Conserved Structure Associated with Different 3′CITEs Is Important for Translation of Umbraviruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Constructs
2.2. In vitro Transcription of Full-Length Viral Genomes
2.3. RNA Folding and SHAPE Modification of RNA
2.4. Reverse Transcription of Modified RNA
2.5. Agroinfiltration and RNA Gel Blot Analysis
2.6. Protoplast Transfection and In Vivo Luciferase Assays
2.7. RNA Structure Drawing
3. Results
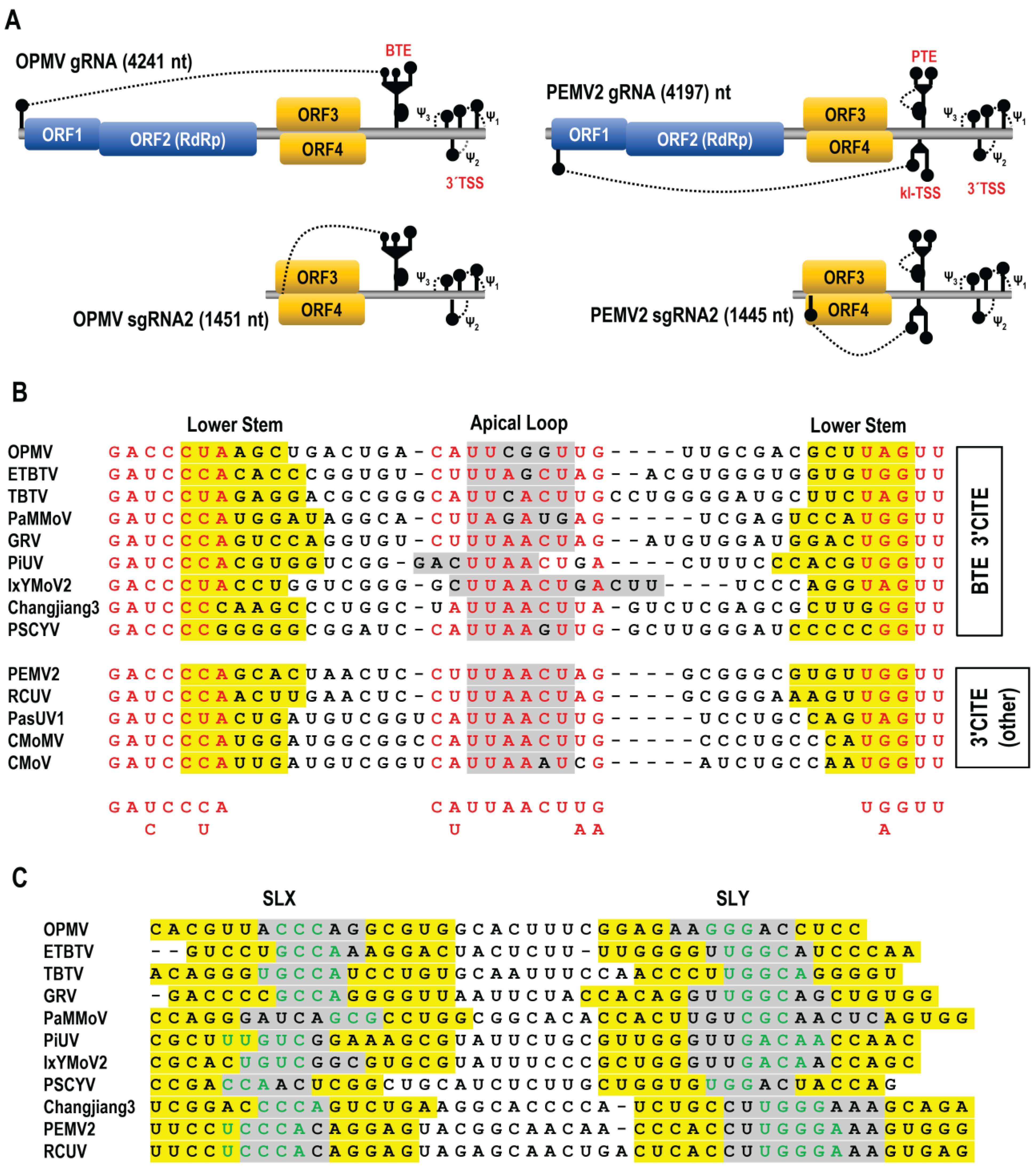
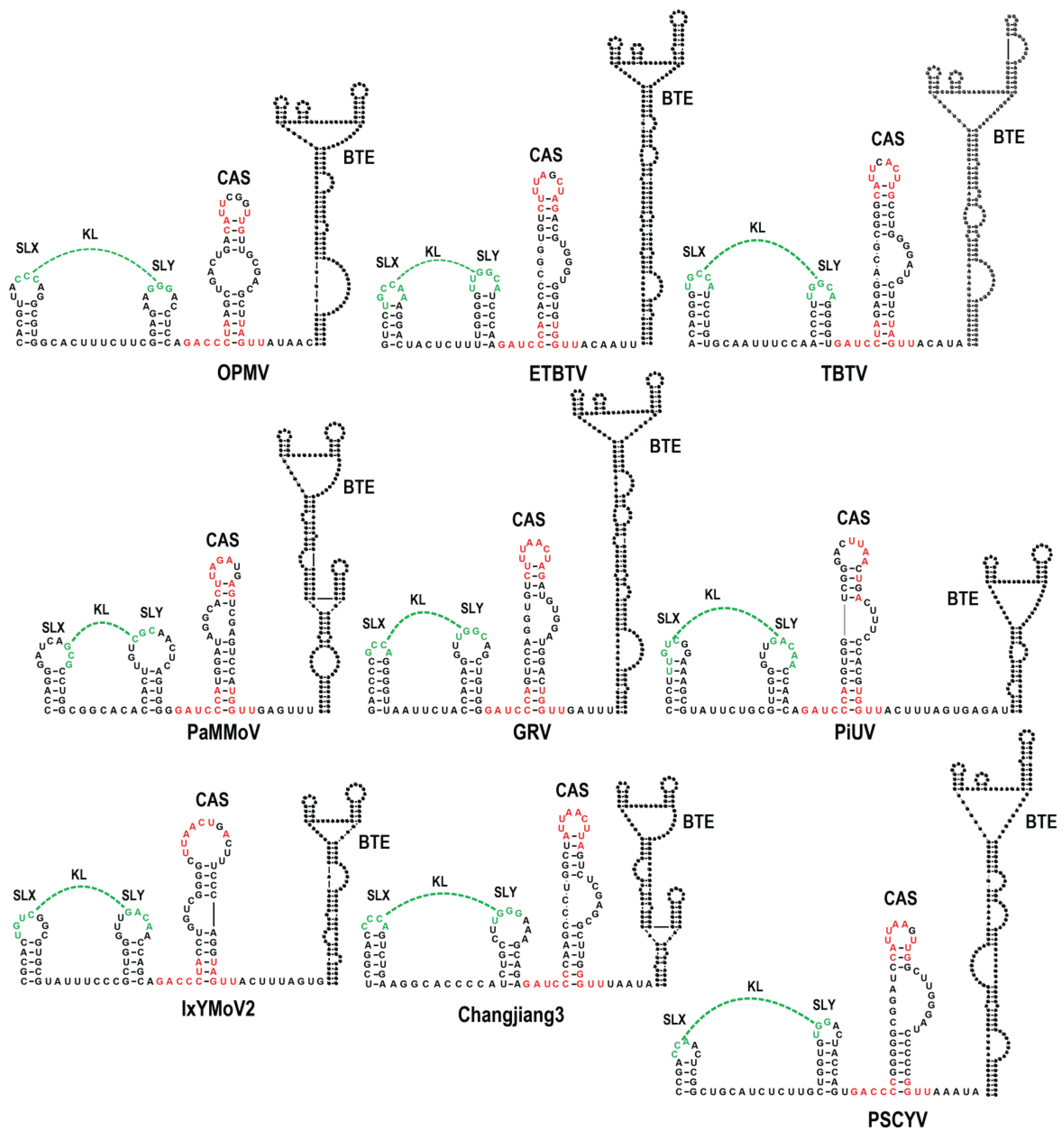
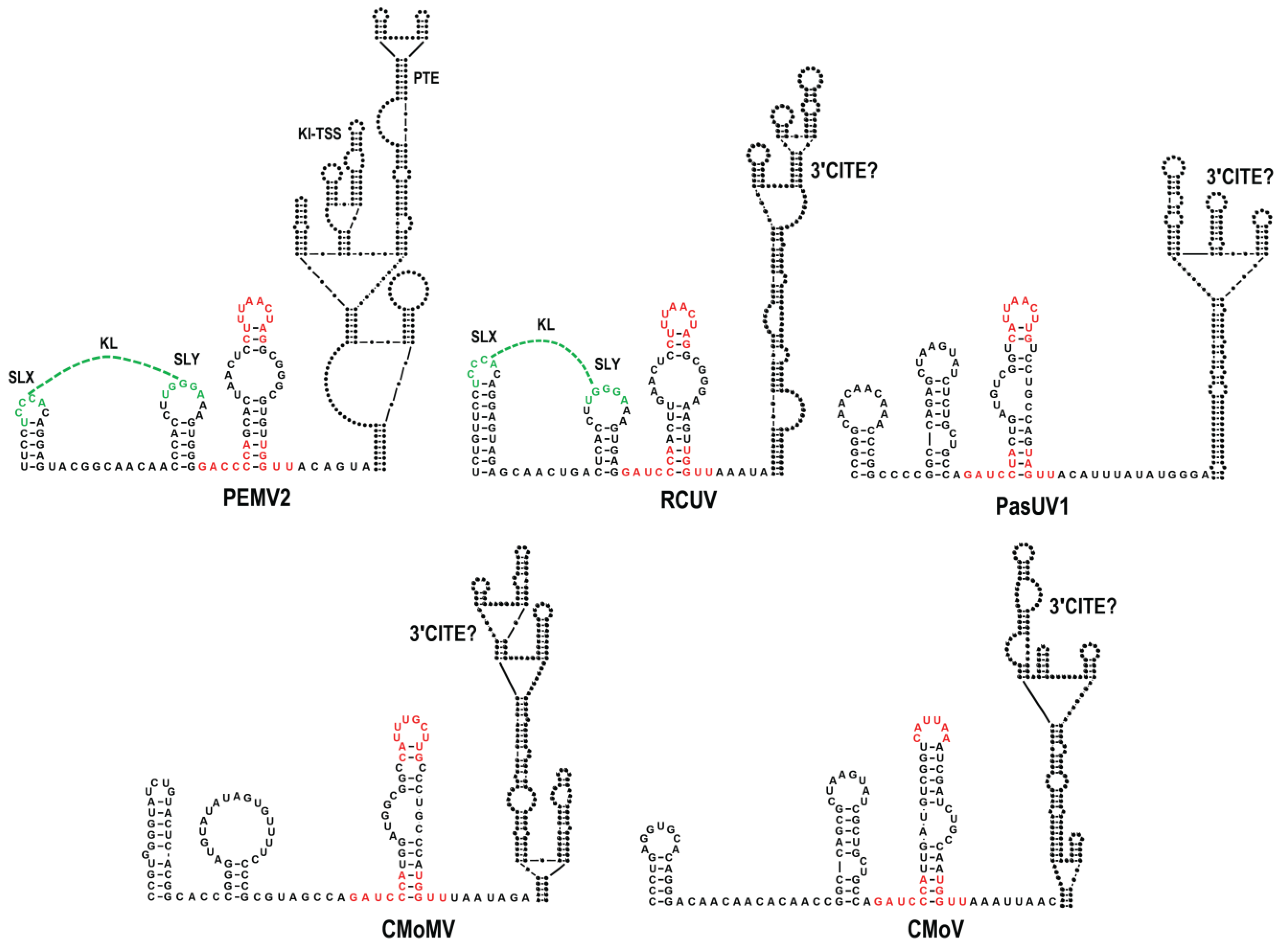
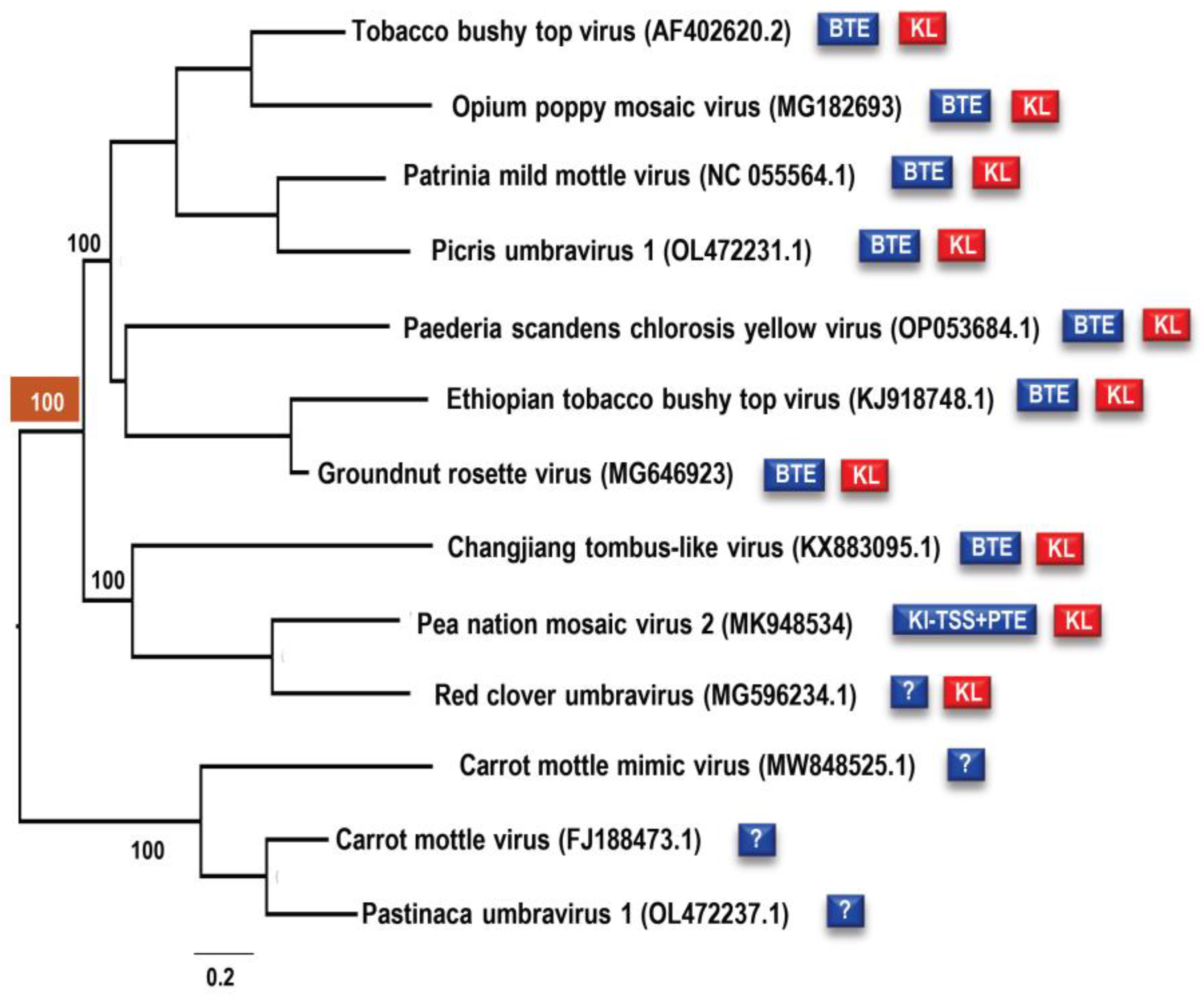
3.1. All Umbraviruses Contain a Conserved Hairpin in Their 3′ UTRs Located Upstream of Known or Putative 3′CITEs
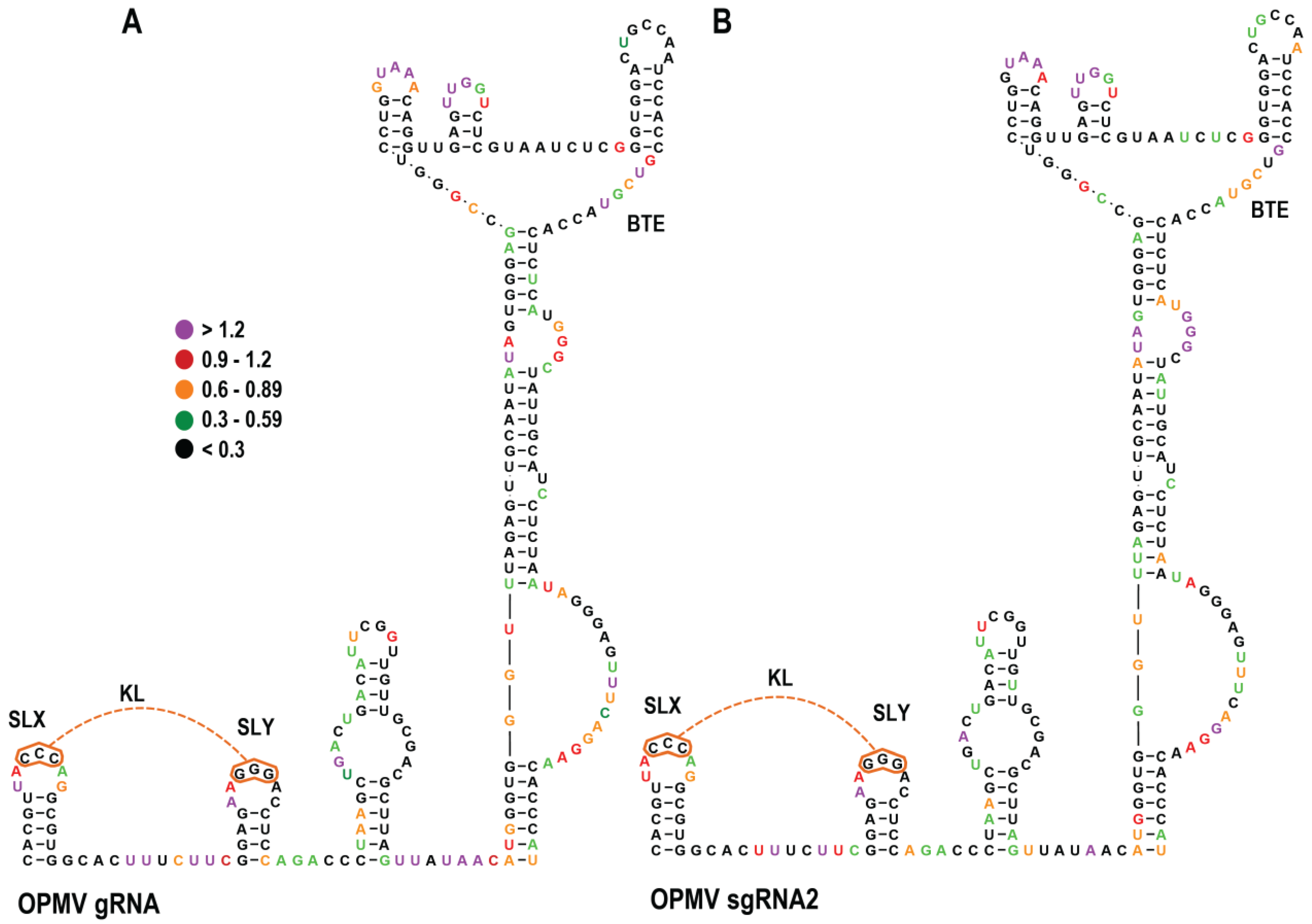
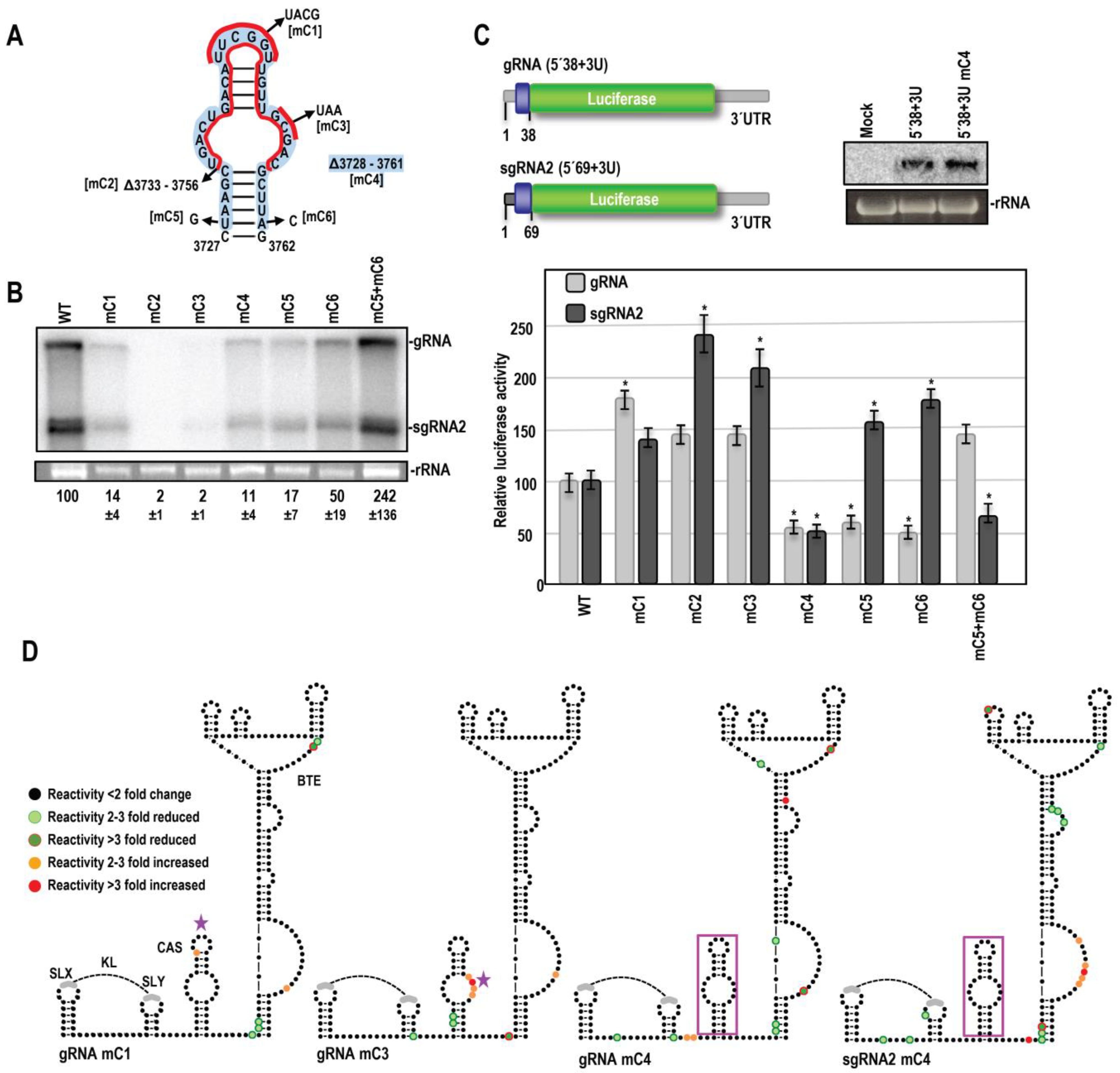
3.2. SHAPE RNA Structure Probing of OPMV gRNA and sgRNA CAS Region
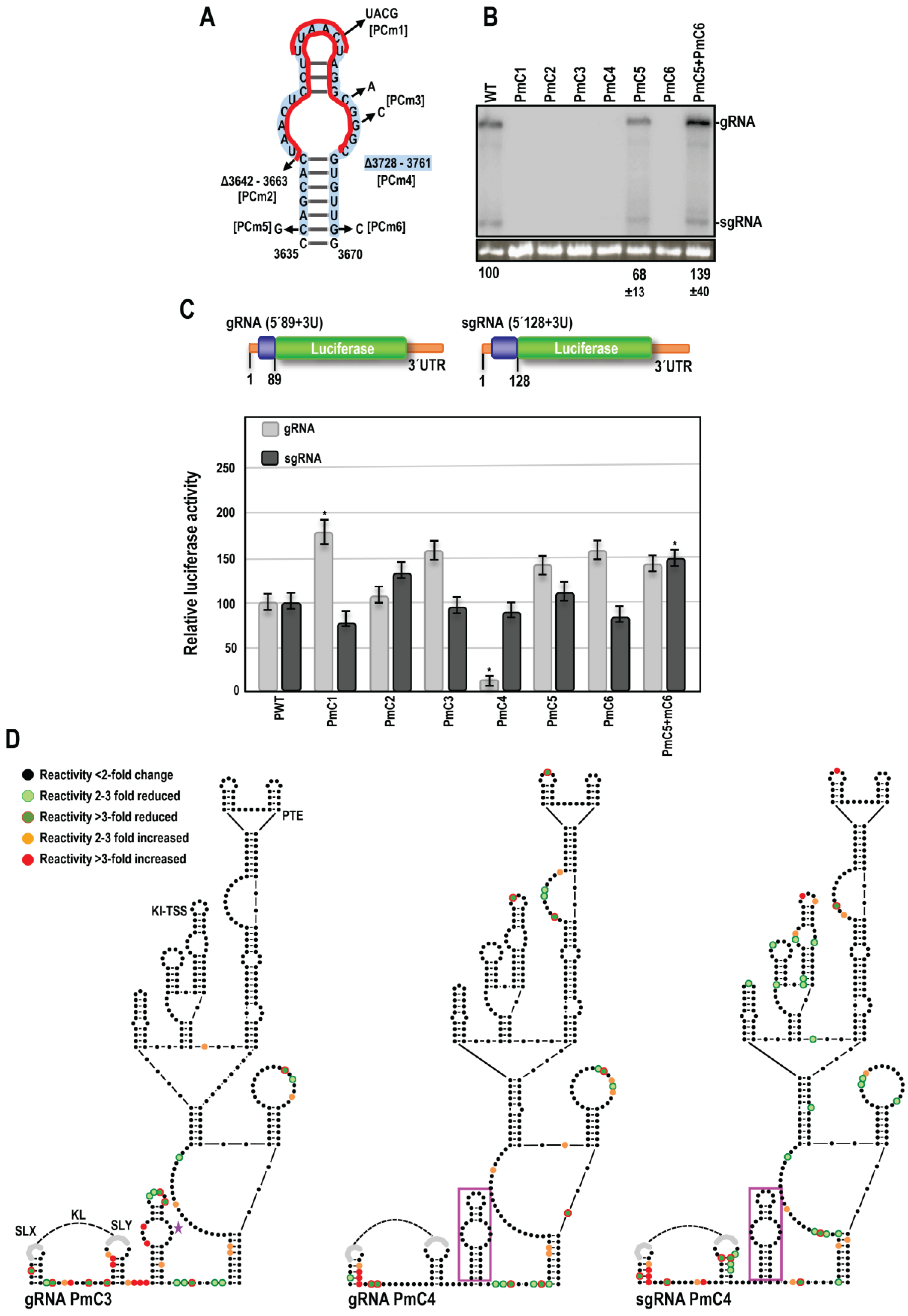
3.3. OPMV CAS Is Important for Viral Accumulation In Vivo
3.4. OPMV CAS Is Required for Efficient Translation of gRNA and sgRNA2 Reporter Constructs In Vivo
3.5. Effect of OPMV CAS Mutations on BTE and KL Structures
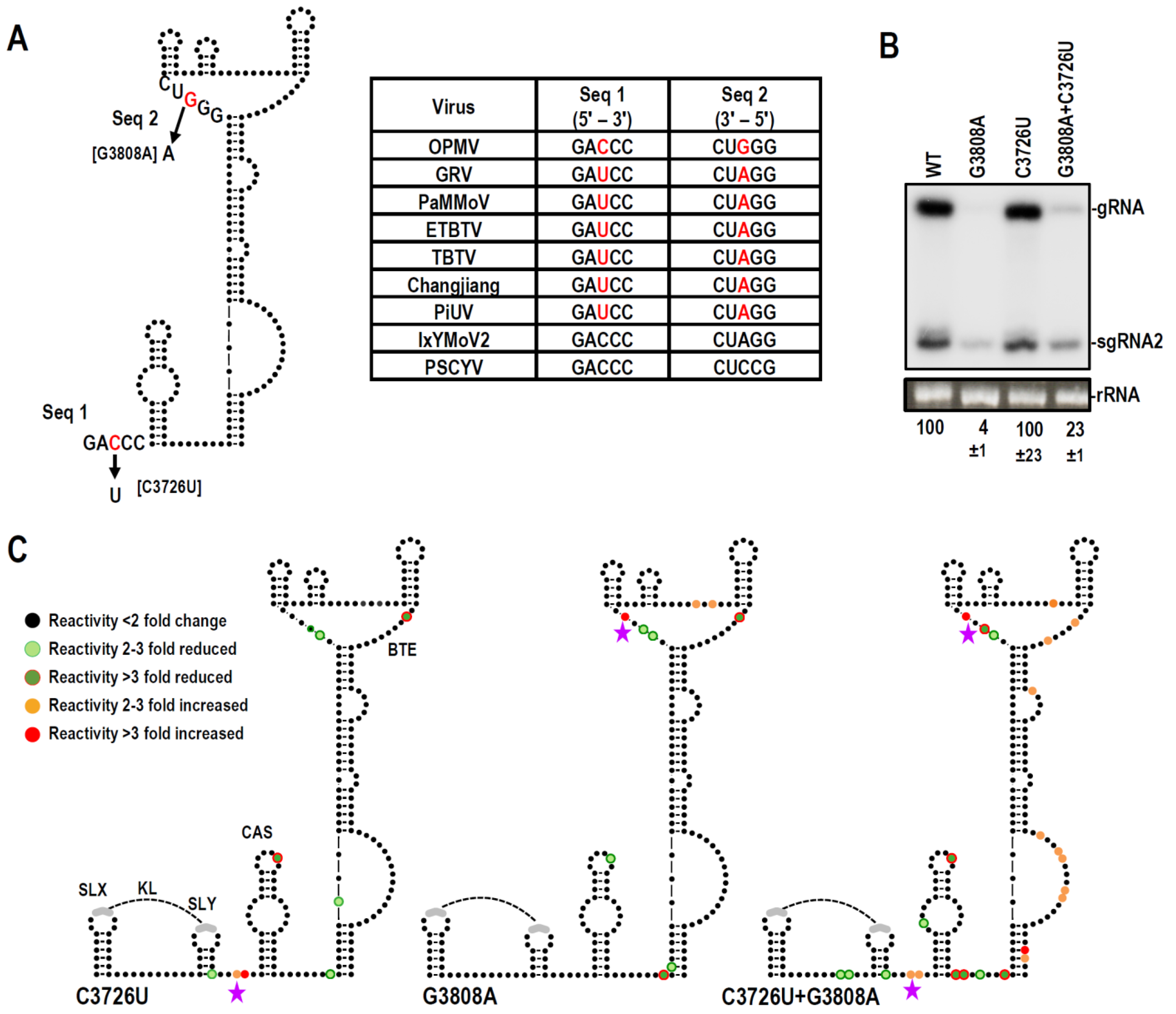
3.6. Examination of Conserved CAS Flanking Sequence and a Complementary Sequence in the BTE 17-nt Signature Motif
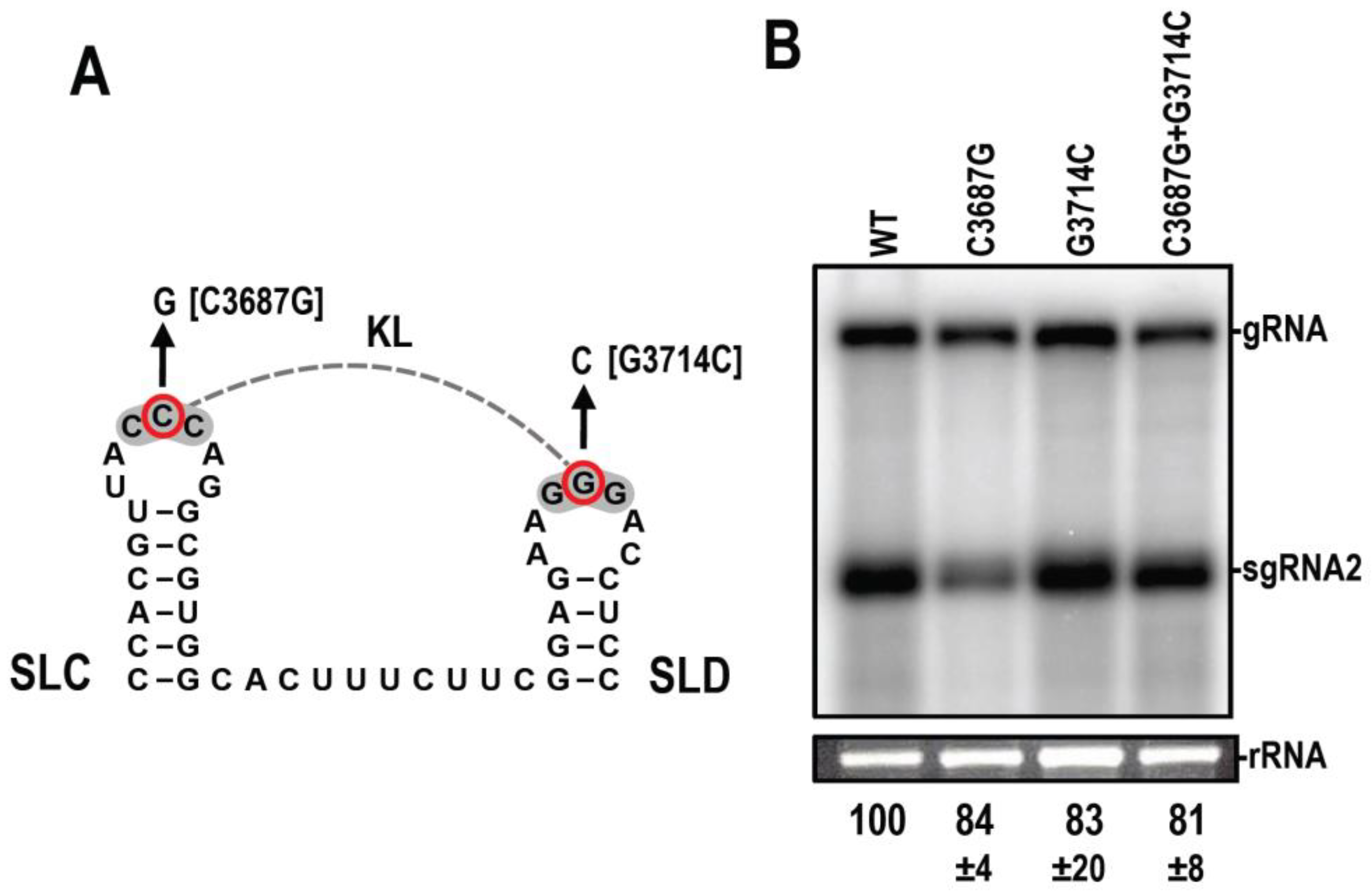
3.7. Disrupting the KL between SLX and SLY Does Not Affect Accumulation in N. benthamiana
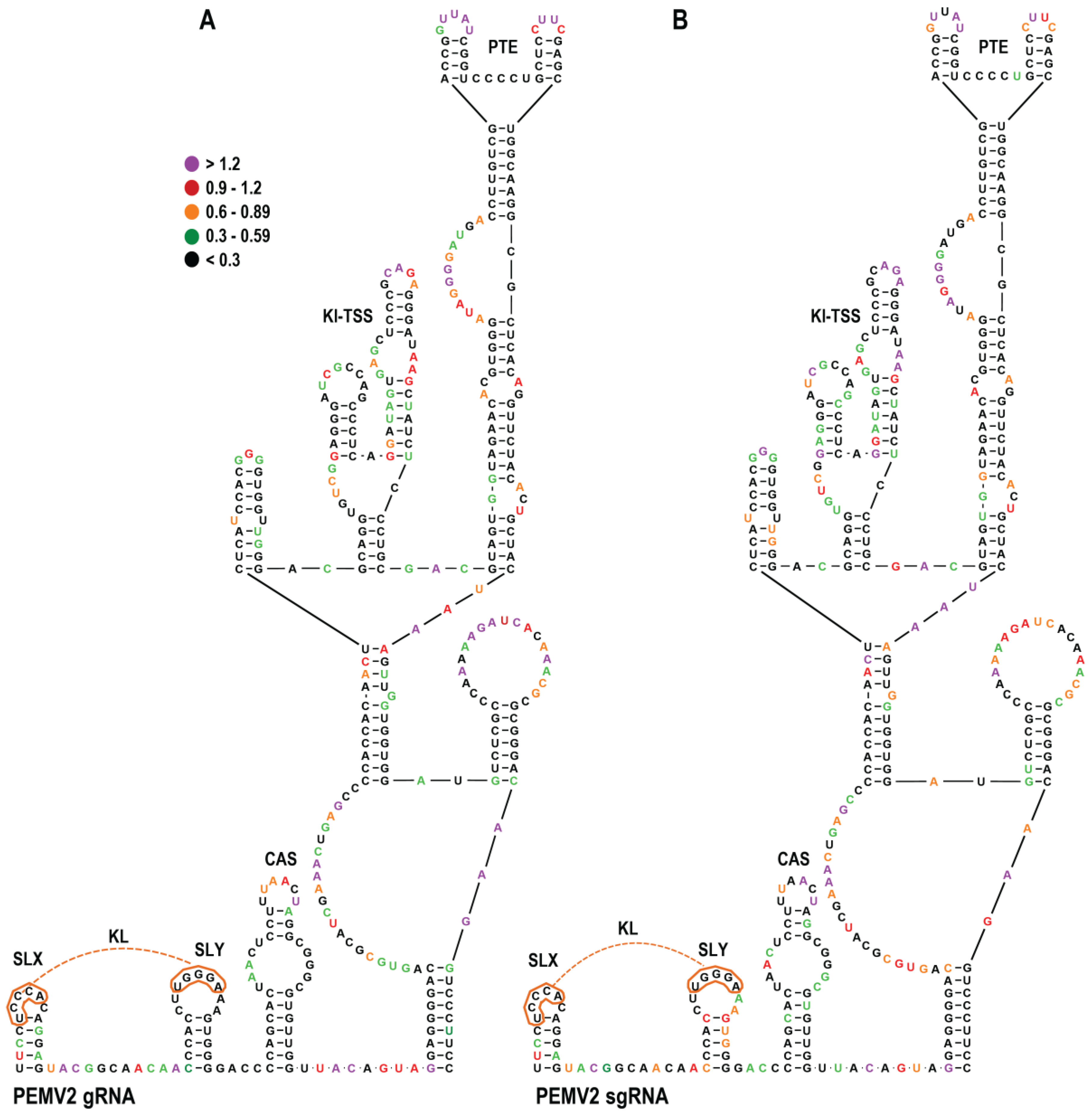
3.8. SHAPE RNA Structure Probing of the PEMV2 gRNA and sgRNA3′UTR
3.9. CAS Is Required for Efficient Accumulation of PEMV2 RNA In Vivo
3.10. PEMV2 CAS Is Required for Efficient Translation of gRNA but Not sgRNA Reporter Constructs In Vivo
3.11. PEMV2 CAS Mutations Affect the Structure of Upstream Sequences
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Firth, A.E.; Brierley, I. Non-canonical translation in RNA viruses. J. Gen. Virol. 2012, 93, 1385–1409. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, I.I.; Vassilenko, K.S.; Terenin, I.M.; Kalinina, N.O.; Agol, V.I.; Dmitriev, S.E. Non-canonical translation initiation mechanisms employed by eukaryotic viral mRNAs. Biochem. Mosc. 2021, 86, 1060–1094. [Google Scholar] [CrossRef]
- Simon, A.E.; Mäkinen, K.; Li, Y.; Verchot, J. Plant Viruses. In Field’s Virology, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 4. [Google Scholar]
- Simon, A.E.; Miller, W.A. 3′ Cap-independent translation enhancers of plant viruses. Annu. Rev. Microbiol. 2013, 67, 21–42. [Google Scholar] [CrossRef]
- Truniger, V.; Miras, M.; Aranda, M.A. Structural and functional diversity of plant virus 3-cap-independent translation enhancers (3′-CITEs). Front. Plant Sci. 2017, 8, 2047. [Google Scholar] [CrossRef] [PubMed]
- Geng, G.; Yu, C.; Li, X.; Yuan, X. A unique internal ribosome entry site representing a dynamic equilibrium state of RNA tertiary structure in the 5′-UTR of wheat yellow mosaic virus RNA1. Nucleic Acids Res. 2020, 48, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Mesa, H.; Gannon, M.; Holshbach, E.; Zhang, J.; Roberts, R.; Buettner, M.; Rakotondrafaraa, A.M. The triticum mosaic virus internal ribosome entry site relies on a picornavirus-like YX-AUG motif to designate the preferred translation initiation site and to likely target the 18S rRNA. J. Virol. 2019, 93, e01705-18. [Google Scholar] [CrossRef]
- Basso, J.; Dallaire, L.P.; Charest, P.J.; Devantier, Y.; Laliberte, J.F. Evidence for an internal ribosome entry site within the 5’ non-translated region of turnip mosaic potyvirus RNA. J. Gen. Virol. 1994, 75, 3157–3165. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.Z.; Kasprzak, W.K.; Shapiro, B.A.; Simon, A.E. Structural characterization of a new subclass of panicum mosaic virus-like 3′ cap-independent translation enhancer. Nucleic Acids Res. 2022, 50, 1601–1619. [Google Scholar] [CrossRef]
- Timmer, R.T.; Benkowski, L.A.; Schodin, D.; Lax, S.R.; Metz, A.M.; Ravel, J.M.; Browning, K.S. The 5′ and 3′ untranslated regions of satellite tobacco necrosis virus-RNA affect translational efficiency and dependence on a 5′ cap structure. J. Biol. Chem. 1993, 268, 9504–9510. [Google Scholar] [CrossRef]
- Kraft, J.J.; Treder, K.; Peterson, M.S.; Miller, W.A. Cation-dependent folding of 3′ cap-independent translation elements facilitates interaction of a 17-nucleotide conserved sequence with eIF4G. Nucleic Acids Res. 2013, 41, 3398–3413. [Google Scholar] [CrossRef]
- Treder, K.; Pettit Kneller, E.L.; Allen, E.M.; Wang, Z.; Browning, K.S.; Miller, W.A. The 3′ cap-independent translation element of barley yellow dwarf virus binds EIF4F via the EIF4G subunit to initiate translation. RNA 2008, 14, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.D.; Kraft, J.J.; Miller, W.A.; Goss, D.J. Recruitment of the 40S ribosome subunit to the 3′-untranslated region (UTR) of a viral mRNA, via the eIF4 complex, facilitates cap-independent translation. J. Biol. Chem. 2015, 290, 11268–11281. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, U.; Powell, P.; Goss, D.J. Eukaryotic initiation factor (eIF) 3 mediates Barley Yellow Dwarf Viral mRNA 3′-5′ UTR interactions and 40S ribosomal subunit binding to facilitate cap-independent translation. Nucleic Acids Res. 2019, 47, 6225–6235. [Google Scholar] [CrossRef] [PubMed]
- Powell, P.; Bhardwaj, U.; Goss, D. Eukaryotic initiation factor 4F promotes a reorientation of eukaryotic initiation factor 3 binding on the 5′ and the 3′ UTRs of barley yellow dwarf virus mRNA. Nucleic Acids Res. 2022, 50, 4988–4999. [Google Scholar] [CrossRef] [PubMed]
- Stupina, V.A.; Maskauskas, A.; McCormack, J.C.; Yingling, Y.G.; Shapiro, B.A.; Dinman, J.D.; Simon, A.E. The 3′ proximal translational enhancer of Turnip crinkle virus binds to 60S ribosomal subunits. RNA 2008, 14, 2379–2393. [Google Scholar] [CrossRef]
- Gao, F.; Kasprzak, W.K.; Szarko, C.; Shapiro, B.A.; Simon, A.E. The 3′ untranslated region of pea enation mosaic virus contains two t-shaped, ribosome-binding, cap-independent translation enhancers. J. Virol. 2014, 88, 11696–11712. [Google Scholar] [CrossRef]
- Du, Z.; Alekhina, O.M.; Vassilenko, K.S.; Simon, A.E. Concerted action of two 3′ cap-independent translation enhancers increases the competitive strength of translated viral genomes. Nucleic Acids Res. 2017, 45, 9558–9572. [Google Scholar] [CrossRef]
- Weingarten-Gabbay, S.; Elias-Kirma, S.; Nir, R.; Gritsenko, A.A.; Stern-Ginossar, N.; Yakhini, Z.; Weinberger, A.; Segal, E. Comparative genetics: Systematic discovery of cap-independent translation sequences in human and viral genomes. Science 2016, 351, aad4939. [Google Scholar] [CrossRef]
- Ilyas, M.; Du, Z.; Simon, A.E. Opium poppy mosaic virus has an Xrn-resistant, translated subgenomic RNA and a BTE 3′ CITE. J. Virol. 2021, 95, e2109-20. [Google Scholar] [CrossRef]
- Wang, Z.; Treder, K.; Miller, W.A. Structure of a viral cap-independent translation element that functions via high affinity binding to the EIF4E subunit of EIF4F. J. Biol. Chem. 2009, 284, 14189–14202. [Google Scholar] [CrossRef]
- Wang, Z.; Parisien, M.; Scheets, K.; Miller, W.A. The cap-binding translation initiation factor, EIF4E, binds a pseudoknot in a viral cap-independent translation element. Structure 2011, 19, 868–880. [Google Scholar] [CrossRef] [PubMed]
- McCormack, J.C.; Yuan, X.; Yingling, Y.G.; Kasprzak, W.; Zamora, R.E.; Shapiro, B.A.; Simon, A.E. Structural domains within the 3′ untranslated region of turnip crinkle virus. J. Virol. 2008, 82, 8706–8720. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Wang, J.; Yu, P.; Eyler, D.; Xu, H.; Starich, M.R.; Tiede, D.M.; Simon, A.E.; Kasprzak, W.; Schwieters, C.D.; et al. Solution structure of the cap-independent translational enhancer and ribosome-binding element in the 3′ UTR of turnip crinkle virus. Proc. Natl. Acad. Sci. USA 2010, 107, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.; Kasprzak, W.K.; Kim, T.; Gao, F.; Young, M.Y.L.; Yuan, X.; Shapiro, B.A.; Seog, J.; Simon, A.E. Folding behavior of a T-shaped, ribosome-binding translation enhancer implicated in a wide-spread conformational switch. eLife 2017, 6, e22883. [Google Scholar] [CrossRef] [PubMed]
- Stupina, V.A.; Yuan, X.F.; Meskauskas, A.; Dinman, J.D.; Simon, A.E. Ribosome binding to a 5′ translational enhancer is altered in the presence of the 3′ untranslated region in cap-independent translation of turnip crinkle virus. J. Virol. 2011, 85, 4638–4653. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Kasprzak, W.; Stupina, V.A.; Shapiro, B.A.; Simon, A.E. A ribosome-binding, 3′ translational enhancer has a T-shaped structure and engages in a long-distance RNA-RNA interaction. J. Virol. 2012, 86, 9828–9842. [Google Scholar] [CrossRef]
- Newburn, L.R.; Wu, B.; Andrew White, K. Investigation of novel RNA elements in the 3′ UTRs of tobacco necrosis virus-D. Viruses 2020, 12, 856. [Google Scholar] [CrossRef]
- Iwakawa, H.; Tajima, Y.; Taniguchi, T.; Kaido, M.; Mise, K.; Tomari, Y.; Taniguchi, H.; Okuno, T. Poly(A)-binding protein facilitates translation of an uncapped/nonpolyadenylated viral RNA by binding to the 3′ untranslated region. J. Virol. 2012, 86, 7836–7849. [Google Scholar] [CrossRef]
- Gao, F.; Simon, A.E. Differential use of 3′CITEs by the subgenomic RNA of pea enation mosaic virus 2. Virology 2017, 510, 194–204. [Google Scholar] [CrossRef]
- Ryabov, E.; Taliansky, M.E.; Robinson, D.; Waterhouse, P.; Murant, A.F.; de Zoeten, G.; Falk, B.; Vetten, H.; Gibbs, M. Genus Umbravirus. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E., Lefkowitz, E.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1191–1195. [Google Scholar]
- Gao, F.; Alekhina, O.M.; Vassilenko, K.S.; Simon, A.E. Unusual dicistronic expression from closely spaced initiation codons in an umbravirus subgenomic RNA. Nucleic Acids Res. 2018, 46, 11726–11742. [Google Scholar] [CrossRef]
- Gao, F.; Simon, A.E. Multiple cis-acting elements modulate programmed-1 ribosomal frameshifting in pea enation mosaic virus. Nucleic Acids Res. 2016, 44, 878–895. [Google Scholar] [CrossRef]
- May, J.P.; Johnson, P.Z.; Ilyas, M.; Gao, F.; Simon, E. The multifunctional long-distance movement protein of pea enation mosaic virus 2 protects viral and host transcripts from nonsense-mediated decay. mBio 2020, 11, e00204-20. [Google Scholar] [CrossRef] [PubMed]
- Mushegian, A.R.; Slena, S.F. Evolution of plant virus movement proteins from the 30K superfamily and of their homologs integrated in plant genomes. Virology 2014, 476, 304–315. [Google Scholar] [CrossRef]
- Gao, F.; Gulay, S.P.; Kasprzak, W.; Dinman, J.D.; Shapiro, B.A.; Simon, A.E. The kissing-loop T-shaped structure translational enhancer of pea enation mosaic virus can bind simultaneously to ribosomes and a 5′ proximal hairpin. J. Virol. 2013, 87, 11987–12002. [Google Scholar] [CrossRef]
- Liu, H.; Naismith, J.H. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 2008, 8, 91. [Google Scholar] [CrossRef]
- Wilkinson, K.A.; Merino, E.J.; Weeks, K.M. Selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE): Quantitative RNA structure analysis at single nucleotide resolution. Nat. Protoc. 2006, 1, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Carino, E.; Bera, S.; Gao, F.; May, J.P.; Simon, A.E. Structural analysis and whole genome mapping of a new type of plant virus subviral RNA: Umbravirus-like associated RNAs. Viruses 2021, 13, 646. [Google Scholar] [CrossRef]
- Karabiber, F.; McGinnis, J.L.; Favorov, O.V.; Weeks, K.M. QuShape: Rapid, accurate, and best-practices quantification of nucleic acid probing information, resolved by capillary electrophoresis. RNA 2013, 19, 63–73. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Johnson, P.Z.; Kasprzak, W.K.; Shapiro, B.A.; Simon, A.E. RNA2Drawer: Geometrically strict drawing of nucleic acid structures with graphical structure editing and highlighting of complementary subsequences. RNA Biol. 2019, 16, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Chkuaseli, T.; White, K.A. Intragenomic long-distance RNA–RNA interactions in plus-strand RNA plant viruses. Front. Microbiol. 2018, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, C.; Liu, S.; Wang, G.; Shi, K.; Li, X.; Yuan, X. Structural alteration of a BYDV-like translation element (BTE) that attenuates p35 expression in three mild tobacco bushy top virus isolates. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Dascenzo, L.; Leonarski, F.; Vicens, Q.; Auffinger, P. Revisiting GNRA and UNCG folds: U-turns versus Z-turns in RNA hairpin loops. RNA 2017, 23, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, Q.; Miller, W.A.; Goss, D.J. Eukaryotic translation initiation factor 4G (eIF4G) coordinates interactions with eIF4A, eIF4B, and eIF4E in binding and translation of the barley yellow dwarf virus 3 cap-independent translation element (BTE). J. Biol. Chem. 2017, 292, 5921–5931. [Google Scholar] [CrossRef] [PubMed]
- Perdrizet, G.A.; Artsimovitch, I.; Furman, R.; Sosnick, T.R.; Pan, T. Transcriptional pausing coordinates folding of the aptamer domain and the expression platform of a riboswitch. Proc. Natl. Acad. Sci. USA 2012, 109, 3323–3328. [Google Scholar] [CrossRef]
- Johnson, P.Z.; Reuning, H.M.; Bera, S.; Gao, F.; Du, Z.Y.; Simon, A.E. Novel 3′ proximal replication elements in umbravirus genomes. Viruses 2022, 14, 2615. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Shi, K.; Meskauskas, A.; Simon, A.E. The 3′ end of turnip crinkle virus contains a highly interactive structure including a translational enhancer that is disrupted by binding to the RNA-dependent RNA polymerase. RNA 2009, 15, 1849–1864. [Google Scholar] [CrossRef]
- Pu, H.; Li, J.; Li, D.; Han, C.; Yu, J. Identification of an internal RNA element essential for replication and translational enhancement of tobacco necrosis virus A. PLoS ONE 2013, 8, e57938. [Google Scholar] [CrossRef]
- Simon, A.E. 3’UTRs of carmoviruses. Virus Res. 2015, 206, 27–36. [Google Scholar] [CrossRef]
- Truniger, V.; Nieto, C.; González-Ibeas, D.; Aranda, M. Mechanism of plant EIF4E-mediated resistance against a carmovirus (Tombusviridae): Cap-independent translation of a viral RNA controlled in cis by an (a)virulence determinant. Plant J. 2008, 56, 716–727. [Google Scholar] [CrossRef]
- Cheng, C.P.; Panavas, T.; Luo, G.; Nagy, P.D. Heterologous RNA replication enhancer stimulates in vitro RNA synthesis and template-switching by the carmovirus, but not by the tombusvirus, RNA-dependent RNA polymerase: Implication for modular evolution of RNA viruses. Virology 2005, 341, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Miras, M.; Sempere, R.N.; Kraft, J.J.; Miller, W.A.; Aranda, M.A.; Truniger, V. Interfamilial recombination between viruses led to acquisition of a novel translation-enhancing RNA element that allows resistance breaking. New Phytol. 2014, 202, 233–246. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bera, S.; Ilyas, M.; Mikkelsen, A.A.; Simon, A.E. Conserved Structure Associated with Different 3′CITEs Is Important for Translation of Umbraviruses. Viruses 2023, 15, 638. https://doi.org/10.3390/v15030638
Bera S, Ilyas M, Mikkelsen AA, Simon AE. Conserved Structure Associated with Different 3′CITEs Is Important for Translation of Umbraviruses. Viruses. 2023; 15(3):638. https://doi.org/10.3390/v15030638
Chicago/Turabian StyleBera, Sayanta, Muhammad Ilyas, Anna A. Mikkelsen, and Anne E. Simon. 2023. "Conserved Structure Associated with Different 3′CITEs Is Important for Translation of Umbraviruses" Viruses 15, no. 3: 638. https://doi.org/10.3390/v15030638
APA StyleBera, S., Ilyas, M., Mikkelsen, A. A., & Simon, A. E. (2023). Conserved Structure Associated with Different 3′CITEs Is Important for Translation of Umbraviruses. Viruses, 15(3), 638. https://doi.org/10.3390/v15030638







