Congenital Zika Syndrome and Disabilities of Feeding and Breastfeeding in Early Childhood: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Exposure/Outcomes
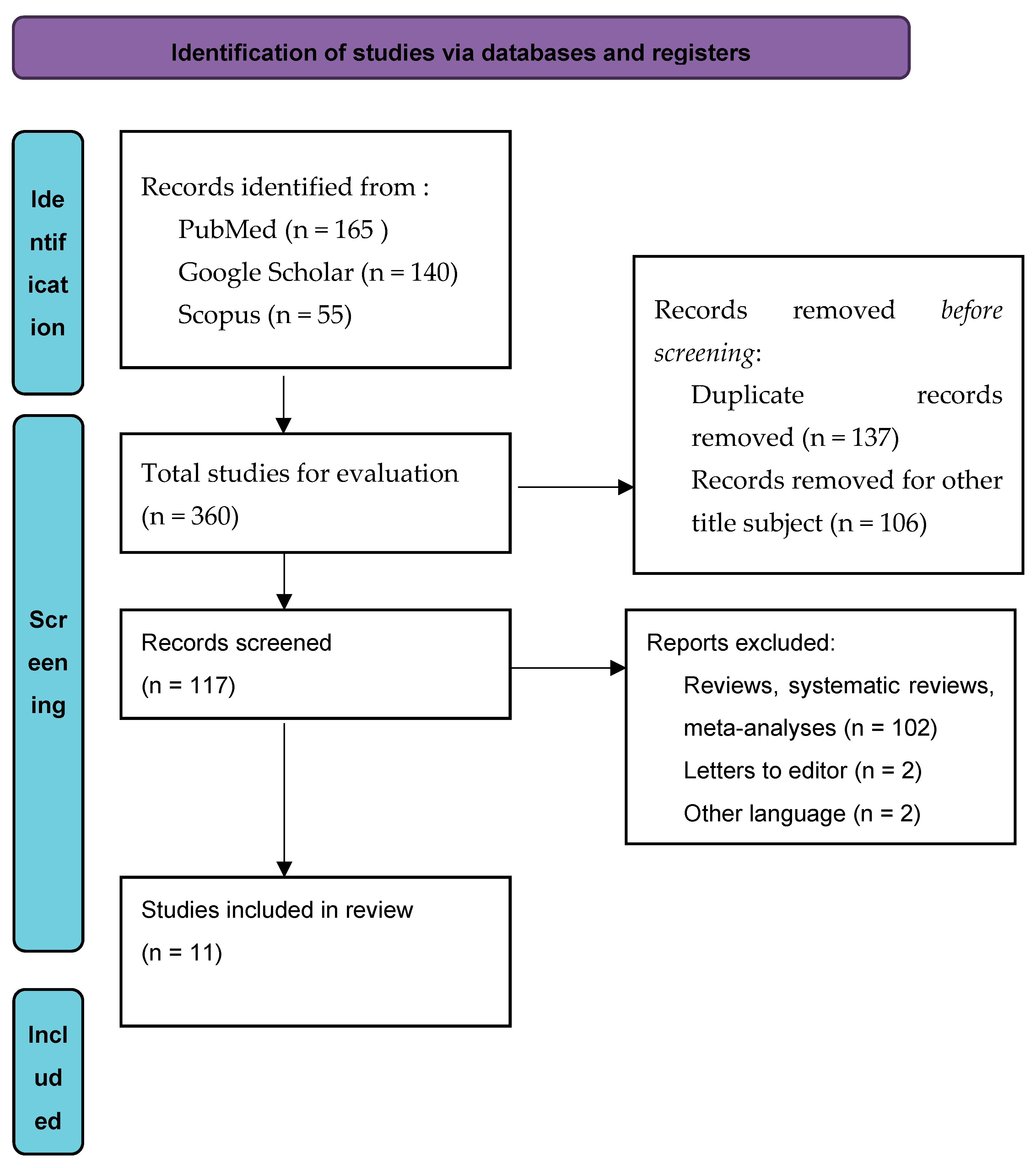
2.3. Search Strategy
2.4. Study Selection
2.5. Methοdological Quality and Risk of Bias
3. Results
3.1. Feeding Difficulties
3.2. Breasteeding Difficulties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zanluca, C.; de Melo, V.C.A.; Mosimann, A.L.P.; dos Santos, G.I.V.; dos Santos, C.N.D.; Luz, K. First Report of Autochthonous Transmission of Zika Virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef]
- Assesssment and Management of Guillain-Barré Syndrome in the Context of Zika Virus Infection: Interim Guidance Update. Available online: https://www.who.int/publications-detail-redirect/WHO-ZIKV-MOC-16.4-Rev.1 (accessed on 14 October 2022).
- CDC. Zika Outcomes Investigation (ZODIAC)|CDC. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/pregnancy/zika/research/zodiac.html (accessed on 13 February 2020).
- Soares, F.; Abranches, A.D.; Villela, L.; Lara, S.; Araújo, D.; Nehab, S.; Silva, L.; Amaral, Y.; Junior, S.C.G.; Pone, S.; et al. Zika Virus Infection in Pregnancy and Infant Growth, Body Composition in the First Three Months of Life: A Cohort Study. Sci. Rep. 2019, 9, 19198. [Google Scholar] [CrossRef]
- Heukelbach, J.; Alencar, C.H.; Kelvin, A.A.; de Oliveira, W.K.; Pamplona de Góes Cavalcanti, L. Zika Virus Outbreak in Brazil. J. Infect. Dev. Ctries 2016, 10, 116–120. [Google Scholar] [CrossRef]
- WHO. Defining the syndrome associated with congenital Zika virus infection. Bull. World Health Organ. 2016, 94, 406. [Google Scholar] [CrossRef]
- Zorrilla, C.D.; García García, I.; García Fragoso, L.; De La Vega, A. Zika Virus Infection in Pregnancy: Maternal, Fetal, and Neonatal Considerations. J. Infect. Dis. 2017, 216 (Suppl. 10), S891–S896. [Google Scholar] [CrossRef]
- Gullo, G.; Scaglione, M.; Cucinella, G.; Riva, A.; Coldebella, D.; Cavaliere, A.F.; Signore, F.; Buzzaccarini, G.; Spagnol, G.; Laganà, A.S.; et al. Congenital Zika Syndrome: Genetic Avenues for Diagnosis and Therapy, Possible Management and Long-Term Outcomes. J. Clin. Med. 2022, 11, 1351. [Google Scholar] [CrossRef]
- CDC. Congenital Zika Syndrome & Other Birth Defects|CDC. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/pregnancy/zika/testing-follow-up/zika-syndrome-birth-defects.html (accessed on 29 October 2020).
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef]
- Del Campo, M.; Feitosa, I.M.L.; Ribeiro, E.M.; Horovitz, D.D.G.; Pessoa, A.L.S.; França, G.V.A.; García-Alix, A.; Doriqui, M.J.R.; Wanderley, H.Y.C.; Sanseverino, M.V.T.; et al. The Phenotypic Spectrum of Congenital Zika Syndrome. Am. J. Med. Genet. A 2017, 173, 841–857. [Google Scholar] [CrossRef]
- Devakumar, D.; Bamford, A.; Ferreira, M.U.; Broad, J.; Rosch, R.E.; Groce, N.; Breuer, J.; Cardoso, M.A.; Copp, A.J.; Alexandre, P.; et al. Infectious Causes of Microcephaly: Epidemiology, Pathogenesis, Diagnosis, and Management. Lancet Infect. Dis. 2018, 18, e1–e13. [Google Scholar] [CrossRef]
- Gérardin, P.; Ramos, R.C.; Jungmann, P.; de Oliveira, J.R.M.; Amara, A.; Gressens, P. Zika Epidemic: A Step towards Understanding the Infectious Causes of Microcephaly? Lancet Infect. Dis. 2018, 18, 15–16. [Google Scholar] [CrossRef]
- Birth Defects|Zika virus|CDC. Available online: https://www.cdc.gov/zika/healtheffects/birth_defects.html (accessed on 14 February 2020).
- Driggers, R.W.; Ho, C.-Y.; Korhonen, E.M.; Kuivanen, S.; Jääskeläinen, A.J.; Smura, T.; Rosenberg, A.; Hill, D.A.; DeBiasi, R.L.; Vezina, G.; et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N. Engl. J. Med. 2016, 374, 2142–2151. [Google Scholar] [CrossRef]
- Teixeira, F.M.E.; Pietrobon, A.J.; Oliveira, L.d.M.; Oliveira, L.M.d.S.; Sato, M.N. Maternal-Fetal Interplay in Zika Virus Infection and Adverse Perinatal Outcomes. Front. Immunol. 2020, 11, 175. [Google Scholar] [CrossRef]
- DeSilva, M.; Munoz, F.M.; Sell, E.; Marshall, H.; Tse Kawai, A.; Kachikis, A.; Heath, P.; Klein, N.P.; Oleske, J.M.; Jehan, F.; et al. Congenital Microcephaly: Case Definition & Guidelines for Data Collection, Analysis, and Presentation of Safety Data after Maternal Immunisation. Vaccine 2017, 35 Pt A, 6472–6482. [Google Scholar] [CrossRef]
- Peçanha, P.M.; Gomes Junior, S.C.; Pone, S.M.; Pone, M.V.d.S.; Vasconcelos, Z.; Zin, A.; Vilibor, R.H.H.; Costa, R.P.; Meio, M.D.B.B.; Nielsen-Saines, K.; et al. Neurodevelopment of Children Exposed Intra-Uterus by Zika Virus: A Case Series. PLoS ONE 2020, 15, e0229434. [Google Scholar] [CrossRef]
- Jones, B. Normal and Abnormal Swallowing: Imaging in Diagnosis and Therapy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Palmer, J.B.; Rudin, N.J.; Lara, G.; Crompton, A.W. Coordination of Mastication and Swallowing. Dysphagia 1992, 7, 187–200. [Google Scholar] [CrossRef]
- Matsuo, K.; Palmer, J.B. Anatomy and Physiology of Feeding and Swallowing–Normal and Abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707. [Google Scholar] [CrossRef]
- Nishino, T.; Yonezawa, T.; Honda, Y. Effects of Swallowing on the Pattern of Continuous Respiration in Human Adults. Am. Rev. Respir. Dis. 1985, 132, 1219–1222. [Google Scholar] [CrossRef]
- Kuhlemeier, K.V. Epidemiology and Dysphagia. Dysphagia 1994, 9, 209–217. [Google Scholar] [CrossRef]
- Bakheit, A.M.O. Management of Neurogenic Dysphagia. Postgrad. Med. J. 2001, 77, 694–699. [Google Scholar] [CrossRef]
- Leal, M.C.; van der Linden, V.; Bezerra, T.P.; de Valois, L.; Borges, A.C.G.; Antunes, M.M.C.; Brandt, K.G.; Moura, C.X.; Rodrigues, L.C.; Ximenes, C.R. Characteristics of Dysphagia in Infants with Microcephaly Caused by Congenital Zika Virus Infection, Brazil, 2015. Emerg. Infect. Dis. 2017, 23, 1253–1259. [Google Scholar] [CrossRef]
- da Silva, A.A.M.; Ganz, J.S.S.; Sousa, P.d.S.; Doriqui, M.J.R.; Ribeiro, M.R.C.; Branco, M.d.R.F.C.; Queiroz, R.C.d.S.; Pacheco, M.d.J.T.; da Costa, F.R.V.; Silva, F.d.S.; et al. Early Growth and Neurologic Outcomes of Infants with Probable Congenital Zika Virus Syndrome. Emerg. Infect. Dis. 2016, 22, 1953. [Google Scholar] [CrossRef]
- World Health Organization. Screening, Assessment and Management of Neonates and Infants with Complications Associated with Zika Virus Exposure in Utero: Rapid Advice Guideline. WHO/ZIKV/MOC/16.3 Rev.1; 2016. Available online: https://apps.who.int/iris/handle/10665/204475 (accessed on 15 October 2022).
- van der Linden, V. Description of 13 Infants Born During October 2015–January 2016 with Congenital Zika Virus Infection Without Microcephaly at Birth—Brazil. MMWR Morb. Mortal Wkly. Rep. 2016, 65, 1343–1348. [Google Scholar] [CrossRef]
- dos Santos, S.F.M.; Soares, F.V.M.; de Abranches, A.D.; da Costa, A.C.C.; Moreira, M.E.L.; de Matos Fonseca, V. Infants with Microcephaly Due to ZIKA Virus Exposure: Nutritional Status and Food Practices. Nutr. J. 2019, 18, 4. [Google Scholar] [CrossRef]
- Duijts, L.; Jaddoe, V.W.V.; Hofman, A.; Moll, H.A. Prolonged and Exclusive Breastfeeding Reduces the Risk of Infectious Diseases in Infancy. Pediatrics 2010, 126, e18–e25. [Google Scholar] [CrossRef]
- Leventakou, V.; Roumeliotaki, T.; Koutra, K.; Vassilaki, M.; Mantzouranis, E.; Bitsios, P.; Kogevinas, M.; Chatzi, L. Breastfeeding Duration and Cognitive, Language and Motor Development at 18 Months of Age: Rhea Mother-Child Cohort in Crete, Greece. J. Epidemiol. Community Health 2015, 69, 232–239. [Google Scholar] [CrossRef]
- Sampieri, C.L.; Montero, H. Breastfeeding in the Time of Zika: A Systematic Literature Review. PeerJ 2019, 7, e6452. [Google Scholar] [CrossRef]
- World Health Organization. Infant Feeding in Areas of Zika Virus Transmission; World Health Organization: Geneva, Switzerland, 2016.
- Developmental Stages in Infant and Toddler Feeding. Infant & Toddler Forum. Available online: https://infantandtoddlerforum.org/toddlers-to-preschool/growth-and-development-of-toddlers/developmental-stages/ (accessed on 15 January 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 8 October 2022).
- Satterfield-Nash, A.; Kotzky, K.; Allen, J.; Bertolli, J.; Moore, C.A.; Pereira, I.O.; Pessoa, A.; Melo, F.; Santelli, A.C.F.E.S.; Boyle, C.A.; et al. Health and Development at Age 19-24 Months of 19 Children Who Were Born with Microcephaly and Laboratory Evidence of Congenital Zika Virus Infection During the 2015 Zika Virus Outbreak-Brazil, 2017. MMWR Morb. Mortal Wkly. Rep. 2017, 66, 1347–1351. [Google Scholar] [CrossRef]
- Ferreira, H.N.C.; Schiariti, V.; Regalado, I.C.R.; Sousa, K.G.; Pereira, S.A.; Fechine, C.P.N.D.S.; Longo, E. Functioning and Disability Profile of Children with Microcephaly Associated with Congenital Zika Virus Infection. Int. J. Environ. Res. Public Health 2018, 15, 1107. [Google Scholar] [CrossRef]
- dos Santos, S.F.M.; Soares, F.V.M.; de Abranches, A.D.; da Costa, A.C.C.; Gomes-Júnior, S.C.d.S.; Fonseca, V.d.M.; Moreira, M.E.L. Nutritional Profile of Newborns with Microcephaly and Factors Associated with Worse Outcomes. Clinics 2019, 74, e798. [Google Scholar] [CrossRef]
- de Carvalho-Sauer, R.d.C.O.; Costa, M.d.C.N.; Paixão, E.S.; de Jesus Silva, N.; Barreto, F.R.; Teixeira, M.G. Cross-Sectional Study of the Anthropometric Characteristics of Children with Congenital Zika Syndrome up to 12 Months of Life. BMC Pediatrics 2020, 20, 479. [Google Scholar] [CrossRef]
- Fábia Cabral Cavalcanti, A.; Aguiar, Y.P.C.; Oliveira Melo, A.S.D.; Leite Cavalcanti, A.; D’Ávila, S. Breastfeeding Behavior in Brazilian Children with Congenital Zika Syndrome. Int. J. Dent. 2020, 2020, e1078250. [Google Scholar] [CrossRef]
- de Oliveira, A.M.M.; de Melo, E.G.M.; Mendes, M.L.T.; Oliveira, S.J.G.d.S.; Tavares, C.S.S.; Vaez, A.C.; de Vasconcelos, S.J.A.; Santos, H.P.; Santos, V.S.; Martins-Filho, P.R.S. Oral and Maxillofacial Conditions, Dietary Aspects, and Nutritional Status of Children with Congenital Zika Syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 71–77. [Google Scholar] [CrossRef]
- Medeiros, A.M.C.; Jardim-Botelho, A.; Santos, E.M.d.S.; Lopes, A.d.S.A.; Santos, F.B.; de Sá, T.P.L.; Barreto, Í.D.C.; dos Santos, C.A.; Cuevas, L.E.; Gurgel, R.Q. Feeding Methods and Weight Evolution in Newborns with Congenital Microcephaly Due for Zika Virus. Audiol. Commun. Res. 2021, 26. [Google Scholar] [CrossRef]
- Lowe, R.; Barcellos, C.; Brasil, P.; Cruz, O.G.; Honório, N.A.; Kuper, H.; Carvalho, M.S. The Zika Virus Epidemic in Brazil: From Discovery to Future Implications. Int. J. Environ. Res. Public Health 2018, 15, 96. [Google Scholar] [CrossRef]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; Kerkhove, M.D.V.; Abadie, V.; Garel, C.; et al. Association between Zika Virus and Microcephaly in French Polynesia, 2013–2015: A Retrospective Study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef]
- Prata-Barbosa, A.; Martins, M.M.; Guastavino, A.B.; Cunha, A.J.L.A.d. Effects of Zika Infection on Growth. J. De Pediatr. 2019, 95, 30–41. [Google Scholar] [CrossRef]
- Taylor, H.; Pennington, L.; Craig, D.; Morris, C.; McConachie, H.; Cadwgan, J.; Sellers, D.; Andrew, M.; Smith, J.; Garland, D.; et al. Children with Neurodisability and Feeding Difficulties: A UK Survey of Parent-Delivered Interventions. BMJ Paediatr. Open 2021, 5, e001095. [Google Scholar] [CrossRef]
- CanChild. Available online: https://www.canchild.ca/en/resources/42-gross-motor-function-classification-system-expanded-revised-gmfcs-e-r (accessed on 16 December 2022).
- Modrell, A.K.; Tadi, P. Primitive Reflexes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Matuszczyk, M.; Mika-Stępkowska, P.; Szmurło, A.; Szary, M.; Perlinski, M.; Kierkuś, J. Dietary Management of Infants and Young Children with Feeding Difficulties and Unsatisfactory Weight Gain Using a Nutritionally Complete Hypercaloric Infant Formula. Practical Considerations from Clinical Cases. Postgrad. Med. 2021, 133, 707–715. [Google Scholar] [CrossRef]
- Carroll, C.; Booth, A.; Campbell, F.; Relton, C. What Are the Implications of Zika Virus for Infant Feeding? A Synthesis of Qualitative Evidence Concerning Congenital Zika Syndrome (CZS) and Comparable Conditions. PLoS Negl. Trop. Dis. 2020, 14, e0008731. [Google Scholar] [CrossRef]
- Cavalcanti, A.L.; Costa, G.M.C.; Celino, S.D.d.M.; Corrêa, R.R.; Ramos, R.A.; Cavalcanti, A.F.C. Born in Chains: Perceptions of Brazilian Mothers Deprived of Freedom about Breastfeeding. Pesqui. Bras. Odontopediatria Clín. Integr 2018, 18, 4144. [Google Scholar] [CrossRef]
- Batista, C.L.C.; Ribeiro, V.S.; Nascimento, M.d.D.S.B.; Rodrigues, V.P. Association between Pacifier Use and Bottle-Feeding and Unfavorable Behaviors during Breastfeeding. J. De Pediatr. 2018, 94, 596–601. [Google Scholar] [CrossRef]
- WHO. Statement on the 2nd Meeting of IHR Emergency Committee on Zika Virus and Observed Increase in Neurological Disorders and Neonatal Malformations. Available online: https://www.who.int/news/item/08-03-2016-who-statement-on-the-2nd-meeting-of-ihr-emergency-committee-on-zika-virus-and-observed-increase-in-neurological-disorders-and-neonatal-malformations (accessed on 16 December 2022).
- World Health Organization. Division of Diarrhoeal and Acute Respiratory Disease Control; Fund (UNICEF), U.N.C. Breastfeeding Counselling: A Training Course; WHO/CDR/93.3-6; World Health Organization: Geneva, Switzerland, 1993. Available online: https://apps.who.int/iris/handle/10665/63428 (accessed on 16 December 2022).
- Davanzo, R.; Cannioto, Z.; Ronfani, L.; Monasta, L.; Demarini, S. Breastfeeding and Neonatal Weight Loss in Healthy Term Infants. J. Hum. Lancet 2013, 29, 45–53. [Google Scholar] [CrossRef]
- Belfort, M.B. The Science of Breastfeeding and Brain Development. Breastfeed Med. 2017, 12, 459–461. [Google Scholar] [CrossRef]
| Studies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Criteria | Leal [25], 2017 Brazil | Satterfield -Nash [37], 2017 Brazil | Ferreira [38], 2018 Brazil | dos Santos [29], 2019 Brazil | dos Santos [39], 2019 Brazil | Soares [4], 2019 Brazil | Carvalho- Sauer [40], 2020 Brazil | Cavalcanti [41], 2020 Brazil | Peçanha [18], 2020 Brazil | Oliveira [42], 2020 Brazil | Medeiros [43], 2021 Brazil |
| 1. Research question clearly stated |  |  |  |  |  |  |  |  |  |  |  |
| 2. Study population clearly specified and defined |  |  |  |  |  |  |  |  |  |  |  |
| 3. Participation rate of eligible persons at least 50% |  |  |  |  |  |  |  |  |  |  |  |
| 4. Same or similar study populations, prespecified inclusion/exclusion criteria |  |  |  |  |  |  |  |  |  |  |  |
| 5. Sample size justification |  |  |  |  |  |  |  |  |  |  |  |
| 6. Exposure of interest measured prior to the outcome |  |  |  |  |  |  |  |  |  |  |  |
| 7. Sufficient timeframe between exposure and outcome |  |  |  |  |  |  |  |  |  |  |  |
| 8.The study examined different levels of exposure as related to the outcome |  |  |  |  |  |  |  |  |  |  |  |
| 9. Clearly defined exposure measures |  |  |  |  |  |  |  |  |  |  |  |
| 10. Exposure assessed more than once over time |  |  |  |  |  |  |  |  |  |  |  |
| 11. Outcome measures clearly defined |  |  |  |  |  |  |  |  |  |  |  |
| 12. Outcome assessors blinded to the exposure status |  |  |  |  |  |  |  |  |  |  |  |
| 13. Loss to follow-up after baseline 20% or less |  |  |  |  |  |  |  |  |  |  |  |
| 14. Confounding variables measured and adjusted statistically |  |  |  |  |  |  |  |  |  |  |  |
 : low risk of bias;
: low risk of bias;  : some concerns (unclear);
: some concerns (unclear);  : high risk of bias.
: high risk of bias.| Author/Year/ Country | Design | N | Exposure | Data Collection | Feeding Outcomes |
|---|---|---|---|---|---|
| Leal [25], 2017 Brazil | A descriptive, retrospective case-series study | 9 | Children with dysphagia and CZS 8–24 months of age | From the medical records of three tertiary care institutions | Feeding dysphagia and problems with breastfeeding were manifested after the third month of life in eight of the nine infants. Abnormal swallowing in all infants. |
| Satterfield-Nash [37], 2017 Brazil | Cohort study | 19 | Children with CZS 19–24 months of age | ZODIAC research | 47% of children presented feeding difficulties. |
| Ferreira [38], 2018 Brazil | A descriptive cross-sectional study | 34 | Children 21 months of age with microcephaly due to CZS | From rehabilitation services | More than 70% of children with microcephaly had severe difficulty eating, 52% of them were not breastfeed |
| dos Santos [29], 2019 Brazil | Data from a cohort study “Fernandes Figueira National Institute of Women, Children and Adolescent Health—Oswaldo Cruz Foundation” | 65 | Infants 12–23 months of age with microcephaly | A public institute | 80% of the infants were not exclusively breastfed until the 6th month. 53.6% of the mothers reported difficulties with breastfeeding. At the age of 12–23 months, few infants continued breastfeeding. |
| dos Santos [39], 2019 Brazil | A longitudinal descriptive study | 21 | Full-term neonates exposed to ZIKV intrauterine | A public neonatal intensive care unit | CZS was associated with worse nutritional status. Mean weight of infants consuming only human milk (via breastfeeding and/or expressing breast milk and pasteurized milk from the milk bank) tended to be higher than that of infants consuming only infant formula. |
| Soares [4], 2019 Brazil | Cohort study | 115 | 56 infants who were exposed intrauterine to ZIKV and 59 who were unexposed, all 1–3 months of age | A part of a large cohort study based in Rio de Janeiro, Brazil | 17.9% of infected infants presented dysphagia (hypersalivation, choking and reflux) compared to the non-infected infants. By the third month of age, 48.3% of exposed infants receiving formula milk compared to 22.2% of unexposed infants. |
| Carvalho-Sauer [40], 2020 Brazil | Cross-sectional study | 46 | Children up to 12 months of age with CZS | Sourced from 22 municipalities in the State of Bahia by convenience sampling | 56.8% of children had dysphagia. There was a positive correlation between breastfeeding time and weight at 3 and 6 months of age, and only a minority of these children were still breastfeeding at 12 months. |
| Cavalcanti [41], 2020 Brazil | Observational, longitudinal study | 98 | Children 2–17 months of age with CZS | Interviews with mothers of children with CZS from two rehabilitation centers | 89.9% of children were breastfed at birth; by the age of 6 months, 36.6% continued breastfeeding, 48% had swallowing difficulty and 27.8% had suckling difficulties; use of bottle was reported for 89.9%. |
| Peçanha [18], 2020 Brazil | An exploratory case series | 84 | Asymptomatic children exposed to ZIKV intrauterine (range 6–18 months) | Outpatient clinic at Instituto Fernandes Figueira (IFF)-Fundação Oswaldo Cruz (Fiocruz) | Exclusive breastfeeding was maintained in 58.3% of children up to 6 months. |
| Oliveira [42], 2020 Brazil | Cross-sectional study | 45 | 45 children with CZS and 50 healthy controls, all 6 months of age | Three rehabilitation centers | Difficulty swallowing (60%), excessive salivation (57.8%) and non-exclusive breastfeeding until 6 months (84.4%). Ultraprocessed food intake, lower weight and enteral nutrition through gastrostomy or jejunostomy were noted in children with CZS. |
| Medeiros [43], 2021 Brazil | Retrospective cohort with nested case-control study | 86 | Two groups of neonates, with microcephaly (n = 43) and without microcephaly (n = 43) (from birth to the 37th day of life) | Data were collected from a maternity hospital in northeastern Brazil | During hospitalization, 34.9% of neonates with microcephaly breastfed exclusively, in contrast to the control group, which breastfed at a rate of 47.4%. A nasogastric feeding tube was used in 23.3% of the microcephaly group, while in the control group, it was used in 7.9%. 58% of the neonates in the control and 70% of the neonates in the control group (without microcephaly) were taken to the maternal breast as soon as they were born. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniou, E.; Andronikidi, P.E.; Eskitzis, P.; Iliadou, M.; Palaska, E.; Tzitiridou-Chatzopoulou, M.; Rigas, N.; Orovou, E. Congenital Zika Syndrome and Disabilities of Feeding and Breastfeeding in Early Childhood: A Systematic Review. Viruses 2023, 15, 601. https://doi.org/10.3390/v15030601
Antoniou E, Andronikidi PE, Eskitzis P, Iliadou M, Palaska E, Tzitiridou-Chatzopoulou M, Rigas N, Orovou E. Congenital Zika Syndrome and Disabilities of Feeding and Breastfeeding in Early Childhood: A Systematic Review. Viruses. 2023; 15(3):601. https://doi.org/10.3390/v15030601
Chicago/Turabian StyleAntoniou, Evangelia, Paraskevi Eva Andronikidi, Panagiotis Eskitzis, Maria Iliadou, Ermioni Palaska, Maria Tzitiridou-Chatzopoulou, Nikolaos Rigas, and Eirini Orovou. 2023. "Congenital Zika Syndrome and Disabilities of Feeding and Breastfeeding in Early Childhood: A Systematic Review" Viruses 15, no. 3: 601. https://doi.org/10.3390/v15030601
APA StyleAntoniou, E., Andronikidi, P. E., Eskitzis, P., Iliadou, M., Palaska, E., Tzitiridou-Chatzopoulou, M., Rigas, N., & Orovou, E. (2023). Congenital Zika Syndrome and Disabilities of Feeding and Breastfeeding in Early Childhood: A Systematic Review. Viruses, 15(3), 601. https://doi.org/10.3390/v15030601









