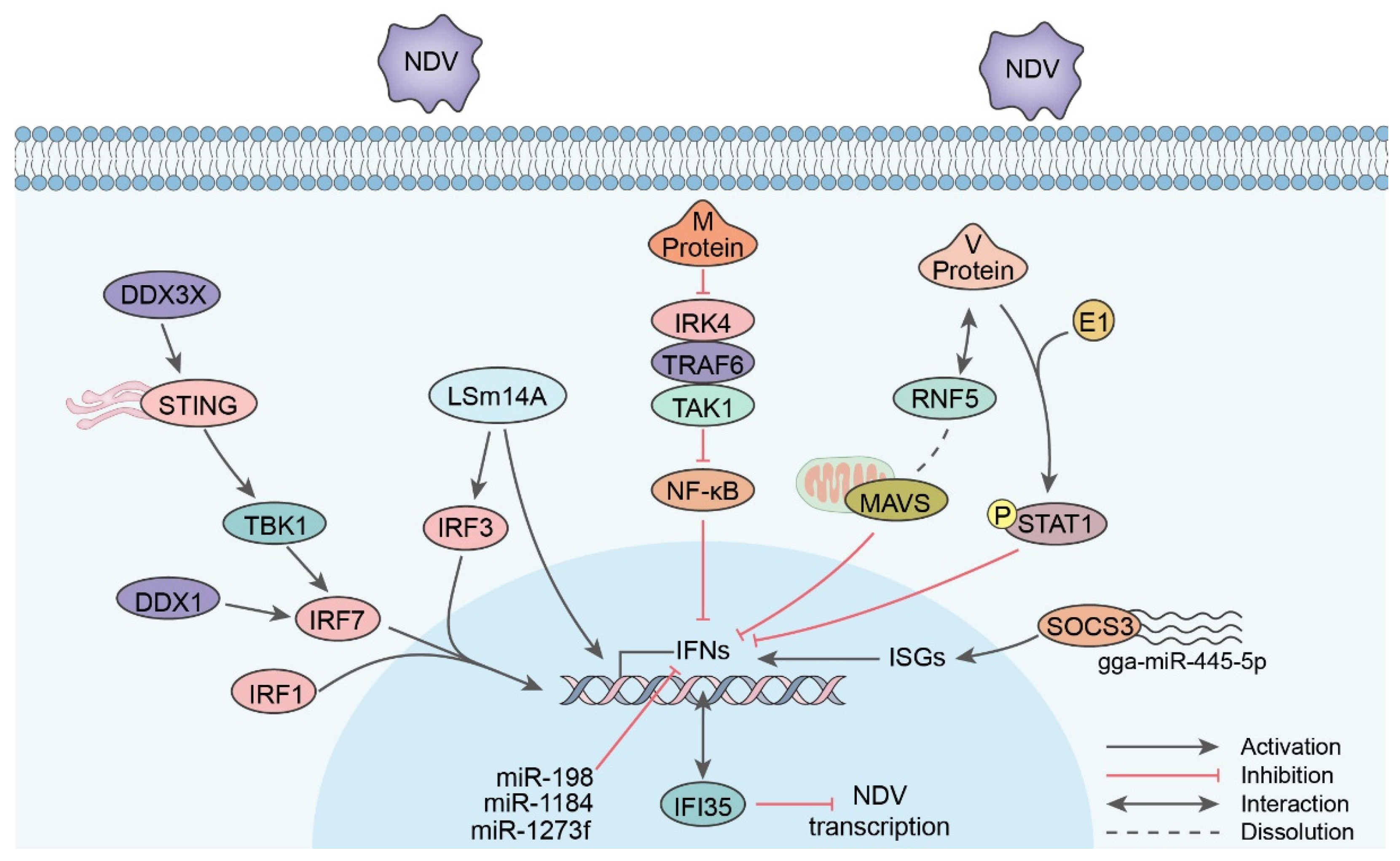
5.1. IFN
5.2. Autophagy
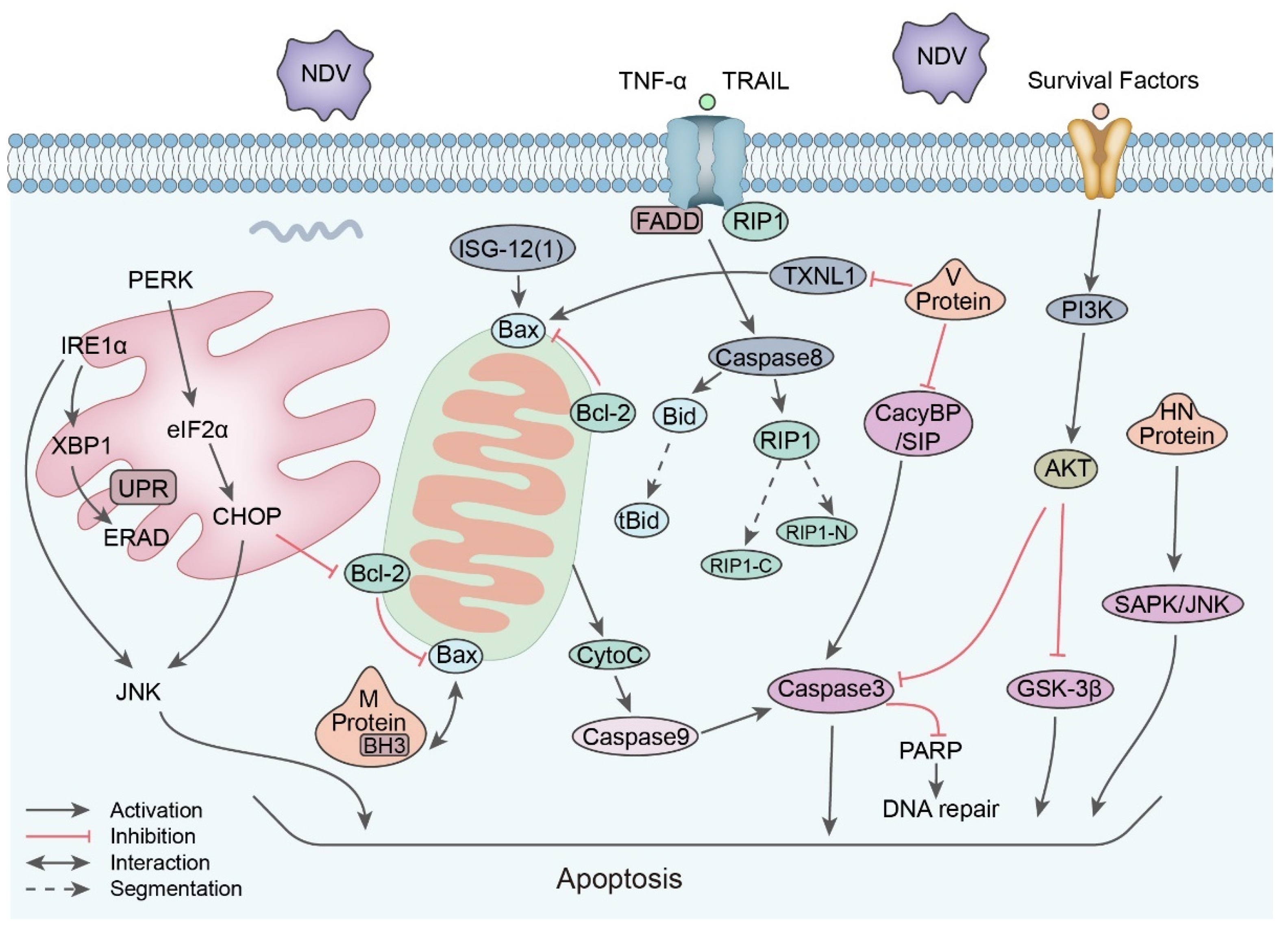
5.3. Apoptosis
5.4. Other Host Interaction Metabolism
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chambers, P.; Millar, N.S.; Bingham, R.W.; Emmerson, P.T. Molecular cloning of complementary DNA to Newcastle disease virus, and nucleotide sequence analysis of the junction between the genes encoding the haemagglutinin-neuraminidase and the large protein. J. Gen. Virol. 1986, 67 Pt 3, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Czeglédi, A.; Ujvári, D.; Somogyi, E.; Wehmann, E.; Werner, O.; Lomniczi, B. Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications. Virus Res. 2006, 120, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Phuangsab, A.; Lorence, R.M.; Reichard, K.W.; Peeples, M.E.; Walter, R.J. Newcastle disease virus therapy of human tumor xenografts: Antitumor effects of local or systemic administration. Cancer Lett. 2001, 172, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, H.; Cong, F.; Wu, W.; Zhao, R.; Kong, X. Phosphoprotein Contributes to the Thermostability of Newcastle Disease Virus. BioMed Res. Int. 2018, 2018, 8917476. [Google Scholar] [CrossRef]
- Pantua, H.D.; McGinnes, L.W.; Peeples, M.E.; Morrison, T.G. Requirements for the assembly and release of Newcastle disease virus-like particles. J. Virol. 2006, 80, 11062–11073. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Cao, H.; Wang, Y.; Zheng, S.J. Engagement of new castle disease virus (NDV) matrix (M) protein with charged multivesicular body protein (CHMP) 4 facilitates viral replication. Virus Res. 2013, 171, 80–88. [Google Scholar] [CrossRef]
- Morrison, T.G. Structure and function of a paramyxovirus fusion protein. Biochim. Biophys. Acta-Biomembr. 2003, 1614, 73–84. [Google Scholar] [CrossRef]
- Romer-Oberdorfer, A.; Werner, O.; Veits, J.; Mebatsion, T.; Mettenleiter, T.C. Contribution of the length of the HN protein and the sequence of the F protein cleavage site to Newcastle disease virus pathogenicity. J. Gen. Virol. 2003, 84, 3121–3129. [Google Scholar] [CrossRef]
- de Leeuw, O.S.; Hartog, L.; Koch, G.; Peeters, B.P.H. Effect of fusion protein cleavage site mutations on virulence of Newcastle disease virus: Non-virulent cleavage site mutants revert to virulence after one passage in chicken brain. J. Gen. Virol. 2003, 84 Pt 2, 475–484. [Google Scholar] [CrossRef]
- Kim, S.H.; Subbiah, M.; Samuel, A.S.; Collins, P.L.; Samal, S.K. Roles of the fusion and hemagglutinin-neuraminidase proteins in replication, tropism, and pathogenicity of avian paramyxoviruses. J. Virol. 2011, 85, 8582–8596. [Google Scholar] [CrossRef]
- Liang, B.; Li, Z.; Jenni, S.; Rahmeh, A.A.; Morin, B.M.; Grant, T.; Grigorieff, N.; Harrison, S.C.; Whelan, S.P.J. Structure of the L Protein of Vesicular Stomatitis Virus from Electron Cryomicroscopy. Cell 2015, 162, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Dortmans, J.C.; Rottier, P.J.; Koch, G.; Peeters, B.P. The viral replication complex is associated with the virulence of Newcastle disease virus. J. Virol. 2010, 84, 10113–10120. [Google Scholar] [CrossRef] [PubMed]
- Nan, F.L.; Zhang, H.; Nan, W.L.; Xie, C.Z.; Ha, Z.; Chen, X.; Xu, X.H.; Qian, J.; Qiu, X.S.; Ge, J.Y.; et al. Lentogenic NDV V protein inhibits IFN responses and represses cell apoptosis. Vet. Microbiol. 2021, 261, 109181. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Chu, Z.; Gao, X.; Yang, M.; Adam, F.E.A.; Theodore, D.W.P.; Liu, H.; Wang, X.; Xiao, S.; Yang, Z. Newcastle disease virus V protein interacts with hnRNP H1 to promote viral replication. Vet. Microbiol. 2021, 260, 109093. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, H.; Yu, S.; Ding, Y.; Wu, W.; Mao, X.; Liao, Y.; Meng, C.; Ur Rehman, Z.; Tan, L.; et al. Newcastle Disease Virus V Protein Degrades Mitochondrial Antiviral Signaling Protein To Inhibit Host Type I Interferon Production via E3 Ubiquitin Ligase RNF5. J. Virol. 2019, 93, e00322-19. [Google Scholar] [CrossRef]
- Yang, Y.; Xue, J.; Teng, Q.; Li, X.; Bu, Y.; Zhang, G. Mechanisms and consequences of Newcastle disease virus W protein subcellular localization in the nucleus or mitochondria. J. Virol. 2021, 95, e02087-20. [Google Scholar] [CrossRef]
- Afonso, C.L. Virulence during Newcastle Disease Viruses Cross Species Adaptation. Viruses 2021, 13, 110. [Google Scholar] [CrossRef]
- Ul-Rahman, A.; Ishaq, H.M.; Raza, M.A.; Shabbir, M.Z. Zoonotic potential of Newcastle disease virus: Old and novel perspectives related to public health. Rev. Med. Virol. 2022, 32, e2246. [Google Scholar] [CrossRef]
- Rahman, A.; Habib, M.; Shabbir, M.Z. Adaptation of Newcastle Disease Virus (NDV) in Feral Birds and their Potential Role in Interspecies Transmission. Open Virol. J. 2018, 12 (Suppl. 2), 52–68. [Google Scholar] [CrossRef]
- Lowenthal, J.; Bean, A.; Kogut, M.; Kapczynski, D.R.; Afonso, C.L.; Miller, P.J. Immune responses of poultry to Newcastle disease virus. Dev. Comp. Immunol. 2013, 41, 447–453. [Google Scholar]
- Hu, Z.L.; He, X.Z.; Deng, J.; Hu, J.; Liu, X.F. Current situation and future direction of Newcastle disease vaccines. Vet. Res. 2022, 53, e2246. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, K.M.; Afonso, C.L.; Yu, Q.Z.; Miller, P.J. Newcastle disease vaccines-A solved problem or a continuous challenge? Vet. Microbiol. 2017, 206, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Ma, S.M.; Schrickel, P.L.; Zhao, P.W.; Wang, J.Y.; Zhang, Y.H.; Li, S.Y.; Wang, C.B. Review detection of Newcastle disease virus. Front. Vet. Sci. 2022, 9, 936251. [Google Scholar] [CrossRef] [PubMed]
- Ganar, K.; Das, M.; Sinha, S.; Kumar, S. Newcastle disease virus: Current status and our understanding. Virus Res. 2014, 184, 71–81. [Google Scholar] [CrossRef]
- Dortmans, J.; Koch, G.; Rottier, P.J.M.; Peeters, B.P.H. Virulence of Newcastle disease virus: What is known so far? Vet. Res. 2011, 42, 122. [Google Scholar] [CrossRef]
- Alexander, D.J. Newcastle disease and other avian paramyxoviruses. Rev. Sci. Tech.-Off. Int. Epizoot. 2000, 19, 443–462. [Google Scholar] [CrossRef]
- Brown, C.; King, D.J.; Seal, B.S. Pathogenesis of Newcastle disease in chickens experimentally infected with viruses of different virulence. Vet. Pathol. 1999, 36, 125–132. [Google Scholar] [CrossRef]
- Cui, N.; Huang, X.; Kong, Z.; Huang, Y.; Huang, Q.; Yang, S.; Zhang, L.; Xu, C.; Zhang, X.; Cui, Y. Newcastle Disease Virus Infection Interferes with the Formation of Intestinal Microflora in Newly Hatched Specific-Pathogen-Free Chicks. Front. Microbiol. 2018, 9, 900. [Google Scholar] [CrossRef]
- Xiang, B.; Chen, R.J.; Liang, J.P.; Chen, L.B.; Lin, Q.Y.; Sun, M.H.; Kang, Y.F.; Ding, C.; Liao, M.; Xu, C.G.; et al. Phylogeny, pathogenicity and transmissibility of a genotype XII Newcastle disease virus in chicken and goose. Transbound. Emerg. Dis. 2020, 67, 159–170. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Ren, S.H.; Butt, S.L.; Manzoor, Z.; Iqbal, J.; Anwar, M.N.; Sun, Y.J.; Qiu, X.S.; Tan, L.; Liao, Y.; et al. Newcastle Disease Virus Induced Pathologies Severely Affect the Exocrine and Endocrine Functions of the Pancreas in Chickens. Genes 2021, 12, 495. [Google Scholar] [CrossRef]
- Thomazelli, L.M.; Sinhorini, J.A.; Oliveira, D.B.L.; Knoebl, T.; Bosqueiro, T.C.M.; Sano, E.; Costa, G.C.V.; Monteiro, C.; Dorlass, E.G.; Utecht, N.; et al. An Outbreak in Pigeons Caused by the Subgenotype VI.2.1.2 of Newcastle Disease Virus in Brazil. Viruses 2021, 13, 2446. [Google Scholar] [CrossRef]
- Ellakany, H.F.; Elbestawy, A.R.; Abd El-Hamid, H.S.; Zedan, R.E.; Gado, A.R.; Taha, A.E.; Soliman, M.A.; Abd El-Hack, M.E.; Swelum, A.A.; Saadeldin, I.M.; et al. Role of Pigeons in the Transmission of Avian Avulavirus (Newcastle Disease-Genotype VIId) to Chickens. Animals 2019, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Elbestawy, A.R.; Ellakany, H.F.; Abd El-Hamid, H.S.; Zedan, R.E.; Gado, A.R.; Sedeik, M.E.; Abd El-Hack, M.E.; Saadeldin, I.M.; Alowaimer, A.N.; Ba-Awadh, H.A.; et al. Muscovy ducks infected with velogenic Newcastle disease virus (genotype VIId) act as carriers to infect in-contact chickens. Poult. Sci. 2019, 98, 4441–4448. [Google Scholar] [CrossRef] [PubMed]
- Nooruzzaman, M.; Barman, L.R.; Mumu, T.T.; Chowdhury, E.H.; Dimitrov, K.M.; Islam, M.R. A Pigeon-Derived Sub-Genotype XXI.1.2 Newcastle Disease Virus from Bangladesh Induces High Mortality in Chickens. Viruses 2021, 13, 1520. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.B.; Yusoff, K.; Ideris, A.; Hair-Bejo, M.; Peeters, B.P.H.; Omar, A.R. Diagnostic and Vaccination Approaches for Newcastle Disease Virus in Poultry: The Current and Emerging Perspectives. BioMed Res. Int. 2018, 2018, 7278459. [Google Scholar] [CrossRef]
- Anis, Z.; Morita, T.; Azuma, K.; Ito, H.; Ito, T.; Shimada, A. Histopathological alterations in immune organs of chickens and ducks after experimental infection with virulent 9a5b newcastle disease virus. J. Comp. Pathol. 2013, 149, 82–93. [Google Scholar] [CrossRef]
- Harrison, L.; Brown, C.; Afonso, C.; Zhang, J.; Susta, L. Early occurrence of apoptosis in lymphoid tissues from chickens infected with strains of Newcastle disease virus of varying virulence. J. Comp. Pathol. 2011, 145, 327–335. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Ren, J.; Liu, H.; Adam, F.A.; Wang, X.; Yang, Z. Insights into the chicken bursa of fabricius response to Newcastle disease virus at 48 and 72 hours post-infection through RNA-seq. Vet. Microbiol. 2019, 236, 108389. [Google Scholar] [CrossRef]
- Lu, A.; Diao, Y.; Chen, H.; Wang, J.; Ge, P.; Sun, X.; Hao, D. Evaluation of histopathological changes, viral load and immune function of domestic geese infected with Newcastle disease virus. Avian Pathol. 2014, 43, 325–332. [Google Scholar] [CrossRef]
- Rasoli, M.; Yeap, S.K.; Tan, S.W.; Moeini, H.; Ideris, A.; Bejo, M.H.; Alitheen, N.B.; Kaiser, P.; Omar, A.R. Alteration in lymphocyte responses, cytokine and chemokine profiles in chickens infected with genotype VII and VIII velogenic Newcastle disease virus. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 11–21. [Google Scholar] [CrossRef]
- Hamisu, T.M.; Aliyu, H.B.; Hair-Bejo, M.; Omar, A.R.; Ideris, A. Alteration in the Population of Intraepithelial Lymphocytes and Virus Shedding in Specific-Pathogen-Free Chickens Following Inoculation with Lentogenic and Velogenic Newcastle Disease Virus Strains. Viral. Immunol. 2022, 35, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Anis, Z.; Morita, T.; Azuma, K.; Ito, H.; Ito, T.; Shimada, A. Comparative study on the pathogenesis of the generated 9a5b Newcastle disease virus mutant isolate between chickens and waterfowl. Vet. Pathol. 2013, 50, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Arslan, M.; Liu, X.; Song, H.; Du, M.; Li, Y.; Zhang, Z. IFN-γ establishes interferon-stimulated gene-mediated antiviral state against Newcastle disease virus in chicken fibroblasts. Acta Biochim. Biophys. Sin. 2020, 52, 268–280. [Google Scholar] [CrossRef]
- Zhang, T.; Ren, M.; Liu, C.; Xu, L.; Wang, F.; Han, Z.; Shao, Y.; Ma, D. Comparative analysis of early immune responses induced by two strains of Newcastle disease virus in chickens. Microbiologyopen 2019, 8, e00701. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chen, Y.; Zhao, W.; Zhang, T.; Liu, C.; Qi, T.; Han, Z.; Shao, Y.; Ma, D.; Liu, S. Infection of Goose with Genotype VIId Newcastle Disease Virus of Goose Origin Elicits Strong Immune Responses at Early Stage. Front. Microbiol. 2016, 7, 1587. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, O.; Abdallah, F.; Ali, H.A.; AlGabr, N.; Mohamed, M.H.A. Different kinetics of chicken interferon-alpha signalling transduction responses following immunization of broiler chickens with different Newcastle disease virus vaccines and infection with virulent genotype VIId strain. Avian Pathol. 2021, 50, 85–97. [Google Scholar] [CrossRef]
- Kang, Y.; Feng, M.; Zhao, X.; Dai, X.; Xiang, B.; Gao, P.; Li, Y.; Li, Y.; Ren, T. Newcastle disease virus infection in chicken embryonic fibroblasts but not duck embryonic fibroblasts is associated with elevated host innate immune response. Virol. J. 2016, 13, 41. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, J.; Hu, S.; Liu, X.; Wang, X.; Zhu, J.; Liu, X. Strong innate immune response and cell death in chicken splenocytes infected with genotype VIId Newcastle disease virus. Virol. J. 2012, 9, 208. [Google Scholar] [CrossRef]
- Kaiser, A.; Willer, T.; Sid, H.; Petersen, H.; Baumgärtner, W.; Steinberg, P.; Rautenschlein, S. Susceptibility of primary chicken intestinal epithelial cells for low pathogenic avian influenza virus and velogenic viscerotropic Newcastle disease virus. Virus Res. 2016, 225, 50–63. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, X.; Song, C.; Sun, Y.; Meng, C.; Liao, Y.; Tan, L.; Ding, Z.; Liu, X.; Ding, C. Deep Sequencing-Based Transcriptome Profiling Reveals Avian Interferon-Stimulated Genes and Provides Comprehensive Insight into Newcastle Disease Virus-Induced Host Responses. Viruses 2018, 10, 162. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.S.; Rehman, S.U.; Yousaf, W.; Hassan, F.U.; Ahmad, W.; Liu, Q.; Pan, H. The Potential of Toll-Like Receptors to Modulate Avian Immune System: Exploring the Effects of Genetic Variants and Phytonutrients. Front. Genet. 2021, 12, 671235. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Park, Y.H.; Chungu, K.; Woo, S.J.; Han, S.T.; Choi, H.J.; Rengaraj, D.; Han, J.Y. Targeted Knockout of MDA5 and TLR3 in the DF-1 Chicken Fibroblast Cell Line Impairs Innate Immune Response Against RNA Ligands. Front. Immunol. 2020, 11, 678. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, Y.; Zhang, X.; Zhang, F.; Zhang, S.; Yu, S.; Qiu, X.; Tan, L.; Song, C.; Gao, S.; et al. Toll-like receptor 3 inhibits Newcastle disease virus replication through activation of pro-inflammatory cytokines and the type-1 interferon pathway. Arch Virol. 2014, 159, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, J.; Ren, J.; Hall, K.H.; Moorman, J.P.; Yao, Z.Q.; Ning, S. Protein phosphatase 1 abrogates IRF7-mediated type I IFN response in antiviral immunity. Eur. J. Immunol. 2016, 46, 2409–2419. [Google Scholar] [CrossRef]
- Elfeil, W.K.; Abouelmaatti, R.R.; Talat, S.; Fawzy, M.; Rady, M.; Diab, M.; Alkahtani, S.; Sultan, H.; Sun, C.; Lei, L.; et al. Molecular characterization of Toll-like receptor type-3 in mallard duck and its response to Newcastle disease virus infection. Environ. Sci. Pollut. Res. Int. 2021, 28, 55786–55795. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, Y.; Wang, H.; Shi, S.; Yan, Y.; Li, J.; Ding, C.; Sun, J. Cloning, expression and functional analysis of the duck Toll-like receptor 5 (TLR5) gene. J. Vet. Sci. 2015, 16, 37–46. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhuang, M.W.; Han, L.; Zhang, J.; Nan, M.L.; Zhan, P.; Kang, D.; Liu, X.; Gao, C.; Wang, P.H. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling. Signal Transduct. Target. Ther. 2020, 5, 299. [Google Scholar] [CrossRef]
- Jiang, Z.; Wei, F.; Zhang, Y.; Wang, T.; Gao, W.; Yu, S.; Sun, H.; Pu, J.; Sun, Y.; Wang, M.; et al. IFI16 directly senses viral RNA and enhances RIG-I transcription and activation to restrict influenza virus infection. Nat. Microbiol. 2021, 6, 932–945. [Google Scholar] [CrossRef]
- Guo, H.Y.; Zhang, X.C.; Jia, R.Y. Toll-Like Receptors and RIG-I-Like Receptors Play Important Roles in Resisting Flavivirus. J. Immunol. Res. 2018, 2018, 6106582. [Google Scholar] [CrossRef]
- Gitlin, L.; Barchet, W.; Gilfillan, S.; Cella, M.; Beutler, B.; Flavell, R.A.; Diamond, M.S.; Colonna, M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 2006, 103, 8459–8464. [Google Scholar] [CrossRef] [PubMed]
- Goubau, D.; Schlee, M.; Deddouche, S.; Pruijssers, A.J.; Zillinger, T.; Goldeck, M.; Schuberth, C.; Van der Veen, A.G.; Fujimura, T.; Rehwinkel, J.; et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature 2014, 514, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.; Wilden, H.; Schirrmacher, V. Importance of retinoic acid-inducible gene I and of receptor for type I interferon for cellular resistance to infection by Newcastle disease virus. Int. J. Oncol. 2012, 40, 287–298. [Google Scholar]
- Sun, Y.; Ding, N.; Ding, S.S.; Yu, S.; Meng, C.; Chen, H.; Qiu, X.; Zhang, S.; Yu, Y.; Zhan, Y.; et al. Goose RIG-I functions in innate immunity against Newcastle disease virus infections. Mol. Immunol. 2013, 53, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. Signaling through RIG-I and type I interferon receptor: Immune activation by Newcastle disease virus in man versus immune evasion by Ebola virus (Review). Int. J. Mol. Med. 2015, 36, 3–10. [Google Scholar] [CrossRef]
- Wilden, H.; Fournier, P.; Zawatzky, R.; Schirrmacher, V. Expression of RIG-I, IRF3, IFN-beta and IRF7 determines resistance or susceptibility of cells to infection by Newcastle Disease Virus. Int. J. Oncol. 2009, 34, 971–982. [Google Scholar]
- Barber, M.R.; Aldridge, J.R., Jr.; Webster, R.G.; Magor, K.E. Association of RIG-I with innate immunity of ducks to influenza. Proc. Natl. Acad. Sci. USA 2010, 107, 5913–5918. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, Y.; Yuan, R.; Feng, M.; Xiang, B.; Sun, M.; Li, Y.; Xie, P.; Tan, Y.; Ren, T. Host Innate Immune Responses of Ducks Infected with Newcastle Disease Viruses of Different Pathogenicities. Front. Microbiol. 2015, 6, 1283. [Google Scholar] [CrossRef]
- Xu, L.; Yu, D.; Fan, Y.; Liu, Y.P.; Yao, Y.G. Evolutionary selection on MDA5 and LGP2 in the chicken preserves antiviral competence in the absence of RIG-I. J. Genet. Genomics 2019, 46, 499–503. [Google Scholar] [CrossRef]
- Karpala, A.J.; Stewart, C.; McKay, J.; Lowenthal, J.W.; Bean, A.G. Characterization of chicken Mda5 activity: Regulation of IFN-β in the absence of RIG-I functionality. J. Immunol. 2011, 186, 5397–5405. [Google Scholar] [CrossRef]
- Diaz-Beneitez, E.; Cubas-Gaona, L.L.; Candelas-Rivera, O.; Benito-Zafra, A.; Sánchez-Aparicio, M.T.; Miorin, L.; Rodríguez, J.F.; García-Sastre, A.; Rodríguez, D. Interaction between chicken TRIM25 and MDA5 and their role in mediated antiviral activity against IBDV infection. Front. Microbiol. 2022, 13, 1068328. [Google Scholar] [CrossRef]
- Liniger, M.; Summerfield, A.; Zimmer, G.; McCullough, K.C.; Ruggli, N. Chicken cells sense influenza A virus infection through MDA5 and CARDIF signaling involving LGP2. J. Virol. 2012, 86, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cui, P.; Ni, R.; Gong, H.; Li, H.; Yan, W.; Fu, X.; Chen, L.; Lei, C.; Wang, H.; et al. Chicken-Derived Pattern Recognition Receptor chLGP2 Inhibits the Replication and Proliferation of Infectious Bronchitis Virus. Front. Microbiol. 2021, 12, 810215. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, K.; Liu, C.; Wang, J.; Tan, D.; Han, X.; Tang, C.; Zhang, Y.; Wang, J. Strong inflammatory responses and apoptosis in the oviducts of egg-laying hens caused by genotype VIId Newcastle disease virus. BMC Vet. Res. 2016, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Zhu, W.; Li, Y.; Gao, P.; Liang, J.; Liu, D.; Ding, C.; Liao, M.; Kang, Y.; Ren, T. Immune responses of mature chicken bone-marrow-derived dendritic cells infected with Newcastle disease virus strains with differing pathogenicity. Arch Virol. 2018, 163, 1407–1417. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Y.; Chen, H.; Dai, Y.; Zhan, Y.; Yu, S.; Qiu, X.; Tan, L.; Song, C.; Ding, C. Activation of the PKR/eIF2α signaling cascade inhibits replication of Newcastle disease virus. Virol. J. 2014, 11, 62. [Google Scholar] [CrossRef]
- Yang, Y.L.; Reis, L.F.; Pavlovic, J.; Aguzzi, A.; Schäfer, R.; Kumar, A.; Williams, B.R.; Aguet, M.; Weissmann, C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. Embo J. 1995, 14, 6095–6106. [Google Scholar] [CrossRef]
- Wan, D.; Jiang, W.; Hao, J. Research Advances in How the cGAS-STING Pathway Controls the Cellular Inflammatory Response. Front. Immunol. 2020, 11, 615. [Google Scholar] [CrossRef]
- Li, S.; Yang, J.; Zhu, Y.; Ji, X.; Wang, K.; Jiang, S.; Luo, J.; Wang, H.; Zheng, W.; Chen, N.; et al. Chicken DNA Sensing cGAS-STING Signal Pathway Mediates Broad Spectrum Antiviral Functions. Vaccines 2020, 8, 369. [Google Scholar] [CrossRef]
- Cao, X.; Xue, Y.J.; Du, J.L.; Xu, Q.; Yang, X.C.; Zeng, Y.; Wang, B.B.; Wang, H.Z.; Liu, J.; Cai, K.Z.; et al. Induction and Suppression of Innate Antiviral Responses by Hepatitis A Virus. Front. Microbiol. 2018, 9, 1865. [Google Scholar] [CrossRef]
- Taschuk, F.; Cherry, S. DEAD-Box Helicases: Sensors, Regulators, and Effectors for Antiviral Defense. Viruses 2020, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, J.; Zhu, W.; Yu, X.; Wang, Z.; Ma, J.; Wang, H.; Yan, Y.; Sun, J.; Cheng, Y. Chicken DDX1 Acts as an RNA Sensor to Mediate IFN-β Signaling Pathway Activation in Antiviral Innate Immunity. Front. Immunol. 2021, 12, 742074. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kirubaharan, J.J.; Chandran, N.D.; Gnanapriya, N. Transcriptional response of chicken embryo cells to Newcastle disease virus (D58 strain) infection. Indian J. Virol. 2013, 24, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Sakai, K.; Matsumoto, M.; Seya, T. DEAD/H BOX 3 (DDX3) helicase binds the RIG-I adaptor IPS-1 to up-regulate IFN-beta-inducing potential. Eur. J. Immunol. 2010, 40, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Susta, L.; Cornax, I.; Diel, D.G.; Garcia, S.C.; Miller, P.J.; Liu, X.; Hu, S.; Brown, C.C.; Afonso, C.L. Expression of interferon gamma by a highly virulent strain of Newcastle disease virus decreases its pathogenicity in chickens. Microb. Pathog. 2013, 61–62, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, J.; Zhao, S.; Li, Y.; Zhang, Y.; Wang, Y.; Yan, Y.; Cheng, Y.; Sun, J. Goose IRF7 is involved in antivirus innate immunity by mediating IFN activation. Dev. Comp. Immunol. 2022, 133, 104435. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ning, Z.; Sun, M.; Gao, S.; Kang, Y.; Xie, P.; Ren, T. Interferon regulatory factor 7- (IRF7-) mediated immune response affects Newcastle disease virus replication in chicken embryo fibroblasts. Acta Vet. Hung. 2014, 62, 500–511. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, J.; Zhang, N.; Yi, J.; Wang, Z.; Ma, J.; Wang, H.; Yan, Y.; Qian, K.; Sun, J.; et al. Functional characterization of goose IRF1 in IFN induction and anti-NDV infection. Vet. Res. 2022, 53, 29. [Google Scholar] [CrossRef]
- Fu, F.; Lin, Z.; Li, Y.; Wang, J.; Li, Y.; Liu, P.; Wang, Z.; Ma, J.; Yan, Y.; Sun, J.; et al. Goose STING mediates IFN signaling activation against RNA viruses. Front. Immunol. 2022, 13, 921800. [Google Scholar] [CrossRef]
- Liu, H.; Tian, J.; Lu, K.; Li, Y.; Guan, Z.; Cao, X.; Li, X.; Chang, Z.; Wang, X.; Sa, X.; et al. Chicken ISG12(2) attenuates Newcastle disease virus and enhances the efficiency of Newcastle disease vaccine via activating immune pathways. Transbound. Emerg. Dis. 2022, 69, 2634–2648. [Google Scholar] [CrossRef]
- Jia, Y.Q.; Wang, X.W.; Chen, X.; Qiu, X.X.; Wang, X.L.; Yang, Z.Q. Characterization of chicken IFI35 and its antiviral activity against Newcastle disease virus. J. Vet. Med. Sci. 2022, 84, 473–483. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, Y.; Zhang, R.; Guan, Y.; Jiang, N.; Zheng, N.; Wu, Z. Nonstructural Protein NSs Hampers Cellular Antiviral Response through LSm14A during Severe Fever with Thrombocytopenia Syndrome Virus Infection. J. Immunol. 2021, 207, 590–601. [Google Scholar] [CrossRef]
- Tian, L.; Wu, C.; Wen, G.; Li, C. Transcriptional responses of LSm14A after infection of blue eggshell layers with Newcastle disease viruses. J. Vet. Med. Sci. 2019, 81, 1468–1474. [Google Scholar] [CrossRef]
- Niu, Q.; Cheng, Y.; Wang, H.; Yan, Y.; Sun, J. Chicken DDX3X Activates IFN-β via the chSTING-chIRF7-IFN-β Signaling Axis. Front. Immunol. 2019, 10, 822. [Google Scholar] [CrossRef]
- Huang, Z.H.; Krishnamurthy, S.; Panda, A.; Samal, S.K. Newcastle disease virus V protein is associated with viral pathogenesis and functions as an alpha interferon antagonist. J. Virol. 2003, 77, 8676–8685. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Fu, Q.; Meng, C.; Yu, S.; Zhan, Y.; Dong, L.; Song, C.; Sun, Y.; Tan, L.; Hu, S.; et al. Newcastle Disease Virus V Protein Targets Phosphorylated STAT1 to Block IFN-I Signaling. PLoS ONE 2016, 11, e0148560. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dang, R.; Yang, Z. The interferon antagonistic activities of the V proteins of NDV correlated with their virulence. Virus Genes 2019, 55, 233–237. [Google Scholar] [CrossRef]
- Wang, X.W.; Jia, Y.Q.; Ren, J.; Huo, N.; Liu, H.J.; Xiao, S.; Wang, X.L.; Yang, Z.Q. Newcastle Disease Virus Nonstructural V Protein Upregulates SOCS3 Expression to Facilitate Viral Replication Depending on the MEK/ERK Pathway. Front. Cell. Infect. Microbiol. 2019, 9, 317. [Google Scholar] [CrossRef]
- Duan, Z.; Xing, J.; Shi, H.; Wang, Y.; Zhao, C. The matrix protein of Newcastle disease virus inhibits inflammatory response through IRAK4/TRAF6/TAK1/NF-κB signaling pathway. Int. J. Biol. Macromol. 2022, 218, 295–309. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Ren, J.; Liu, H.; Xiao, S.; Wang, X.; Yang, Z. MicroRNA gga-miR-455-5p suppresses Newcastle disease virus replication via targeting cellular suppressors of cytokine signaling 3. Vet. Microbiol. 2019, 239, 108460. [Google Scholar] [CrossRef]
- Zhou, C.L.; Tan, L.; Sun, Y.J.; Qiu, X.S.; Meng, C.C.; Liao, Y.; Song, C.P.; Liu, W.W.; Nair, V.; Ding, C. Exosomes Carry microRNAs into Neighboring Cells to Promote Diffusive Infection of Newcastle Disease Virus. Viruses 2019, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.T.; Ou, J.J. Hepatitis C Virus-Induced Autophagy and Host Innate Immune Response. Viruses 2017, 9, 224. [Google Scholar] [CrossRef]
- Heaton, N.S.; Randall, G. Dengue virus and autophagy. Viruses 2011, 3, 1332–1341. [Google Scholar] [CrossRef]
- Chiramel, A.I.; Best, S.M. Role of autophagy in Zika virus infection and pathogenesis. Virus Res. 2018, 254, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Zhou, Z.; Jiang, K.; Yu, S.; Jia, L.; Wu, Y.; Liu, Y.; Meng, S.; Ding, C. Newcastle disease virus triggers autophagy in U251 glioma cells to enhance virus replication. Arch Virol. 2012, 157, 1011–1018. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, S.; Ding, N.; Meng, C.; Meng, S.; Zhang, S.; Zhan, Y.; Qiu, X.; Tan, L.; Chen, H.; et al. Autophagy benefits the replication of Newcastle disease virus in chicken cells and tissues. J. Virol. 2014, 88, 525–537. [Google Scholar] [CrossRef]
- Ye, T.; Jiang, K.; Wei, L.; Barr, M.P.; Xu, Q.; Zhang, G.; Ding, C.; Meng, S.; Piao, H. Oncolytic Newcastle disease virus induces autophagy-dependent immunogenic cell death in lung cancer cells. Am. J. Cancer Res. 2018, 8, 1514–1527. [Google Scholar]
- Kang, Y.; Yuan, R.; Xiang, B.; Zhao, X.; Gao, P.; Dai, X.; Liao, M.; Ren, T. Newcastle disease virus-induced autophagy mediates antiapoptotic signaling responses in vitro and in vivo. Oncotarget 2017, 8, 73981–73993. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, Y.J.; Zhang, F.Q.; Zhang, X.R.; Qiu, X.S.; Yu, L.P.; Wu, Y.T.; Ding, C. Newcastle disease virus NP and P proteins induce autophagy via the endoplasmic reticulum stress-related unfolded protein response. Sci. Rep. 2016, 6, 24721. [Google Scholar] [CrossRef]
- Wang, C.; Wang, T.; Hu, R.; Dai, J.; Liu, H.; Li, N.; Schneider, U.; Yang, Z.; Wang, J. Cyclooxygenase-2 Facilitates Newcastle Disease Virus Proliferation and Is as a Target for Canthin-6-One Antiviral Activity. Front. Microbiol. 2020, 11, 987. [Google Scholar] [CrossRef]
- Ren, S.; Rehman, Z.U.; Shi, M.; Yang, B.; Qu, Y.; Yang, X.F.; Shao, Q.; Meng, C.; Yang, Z.; Gao, X.; et al. Syncytia generated by hemagglutinin-neuraminidase and fusion proteins of virulent Newcastle disease virus induce complete autophagy by activating AMPK-mTORC1-ULK1 signaling. Vet. Microbiol. 2019, 230, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Tang, N.; Liu, P.; Sun, Y.; Lu, S.; Liu, W.; Tan, L.; Song, C.; Qiu, X.; Liao, Y.; et al. Newcastle disease virus degrades SIRT3 via PINK1-PRKN-dependent mitophagy to reprogram energy metabolism in infected cells. Autophagy 2022, 18, 1503–1521. [Google Scholar] [CrossRef]
- Hu, L.; Sun, S.; Wang, T.; Li, Y.; Jiang, K.; Lin, G.; Ma, Y.; Barr, M.P.; Song, F.; Zhang, G.; et al. Oncolytic newcastle disease virus triggers cell death of lung cancer spheroids and is enhanced by pharmacological inhibition of autophagy. Am. J. Cancer Res. 2015, 5, 3612–3623. [Google Scholar] [PubMed]
- Shao, X.; Wang, X.; Guo, X.; Jiang, K.; Ye, T.; Chen, J.; Fang, J.; Gu, L.; Wang, S.; Zhang, G.; et al. STAT3 Contributes to Oncolytic Newcastle Disease Virus-Induced Immunogenic Cell Death in Melanoma Cells. Front. Oncol. 2019, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari Nejad, A.S.; Fotouhi, F.; Mehrbod, P.; Keshavarz, M.; Alikhani, M.Y.; Ghaemi, A. Oncolytic effects of Hitchner B1 strain of newcastle disease virus against cervical cancer cell proliferation is mediated by the increased expression of cytochrome C, autophagy and apoptotic pathways. Microb. Pathog. 2020, 147, 104438. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Lam, K.M.; Vasconcelos, A.C. Newcastle disease virus-induced apoptosis in chicken peripheral blood lymphocytes. Vet. Immunol. Immunopathol. 1994, 44, 45–56. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, Y.; Qiao, C.; Yuan, Y.; Sun, Y.; Qiu, X.; Meng, C.; Song, C.; Liao, Y.; Munir, M.; et al. NDV entry into dendritic cells through macropinocytosis and suppression of T lymphocyte proliferation. Virology 2018, 518, 126–135. [Google Scholar] [CrossRef]
- Kristeen-Teo, Y.W.; Yeap, S.K.; Tan, S.W.; Omar, A.R.; Ideris, A.; Tan, S.G.; Alitheen, N.B. The effects of different velogenic NDV infections on the chicken bursa of Fabricius. BMC Vet. Res. 2017, 13, 151. [Google Scholar] [CrossRef]
- Kang, Y.; Yuan, R.; Zhao, X.; Xiang, B.; Gao, S.; Gao, P.; Dai, X.; Feng, M.; Li, Y.; Xie, P.; et al. Transient activation of the PI3K/Akt pathway promotes Newcastle disease virus replication and enhances anti-apoptotic signaling responses. Oncotarget 2017, 8, 23551–23563. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, H.X.; Mao, X.; Fang, H.; Wang, H.; Li, Y.; Sun, Y.; Meng, C.; Tan, L.; Song, C.; et al. RIP1 is a central signaling protein in regulation of TNF-α/TRAIL mediated apoptosis and necroptosis during Newcastle disease virus infection. Oncotarget 2017, 8, 43201–43217. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, H.; Xie, N.; Liu, D.; Jiang, Y.; Liu, Z.; Ye, D.; Liu, S.; Chen, X.; Li, C.; et al. Bcl-3 promotes TNF-induced hepatocyte apoptosis by regulating the deubiquitination of RIP1. Cell Death Differ. 2022, 29, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, W.; Niu, Q.; Sun, Y.; Meng, C.; Tan, L.; Song, C.; Qiu, X.; Liao, Y.; Ding, C. eIF2α-CHOP-BCl-2/JNK and IRE1α-XBP1/JNK signaling promote apoptosis and inflammation and support the proliferation of Newcastle disease virus. Cell Death Dis. 2019, 10, 891. [Google Scholar] [CrossRef]
- Mohammed, M.S.; Al-Taee, M.F.; Al-Shammari, A.M. Caspase Dependent and Independent Anti-hematological Malignancy Activity of AMHA1 Attenuated Newcastle Disease Virus. Int. J. Mol. Cell Med. 2019, 8, 211–223. [Google Scholar] [PubMed]
- Molouki, A.; Hsu, Y.T.; Jahanshiri, F.; Abdullah, S.; Rosli, R.; Yusoff, K. The matrix (M) protein of Newcastle disease virus binds to human bax through its BH3 domain. Virol. J. 2011, 8, 385. [Google Scholar] [CrossRef]
- Chu, Z.; Wang, C.; Tang, Q.; Shi, X.; Gao, X.; Ma, J.; Lu, K.; Han, Q.; Jia, Y.; Wang, X.; et al. Newcastle Disease Virus V Protein Inhibits Cell Apoptosis and Promotes Viral Replication by Targeting CacyBP/SIP. Front. Cell Infect Microbiol. 2018, 8, 304. [Google Scholar] [CrossRef]
- Yang, M.; Ma, J.; Chu, Z.; Cao, X.; Lu, K.; Shi, X.; Tong, L.; Yan, C.; Liu, H.; Wang, X.; et al. Musashi1 inhibit the release of Newcastle disease viruses through preventing apoptosis of DF-1 cells. Poult. Sci. 2021, 100, 101105. [Google Scholar] [CrossRef]
- Ravindra, P.V.; Tiwari, A.K.; Sharma, B.; Rajawat, Y.S.; Ratta, B.; Palia, S.; Sundaresan, N.R.; Chaturvedi, U.; Gangaplara, A.; Chindera, K.; et al. HN protein of Newcastle disease virus causes apoptosis in chicken embryo fibroblast cells. Arch. Virol. 2008, 153, 749–754. [Google Scholar] [CrossRef]
- Mohebbi, A.; Ebrahimzadeh, M.S.; Baghban Rahimi, S.; Saeidi, M.; Tabarraei, A.; Mohebbi, S.R.; Shirian, S.; Gorji, A.; Ghaemi, A. Non-replicating Newcastle Disease Virus as an adjuvant for DNA vaccine enhances antitumor efficacy through the induction of TRAIL and granzyme B expression. Virus Res. 2019, 261, 72–80. [Google Scholar] [CrossRef]
- Shan, P.; Tang, B.; Xie, S.; Zhang, Z.; Fan, J.; Wei, Z.; Song, C. NDV-D90 inhibits 17β-estradiol-mediated resistance to apoptosis by differentially modulating classic and nonclassic estrogen receptors in breast cancer cells. J. Cell Biochem. 2021, 122, 3–15. [Google Scholar] [CrossRef]
- Mansour, M.; Palese, P.; Zamarin, D. Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J. Virol. 2011, 85, 6015–6023. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Nejad, A.S.M.; Esghaei, M.; Bokharaei-Salim, F.; Dianat-Moghadam, H.; Keyvani, H.; Ghaemi, A. Oncolytic Newcastle disease virus reduces growth of cervical cancer cell by inducing apoptosis. Saudi J. Biol. Sci. 2020, 27, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shao, X.; Gu, L.; Jiang, K.; Wang, S.; Chen, J.; Fang, J.; Guo, X.; Yuan, M.; Shi, J.; et al. Targeting STAT3 enhances NDV-induced immunogenic cell death in prostate cancer cells. J. Cell Mol. Med. 2020, 24, 4286–4297. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Chen, Y.; Hong, X.; Liu, X.; Su, X.; Li, S.; Dong, X.; Zhao, G.; Li, Y. Newcastle disease virus enhances the growth-inhibiting and proapoptotic effects of temozolomide on glioblastoma cells in vitro and in vivo. Sci. Rep. 2018, 8, 11470. [Google Scholar] [CrossRef] [PubMed]
- Kazimirsky, G.; Jiang, W.; Slavin, S.; Ziv-Av, A.; Brodie, C. Mesenchymal stem cells enhance the oncolytic effect of Newcastle disease virus in glioma cells and glioma stem cells via the secretion of TRAIL. Stem Cell Res. Ther. 2016, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.L.; Yu, Y.H.; Tian, H.; Ren, G.P.; Wang, H.; Zhou, B.; Han, X.H.; Yu, Q.Z.; Li, D.S. Genetically engineered Newcastle disease virus expressing interleukin-2 and TNF-related apoptosis-inducing ligand for cancer therapy. Cancer Biol. Ther. 2014, 15, 1226–1238. [Google Scholar] [CrossRef]
- Fan, X.; Lu, H.; Cui, Y.; Hou, X.; Huang, C.; Liu, G. Overexpression of p53 delivered using recombinant NDV induces apoptosis in glioma cells by regulating the apoptotic signaling pathway. Exp. Ther. Med. 2018, 15, 4522–4530. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Liu, H.; Wang, X.; Chu, Z.; Hu, R.; Ren, J.; Xiao, S.; Zhang, S.; Wang, X.; et al. High level expression of ISG12(1) promotes cell apoptosis via mitochondrial-dependent pathway and so as to hinder Newcastle disease virus replication. Vet. Microbiol. 2019, 228, 147–156. [Google Scholar] [CrossRef]
- Del Vesco, A.P.; Jang, H.J.; Monson, M.S.; Lamont, S.J. Role of the chicken oligoadenylate synthase-like gene during in vitro Newcastle disease virus infection. Poult. Sci. 2021, 100, 101067. [Google Scholar] [CrossRef]
- Wang, C.; Chu, Z.; Liu, W.; Pang, Y.; Gao, X.; Tang, Q.; Ma, J.; Lu, K.; Adam, F.E.A.; Dang, R.; et al. Newcastle disease virus V protein inhibits apoptosis in DF-1 cells by downregulating TXNL1. Vet. Res. 2018, 49, 102. [Google Scholar] [CrossRef]
- Meng, G.; Xia, M.; Wang, D.; Chen, A.; Wang, Y.; Wang, H.; Yu, D.; Wei, J. Mitophagy promotes replication of oncolytic Newcastle disease virus by blocking intrinsic apoptosis in lung cancer cells. Oncotarget 2014, 5, 6365–6374. [Google Scholar] [CrossRef] [PubMed]
- Ginting, T.E.; Christian, S.; Larasati, Y.O.; Suryatenggara, J.; Suriapranata, I.M.; Mathew, G. Antiviral interferons induced by Newcastle disease virus (NDV) drive a tumor-selective apoptosis. Sci. Rep. 2019, 9, 15160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, R.; Li, Y.R.; Sun, Y.J.; Song, C.P.; Zhan, Y.; Tan, L.; Liao, Y.; Meng, C.C.; Qiu, X.S.; et al. Newcastle disease virus induces G(0)/G(1) cell cycle arrest in asynchronously growing cells. Virology 2018, 520, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.L.; Gao, X.L.; Liu, H.J.; Ma, J.G.; Wang, C.Y.; Lu, K.J.; Han, Q.S.; Wang, Y.H.; Wang, C.Y.; Adam, F.E.A.; et al. Newcastle disease virus selectively infects dividing cells and promotes viral proliferation. Vet. Res. 2019, 50, 27. [Google Scholar] [CrossRef] [PubMed]
- Nan, F.L.; Zheng, W.; Nan, W.L.; Yu, T.; Xie, C.Z.; Zhang, H.; Xu, X.H.; Li, C.H.; Ha, Z.; Zhang, J.Y.; et al. Newcastle Disease Virus Inhibits the Proliferation of T Cells Induced by Dendritic Cells In Vitro and In Vivo. Front. Immunol. 2021, 11, 619829. [Google Scholar] [CrossRef]
- Liu, P.R.; Tang, N.; Meng, C.C.; Yin, Y.C.; Qiu, X.S.; Tan, L.; Sun, Y.J.; Song, C.P.; Liu, W.W.; Liao, Y.; et al. SLC1A3 facilitates Newcastle disease virus replication by regulating glutamine catabolism. Virulence 2022, 13, 1407–1422. [Google Scholar] [CrossRef]
- Xue, M.G.; Zhao, B.S.; Zhang, Z.J.; Lu, M.J.; Harder, O.; Chen, P.; Lu, Z.K.; Li, A.Z.; Ma, Y.M.; Xu, Y.S.; et al. Viral N-6-methyladenosine upregulates replication and pathogenesis of human respiratory syncytial virus. Nat. Commun. 2019, 10, 4595. [Google Scholar] [CrossRef]
- Wan, Y.L.; Chen, Y.; Wang, T.; Zhao, B.; Zeng, W.; Zhang, L.Y.; Zhang, Y.M.; Cao, S.Y.; Wang, J.Y.; Xue, Q.H.; et al. PPRV-Induced Autophagy Facilitates Infectious Virus Transmission by the Exosomal Pathway. J. Virol. 2022, 96, e00244-22. [Google Scholar] [CrossRef]
- Xu, X.H.; Qian, J.; Ding, J.X.; Li, J.D.; Nan, F.L.; Wang, W.Q.; Qin, Q.; Fei, Y.D.; Xue, C.; Wang, J.Z.; et al. Detection of viral components in exosomes derived from NDV-infected DF-1 cells and their promoting ability in virus replication. Microb. Pathog. 2019, 128, 414–422. [Google Scholar] [CrossRef]
- Xu, X.H.; Zhang, D.; Ding, W.; Wang, W.Q.; Jin, N.Y.; Ding, Z. NDV related exosomes enhance NDV replication through exporting NLRX1 mRNA. Vet. Microbiol. 2021, 260, 109167. [Google Scholar] [CrossRef]
- Shang, Z.F.; Tan, S.G.; Ma, D.L. Respiratory syncytial virus: From pathogenesis to potential therapeutic strategies. Int. J. Biol. Sci. 2021, 17, 4073–4091. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.T.; Zhang, J.Y.; Chen, Z.Q.; Pan, W.; Chen, Z.R.; Yan, Y.D.; Dai, J.F. Glycosylation of viral proteins: Implication in virus-host interaction and virulence. Virulence 2022, 13, 670–683. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Ding, Z.; Xu, X. Pathologic Mechanisms of the Newcastle Disease Virus. Viruses 2023, 15, 864. https://doi.org/10.3390/v15040864
Zhang D, Ding Z, Xu X. Pathologic Mechanisms of the Newcastle Disease Virus. Viruses. 2023; 15(4):864. https://doi.org/10.3390/v15040864
Chicago/Turabian StyleZhang, Di, Zhuang Ding, and Xiaohong Xu. 2023. "Pathologic Mechanisms of the Newcastle Disease Virus" Viruses 15, no. 4: 864. https://doi.org/10.3390/v15040864
APA StyleZhang, D., Ding, Z., & Xu, X. (2023). Pathologic Mechanisms of the Newcastle Disease Virus. Viruses, 15(4), 864. https://doi.org/10.3390/v15040864




