Vector Competence of Northern European Culex pipiens Biotype pipiens and Culex torrentium to West Nile Virus and Sindbis Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Rearing of Mosquitoes
2.2. Infection of Mosquitoes
2.3. Vector Competence Assay
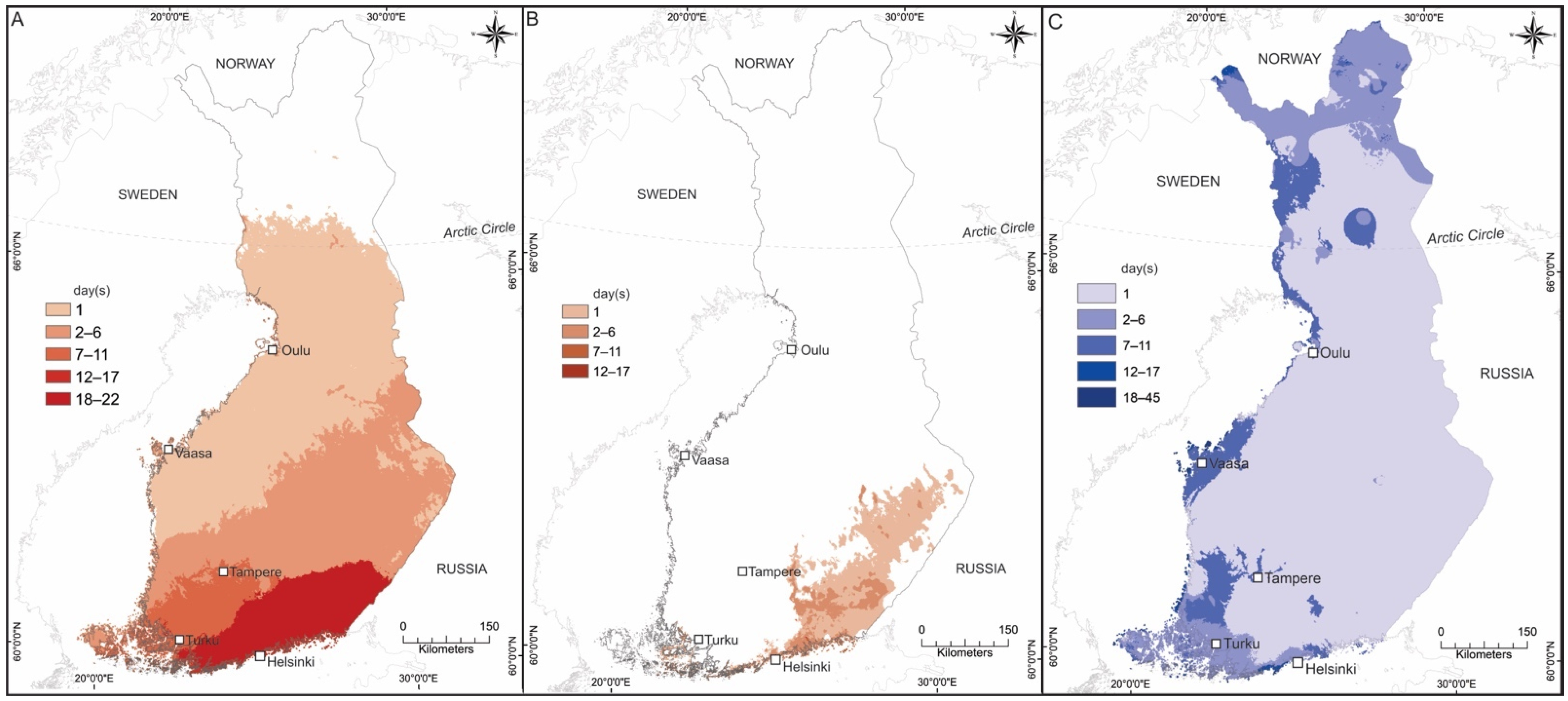
2.4. Air Temperature and Relative Humidity Data
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocklöv, J.; Dubrow, R. Climate change: An enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 2020, 21, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Adouchief, S.; Smura, T.; Sane, J.; Vapalahti, O.; Kurkela, S. Sindbis virus as a human pathogen-epidemiology, clinical picture and pathogenesis. Rev. Med. Virol. 2016, 26, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Finnish Institute for Health and Welfare (THL). Tartuntatautirekisterin Tilastotietokanta. [Statistical Database of National Infectious Disease Registry]. THL: Helsinki, Finland. Available online: https://sampo.thl.fi/pivot/prod/fi/ttr/shp/fact_shp?row=area-12260&column=time-12059&filter=reportgroup-12074 (accessed on 18 December 2022).
- Korhonen, E.M.; Suvanto, M.T.; Uusitalo, R.; Faolotto, G.; Smura, T.; Sane, J.; Vapalahti, O.; Huhtamo, E. Sindbis Virus Strains of Divergent Origin Isolated from Humans and Mosquitoes During a Recent Outbreak in Finland. Vector Borne Zoonotic Dis. 2020, 20, 843–849. [Google Scholar] [CrossRef]
- Suvanto, M.T.; Uusitalo, R.; Otte Im Kampe, E.; Vuorinen, T.; Kurkela, S.; Vapalahti, O.; Dub, T.; Huhtamo, E.; Korhonen, E.M. Sindbis virus outbreak and evidence for geographical expansion in Finland, 2021. Euro Surveill. 2022, 27, 2200580. [Google Scholar] [CrossRef] [PubMed]
- Barzon, L. Ongoing and emerging arbovirus threats in Europe. J. Clin. Virol. 2018, 107, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Young, J.J.; Haussig, J.M.; Aberle, S.W.; Pervanidou, D.; Riccardo, F.; Sekulić, N.; Bakonyi, T.; Gossner, C.M. Epidemiology of human West Nile virus infections in the European Union and European Union enlargement countries, 2010 to 2018. Euro Surveill. 2021, 26, 2001095. [Google Scholar] [CrossRef]
- Ziegler, U.; Santos, P.D.; Groschup, M.H.; Hattendorf, C.; Eiden, M.; Hoper, D.; Eisermann, P.; Keller, M.; Michel, F.; Klopfleisch, R.; et al. West Nile Virus Epidemic in Germany Triggered by Epizootic Emergence, 2019. Viruses 2020, 12, 448. [Google Scholar] [CrossRef]
- Kampen, H.; Holicki, C.M.; Ziegler, U.; Groschup, M.H.; Tews, B.A.; Werner, D. West Nile Virus Mosquito Vectors (Diptera: Culicidae) in Germany. Viruses 2020, 12, 493. [Google Scholar] [CrossRef]
- Sikkema, R.S.; Schrama, M.; van den Berg, T.; Morren, J.; Munger, E.; Krol, L.; van der Beek, J.G.; Blom, R.; Chestakova, I.; van der Linden, A.; et al. Detection of West Nile virus in a common whitethroat (Curruca communis) and Culex mosquitoes in the Netherlands, 2020. Euro Surveill. 2020, 25, 2001704. [Google Scholar] [CrossRef]
- Uejio, C.K.; Kemp, A.; Comrie, A.C. Climatic controls on West Nile virus and Sindbis virus transmission and outbreaks in South Africa. Vector Borne Zoonotic Dis. 2012, 12, 117–125. [Google Scholar] [CrossRef]
- Avizov, N.; Zuckerman, N.; Orshan, L.; Shalom, U.; Yeger, T.; Vapalahti, O.; Israely, T.; Paran, N.; Melamed, S.; Mendelson, E.; et al. High Endemicity and Distinct Phylogenetic Characteristics of Sindbis Virus in Israel. J. Infect. Dis. 2018, 218, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, Z. Mosquito-borne viruses in Europe. Parasitol. Res. 2008, 103 (Suppl. 1), S29–S43. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Smura, T.; Lundström, J.O.; Pettersson, J.H.; Sironen, T.; Vapalahti, O.; Lundkvist, Å.; Hesson, J.C. Introduction and Dispersal of Sindbis Virus from Central Africa to Europe. J. Virol. 2019, 93, e00620-19. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, E.; Olsen, B.; Lundkvist, A.; Hubálek, Z.; Sikutová, S.; Waldenström, J.; Karlsson, M.; Wahlström, M.; Jozan, M.; Falk, K.I. Surveillance for West Nile virus in wild birds from northern Europe. Vector Borne Zoonotic Dis. 2011, 11, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Lühken, R.; Helms, M.; Pluskota, B.; Pfitzner, W.P.; Oerther, S.; Becker, N.; Schmidt-Chanasit, J.; Heitmann, A. Vector Competence of Mosquitoes from Germany for Sindbis Virus. Viruses 2022, 14, 2644. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Culex pipiens Group—Current Known Distribution: March 2021. 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/culex-pipiens-group-current-known-distribution-march-2021 (accessed on 20 December 2022).
- Culverwell, C.L.; Uusitalo, R.J.; Korhonen, E.M.; Vapalahti, O.P.; Huhtamo, E.; Harbach, R.E. The mosquitoes of Finland: Updated distributions and bionomics. Med. Vet. Entomol. 2021, 35, 1–29. [Google Scholar] [CrossRef]
- Hesson, J.C.; Ostman, O.; Schäfer, M.; Lundström, J.O. Geographic distribution and relative abundance of the sibling vector species Culex torrentium and Culex pipiens in Sweden. Vector Borne Zoonotic Dis. 2011, 11, 1383–1389. [Google Scholar] [CrossRef]
- Jansen, S.; Heitmann, A.; Lühken, R.; Leggewie, M.; Helms, M.; Badusche, M.; Rossini, G.; Schmidt-Chanasit, J.; Tannich, E. Culex torrentium: A Potent Vector for the Transmission of West Nile Virus in Central Europe. Viruses 2019, 11, 492. [Google Scholar] [CrossRef]
- Jansen, S.; Cadar, D.; Lühken, R.; Pfitzner, W.P.; Jöst, H.; Oerther, S.; Helms, M.; Zibrat, B.; Kliemke, K.; Becker, N.; et al. Vector Competence of the Invasive Mosquito Species Aedes koreicus for Arboviruses and Interference with a Novel Insect Specific Virus. Viruses 2021, 13, 2507. [Google Scholar] [CrossRef]
- Chao, D.-Y.; Davis, B.S.; Chang, G.-J.J. Development of multiplex real-time reverse transcriptase PCR assays for detecting eight medical important flaviviruses in mosquitoes. J. Clin. Microbiol. 2007, 45, 584–589. [Google Scholar] [CrossRef]
- Eshoo, M.W.; Whitehouse, C.A.; Zoll, S.T.; Massire, C.; Pennella, T.-T.D.; Blyn, L.B.; Sampath, R.; Hall, T.A.; Ecker, J.A.; Desai, A.; et al. Direct broad-range detection of alphaviruses in mosquito extracts. Virology 2007, 368, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.J.; Lanciotti, R.S. Consensus amplification and novel multiplex sequencing method for S segment species identification of 47 viruses of the Orthobunyavirus, Phlebovirus, and Nairovirus genera of the family Bunyaviridae. J. Clin. Microbiol. 2009, 47, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Jöst, H.; Bialonski, A.; Storch, V.; Günther, S.; Becker, N.; Schmidt-Chanasit, J. Isolation and phylogenetic analysis of Sindbis viruses from mosquitoes in Germany. J. Clin. Microbiol. 2010, 8, 1900–1903. [Google Scholar] [CrossRef] [PubMed]
- Aalto, J.; Pirinen, P.; Jylhä, K. New gridded daily climatology of Finland: Permutation-based uncertainty estimates and temporal trends in climate. J. Geophys. Res. Atmos. 2016, 121, 3807–3823. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [PubMed]
- Vogels, C.B.F.; Göertz, G.P.; Pijlman, G.P.; Koenraadt, C.J.M. Vector competence of northern and southern European Culex pipiens pipiens mosquitoes for West Nile virus across a gradient of temperatures. Med. Vet. Entomol. 2017, 31, 358–364. [Google Scholar] [CrossRef]
- Lundström, J.O.; Niklasson, B.; Francy, D.B. Swedish Culex torrentium and Cx. pipiens (Diptera: Culicidae) as experimental vectors of Ockelbo virus. J. Med. Entomol. 1990, 27, 561–563. [Google Scholar] [CrossRef]
- Holicki, C.M.; Ziegler, U.; Răileanu, C.; Kampen, H.; Werner, D.; Schulz, J.; Silaghi, C.; Groschup, M.H.; Vasić, A. West Nile Virus Lineage 2 Vector Competence of Indigenous Culex and Aedes Mosquitoes from Germany at Temperate Climate Conditions. Viruses 2020, 12, 561. [Google Scholar] [CrossRef]
- Uusitalo, R.; Siljander, M.; Culverwell, C.L.; Hendrickx, G.; Lindén, A.; Dub, T.; Aalto, J.; Sane, J.; Marsboom, C.; Suvanto, M.T.; et al. Predicting Spatial Patterns of Sindbis Virus (SINV) Infection Risk in Finland Using Vector, Host and Environmental Data. Int. J. Environ. Res. Public Health 2021, 18, 7064. [Google Scholar] [CrossRef]
- Kampen, H.; Tews, B.A.; Werner, D. First Evidence of West Nile Virus Overwintering in Mosquitoes in Germany. Viruses 2021, 13, 2463. [Google Scholar] [CrossRef]
- Rudolf, I.; Betášová, L.; Blažejová, H.; Venclíková, K.; Straková, P.; Šebesta, O.; Mendel, J.; Bakonyi, T.; Schaffner, F.; Nowotny, N.; et al. West Nile virus in overwintering mosquitoes, central Europe. Parasites Vectors 2017, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Dohm, D.J.; Turell, M.J. Effect of Incubation at Overwintering Temperatures on the Replication of West Nile Virus in New York Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2001, 38, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xing, D.; Li, C.; Dong, Y.; Zhao, T.; Guo, X. Replication and transmission of West Nile virus in simulated overwintering adults of Culex pipiens pallens (Diptera: Culicidae) in China. Acta Trop. 2023, 237, 106720. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K.; Fang, Y.; Martinez, V.M. Effects of Temperature on the Transmission of West Nile Virus by Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 2006, 43, 309–317. [Google Scholar] [CrossRef]

| Virus | Mosquito Species | Temperature | Dpi | n | IR | Viral RNA Copy Number/Body | TR | TE |
|---|---|---|---|---|---|---|---|---|
| WNV | Culex torrentium | 27 ± 5 °C | 14 | 24 | 92% (22/24) | 8.00 (7.25–8.75) | 36% (8/22) | 33% (8/24) |
| 24 ± 5 °C | 14 | 9 | 100% (9/9) | 7.35 (6.51–8.20) | 11% (1/9) | 11% (1/9) | ||
| 21 ± 5 °C | 14 | 30 | 83% (25/30) | 6.93 (6.12–7.73) | 0% (0/25) | 0% (0/30) | ||
| 18 ± 5 °C | 14 | 32 | 97% (31/32) | 6.01 (5.37–6.65) | 0% (0/31) | 0% (0/32) | ||
| Culex pipiens pipiens | 27 ± 5 °C | 14 | 35 | 43% (15/35) | 7.85 (6.66–9.04) | 40% (6/15) | 17% (6/35) | |
| 24 ± 5 °C | 14 | 30 | 83% (25/30) | 7.28 (6.46–8.11) | 8% (2/25) | 7% (2/30) | ||
| 21 ± 5 °C | 14 | 30 | 73% (22/30) | 5.99 (5.26–6.71) | 0% (0/22) | 0% (0/30) | ||
| 18 ± 5 °C | 14 | 30 | 63% (19/30) | 5.72 (4.98–6.45) | 0% (0/19) | 0% (0/30) | ||
| SINV | Culex torrentium | 24 ± 5 °C | 5 | 30 | 87% (26/30) | 7.81 (6.98–8.65) | 69% (18/26) | 60% (18/30) |
| 18 ± 5 °C | 5 | 34 | 97% (33/34) | 7.73 (7.19–8.62) | 18% (6/33) | 18% (6/34) | ||
| Culex pipiens pipiens | 24 ± 5 °C | 5 | 29 | 48% (14/29) | 5.16 (3.90–6.43) | 57% (8/14) | 28% (8/29) | |
| 18 ± 5 °C | 5 | 31 | 23% (7/31) | 4.33 (3.52–5.14) | 29% (2/7) | 6% (2/31) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, S.; Heitmann, A.; Uusitalo, R.; Korhonen, E.M.; Lühken, R.; Kliemke, K.; Lange, U.; Helms, M.; Kirjalainen, L.; Nykänen, R.; et al. Vector Competence of Northern European Culex pipiens Biotype pipiens and Culex torrentium to West Nile Virus and Sindbis Virus. Viruses 2023, 15, 592. https://doi.org/10.3390/v15030592
Jansen S, Heitmann A, Uusitalo R, Korhonen EM, Lühken R, Kliemke K, Lange U, Helms M, Kirjalainen L, Nykänen R, et al. Vector Competence of Northern European Culex pipiens Biotype pipiens and Culex torrentium to West Nile Virus and Sindbis Virus. Viruses. 2023; 15(3):592. https://doi.org/10.3390/v15030592
Chicago/Turabian StyleJansen, Stephanie, Anna Heitmann, Ruut Uusitalo, Essi M. Korhonen, Renke Lühken, Konstantin Kliemke, Unchana Lange, Michelle Helms, Lauri Kirjalainen, Roope Nykänen, and et al. 2023. "Vector Competence of Northern European Culex pipiens Biotype pipiens and Culex torrentium to West Nile Virus and Sindbis Virus" Viruses 15, no. 3: 592. https://doi.org/10.3390/v15030592
APA StyleJansen, S., Heitmann, A., Uusitalo, R., Korhonen, E. M., Lühken, R., Kliemke, K., Lange, U., Helms, M., Kirjalainen, L., Nykänen, R., Gregow, H., Pirinen, P., Rossini, G., Vapalahti, O., Schmidt-Chanasit, J., & Huhtamo, E. (2023). Vector Competence of Northern European Culex pipiens Biotype pipiens and Culex torrentium to West Nile Virus and Sindbis Virus. Viruses, 15(3), 592. https://doi.org/10.3390/v15030592







