Inhibition of Rab1B Impairs Trafficking and Maturation of SARS-CoV-2 Spike Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Antibodies
2.3. Plasmids
2.4. Transfection of Plasmids in HuH7 and HEK293ACE2 Cells
2.5. SDS–PAGE and Western Blot Analysis
2.6. Immunofluorescence (IF) and Confocal Laser Scanning Microscopy (CLSM)
2.7. Colocalization Analysis (Pearson Correlation Coefficient, PCC)
2.8. Deglycosylation Assay
2.9. Ultracentrifugation
2.10. Transfection of SARS-CoV-2 Infected Cells
3. Results
3.1. Subcellular Localization of SARS-CoV-2 Spike Protein
3.1.1. Intracellular Distribution of the Spike Protein
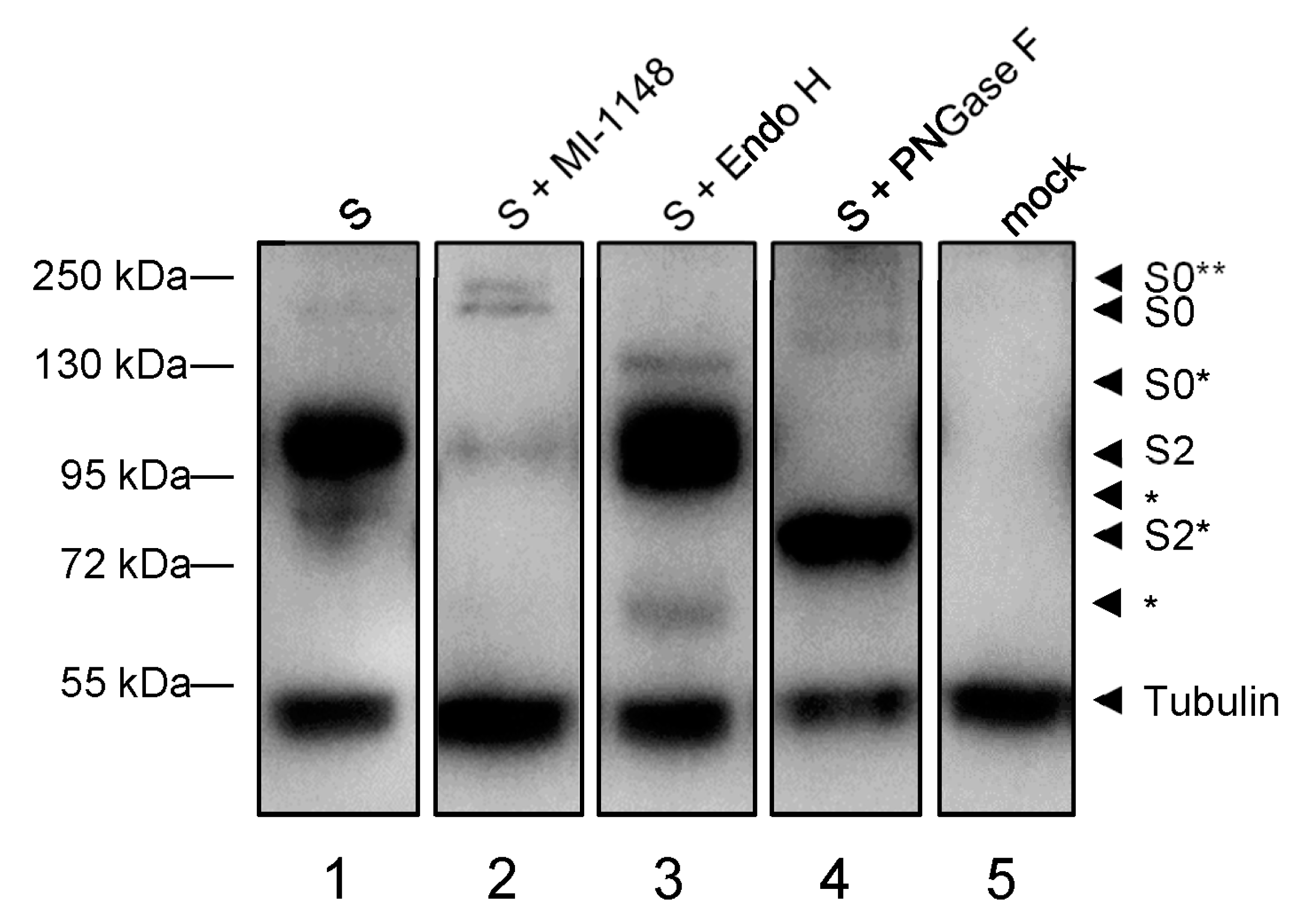
3.1.2. Maturation of the Spike Protein
3.2. Rab1B Is Critical for Transport, Maturation and Release of SARS-CoV-2 S
3.2.1. SARS-CoV-2 Spike Protein Colocalizes with Rab1A and Rab1B
3.2.2. Functional Rab1B Is Necessary for SARS-CoV-2 Spike Protein Maturation
3.3. Inhibition of Rab1 Disturbs the Integrity of Compartments of the Early Secretory Pathway
3.3.1. Rab1 Is Important for ERGIC and Golgi Organization
3.3.2. Inhibition of Rab1B Traps S in the ERGIC
3.4. Rab1B Colocalizes with S in SARS-CoV-2 Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 14 June 2021).
- Caraco, Y.; Crofoot, G.E.; Moncada, P.A.; Galustyan, A.N.; Musungaie, D.B.; Payne, B.; Kovalchuk, E.; Gonzalez, A.; Brown, M.L.; Williams-Diaz, A.; et al. Phase 2/3 Trial of Molnupiravir for Treatment of COVID-19 in Nonhospitalized Adults. NEJM Evid. 2022, 1, 2. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. COVID-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ 2021, 375, n2713. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Bonilla, H.; Jagannathan, P.; Shafer, R.W. SARS-CoV-2 Antiviral Therapy. Clin. Microbiol. Rev. 2021, 34, e0010921. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Xiao, J.; Hooper, A.T.; Hamilton, J.D.; Musser, B.J.; et al. REGEN-COV Antibody Combination and Outcomes in Outpatients with COVID-19. N. Engl. J. Med. 2021, 385, e81. [Google Scholar] [CrossRef]
- Bestle, D.; Heindl, M.R.; Limburg, H.; van Lam van, T.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance 2020, 3, 9. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Pöhlmann, S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell 2020, 78, 779.e5–784.e5. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271.e8–280.e8. [Google Scholar] [CrossRef]
- Kishimoto, M.; Uemura, K.; Sanaki, T.; Sato, A.; Hall, W.W.; Kariwa, H.; Orba, Y.; Sawa, H.; Sasaki, M. TMPRSS11D and TMPRSS13 Activate the SARS-CoV-2 Spike Protein. Viruses 2021, 13, 384. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; McLellan, J.S.; Crispin, M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020, 369, 330–333. [Google Scholar] [CrossRef]
- Aebi, M. N-linked protein glycosylation in the ER. Biochim. Biophys. Acta 2013, 1833, 2430–2437. [Google Scholar] [CrossRef]
- Nal, B.; Chan, C.; Kien, F.; Siu, L.; Tse, J.; Chu, K.; Kam, J.; Staropoli, I.; Crescenzo-Chaigne, B.; Escriou, N.; et al. Differential maturation and subcellular localization of severe acute respiratory syndrome coronavirus surface proteins S, M and E. J. Gen. Virol. 2005, 86, 1423–1434. [Google Scholar] [CrossRef]
- Helenius, A.; Aebi, M. Intracellular functions of N-linked glycans. Science 2001, 291, 2364–2369. [Google Scholar] [CrossRef]
- Galea, G.; Bexiga, M.G.; Panarella, A.; O’Neill, E.D.; Simpson, J.C. A high-content screening microscopy approach to dissect the role of Rab proteins in Golgi-to-ER retrograde trafficking. J. Cell Sci. 2015, 128, 2339–2349. [Google Scholar] [CrossRef]
- McCaughey, J.; Stephens, D.J. COPII-dependent ER export in animal cells: Adaptation and control for diverse cargo. Histochem. Cell Biol. 2018, 150, 119–131. [Google Scholar] [CrossRef]
- Westrate, L.M.; Hoyer, M.J.; Nash, M.J.; Voeltz, G.K. Vesicular and uncoated Rab1-dependent cargo carriers facilitate ER to Golgi transport. J. Cell Sci. 2020, 133, jcs.239814. [Google Scholar] [CrossRef]
- Sannerud, R.; Marie, M.; Nizak, C.; Dale, H.A.; Pernet-Gallay, K.; Perez, F.; Goud, B.; Saraste, J. Rab1 defines a novel pathway connecting the pre-Golgi intermediate compartment with the cell periphery. Mol. Biol. Cell 2006, 17, 1514–1526. [Google Scholar] [CrossRef]
- Martinez, H.; García, I.A.; Sampieri, L.; Alvarez, C. Spatial-Temporal Study of Rab1b Dynamics and Function at the ER-Golgi Interface. PLoS ONE 2016, 11, e0160838. [Google Scholar] [CrossRef]
- Galea, G.; Simpson, J.C. High-content analysis of Rab protein function at the ER-Golgi interface. Bioarchitecture 2015, 5, 44–53. [Google Scholar] [CrossRef]
- Boson, B.; Legros, V.; Zhou, B.; Siret, E.; Mathieu, C.; Cosset, F.-L.; Lavillette, D.; Denolly, S. The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles. J. Biol. Chem. 2020, 296, 100111. [Google Scholar] [CrossRef]
- Lontok, E.; Corse, E.; Machamer, C.E. Intracellular targeting signals contribute to localization of coronavirus spike proteins near the virus assembly site. J. Virol. 2004, 78, 5913–5922. [Google Scholar] [CrossRef]
- Ma, W.; Goldberg, J. Rules for the recognition of dilysine retrieval motifs by coatomer. EMBO J. 2013, 32, 926–937. [Google Scholar] [CrossRef]
- McBride, C.E.; Li, J.; Machamer, C.E. The cytoplasmic tail of the severe acute respiratory syndrome Coronavirus spike protein contains a novel endoplasmic reticulum retrieval signal that binds COPI and promotes interaction with membrane protein. J. Virol. 2007, 81, 2418–2428. [Google Scholar] [CrossRef]
- Schwegmann-Wessels, C.; Al-Falah, M.; Escors, D.; Wang, Z.; Zimmer, G.; Deng, H.; Enjuanes, L.; Naim, H.Y.; Herrler, G. A novel sorting signal for intracellular localization is present in the S protein of a porcine coronavirus but absent from severe acute respiratory syndrome-associated Coronavirus. J. Biol. Chem. 2004, 279, 43661–43666. [Google Scholar] [CrossRef] [PubMed]
- Stertz, S.; Reichelt, M.; Spiegel, M.; Kuri, T.; Martínez-Sobrido, L.; García-Sastre, A.; Weber, F.; Kochs, G. The intracellular sites of early replication and budding of SARS-Coronavirus. Virology 2007, 361, 304–315. [Google Scholar] [CrossRef]
- Tisdale, E.J.; Bourne, J.R.; Khosravi-Far, R.; Der, C.J.; Balch, W.E. GTP-binding mutants of Rab1 and Rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol. 1992, 119, 749–761. [Google Scholar] [CrossRef]
- Nuoffer, C.; Davidson, H.W.; Matteson, J.; Meinkoth, J.; Balch, W.E. A GDP-bound of Rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J. Cell Biol. 1994, 125, 225–237. [Google Scholar] [CrossRef]
- Cattin-Ortolá, J.; Welch, L.; Maslen, S.L.; Skehel, J.M.; Papa, G.; James, L.C.; Munro, S. Sequences in the cytoplasmic tail of SARS-CoV-2 Spike facilitate expression at the cell surface and syncytia formation. Nat. Commun. 2020, 12, 533. [Google Scholar] [CrossRef]
- Schweizer, A.; Fransen, J.A.; Bächi, T.; Ginsel, L.; Hauri, H.P. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J. Cell Biol. 1988, 107, 1643–1653. [Google Scholar] [CrossRef]
- Hardes, K.; Becker, G.L.; Lu, Y.; Dahms, S.O.; Köhler, S.; Beyer, W.; Sandvig, K.; Yamamoto, H.; Lindberg, I.; Walz, L.; et al. Novel Furin Inhibitors with Potent Anti-infectious Activity. ChemMedChem 2015, 10, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Go, E.P.; Ding, H.; Anang, S.; Kappes, J.C.; Desaire, H.; Sodroski, J.G. Analysis of Glycosylation and Disulfide Bonding of Wild-Type SARS-CoV-2 Spike Glycoprotein. J. Virol. 2022, 96, e0162621. [Google Scholar] [CrossRef] [PubMed]
- Bhuin, T.; Roy, J.K. Rab proteins: The key regulators of intracellular vesicle transport. Exp. Cell Res. 2014, 328, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hutagalung, A.H.; Novick, P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011, 91, 119–149. [Google Scholar] [CrossRef] [PubMed]
- Moyer, B.D.; Allan, B.B.; Balch, W.E. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis—Golgi tethering. Traffic 2001, 2, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Garcia-Mata, R.; Brandon, E.; Sztul, E. COPI recruitment is modulated by a Rab1b-dependent mechanism. Mol. Biol. Cell 2003, 14, 2116–2127. [Google Scholar] [CrossRef]
- Ward, T.H.; Polishchuk, R.S.; Caplan, S.; Hirschberg, K.; Lippincott-Schwartz, J. Maintenance of Golgi structure and function depends on the integrity of ER export. J. Cell Biol. 2001, 155, 557–570. [Google Scholar] [CrossRef]
- Haas, A.K.; Yoshimura, S.-i.; Stephens, D.J.; Preisinger, C.; Fuchs, E.; Barr, F.A. Analysis of GTPase-activating proteins: Rab1 and Rab43 are key Rabs required to maintain a functional Golgi complex in human cells. J. Cell Sci. 2007, 120, 2997–3010. [Google Scholar] [CrossRef]
- Pires De Souza, G.A.; Le Bideau, M.; Boschi, C.; Wurtz, N.; Colson, P.; Aherfi, S.; Devaux, C.; La Scola, B. Choosing a cellular model to study SARS-CoV-2. Front. Cell. Infect. Microbiol. 2022, 12, 1003608. [Google Scholar] [CrossRef]
- Satoh, A.; Tokunaga, F.; Kawamura, S.; Ozaki, K. In situ inhibition of vesicle transport and protein processing in the dominant negative Rab1 mutant of Drosophila. J. Cell Sci. 1997, 110 (Suppl. S23), 2943–2953. [Google Scholar] [CrossRef]
- Klaus, J.P.; Eisenhauer, P.; Russo, J.; Mason, A.B.; Do, D.; King, B.; Taatjes, D.; Cornillez-Ty, C.; Boyson, J.E.; Thali, M.; et al. The intracellular cargo receptor ERGIC-53 is required for the production of infectious arenavirus, Coronavirus, and filovirus particles. Cell Host Microbe 2013, 14, 522–534. [Google Scholar] [CrossRef]
- Vollenweider, F.; Kappeler, F.; Itin, C.; Hauri, H.P. Mistargeting of the lectin ERGIC-53 to the endoplasmic reticulum of HeLa cells impairs the secretion of a lysosomal enzyme. J. Cell Biol. 1998, 142, 377–389. [Google Scholar] [CrossRef]
- Mossuto, M.F.; Sannino, S.; Mazza, D.; Fagioli, C.; Vitale, M.; Yoboue, E.D.; Sitia, R.; Anelli, T. A dynamic study of protein secretion and aggregation in the secretory pathway. PLoS ONE 2014, 9, e108496. [Google Scholar] [CrossRef]
- Momose, F.; Sekimoto, T.; Ohkura, T.; Jo, S.; Kawaguchi, A.; Nagata, K.; Morikawa, Y. Apical transport of influenza A virus ribonucleoprotein requires Rab11-positive recycling endosome. PLoS ONE 2011, 6, e21123. [Google Scholar] [CrossRef]
- Amorim, M.J.; Bruce, E.A.; Read, E.K.C.; Foeglein, A.; Mahen, R.; Stuart, A.D.; Digard, P. A Rab11- and microtubule-dependent mechanism for cytoplasmic transport of influenza A virus viral RNA. J. Virol. 2011, 85, 4143–4156. [Google Scholar] [CrossRef]
- Nachmias, D.; Sklan, E.H.; Ehrlich, M.; Bacharach, E. Human immunodeficiency virus type 1 envelope proteins traffic toward virion assembly sites via a TBC1D20/Rab1-regulated pathway. Retrovirology 2012, 9, 7. [Google Scholar] [CrossRef]
- Sklan, E.H.; Serrano, R.L.; Einav, S.; Pfeffer, S.R.; Lambright, D.G.; Glenn, J.S. TBC1D20 is a Rab1 GTPase-activating protein that mediates hepatitis C virus replication. J. Biol. Chem. 2007, 282, 36354–36361. [Google Scholar] [CrossRef]
- Zenner, H.L.; Yoshimura, S.-I.; Barr, F.A.; Crump, C.M. Analysis of Rab GTPase-activating proteins indicates that Rab1a/b and Rab43 are important for Herpes Simplex virus 1 secondary envelopment. J. Virol. 2011, 85, 8012–8021. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Neumann, G.; Kawaoka, Y. Role of the GTPase Rab1b in ebolavirus particle formation. J. Virol. 2010, 84, 4816–4820. [Google Scholar] [CrossRef]
- Lin, J.; Wang, C.; Liang, W.; Zhang, J.; Zhang, L.; Lv, H.; Dong, W.; Zhang, Y. Rab1A is required for assembly of classical swine fever virus particle. Virology 2018, 514, 18–29. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veeck, C.; Biedenkopf, N.; Rohde, C.; Becker, S.; Halwe, S. Inhibition of Rab1B Impairs Trafficking and Maturation of SARS-CoV-2 Spike Protein. Viruses 2023, 15, 824. https://doi.org/10.3390/v15040824
Veeck C, Biedenkopf N, Rohde C, Becker S, Halwe S. Inhibition of Rab1B Impairs Trafficking and Maturation of SARS-CoV-2 Spike Protein. Viruses. 2023; 15(4):824. https://doi.org/10.3390/v15040824
Chicago/Turabian StyleVeeck, Christopher, Nadine Biedenkopf, Cornelius Rohde, Stephan Becker, and Sandro Halwe. 2023. "Inhibition of Rab1B Impairs Trafficking and Maturation of SARS-CoV-2 Spike Protein" Viruses 15, no. 4: 824. https://doi.org/10.3390/v15040824
APA StyleVeeck, C., Biedenkopf, N., Rohde, C., Becker, S., & Halwe, S. (2023). Inhibition of Rab1B Impairs Trafficking and Maturation of SARS-CoV-2 Spike Protein. Viruses, 15(4), 824. https://doi.org/10.3390/v15040824





