Synthesis of 4′-Substituted Carbocyclic Uracil Derivatives and Their Monophosphate Prodrugs as Potential Antiviral Agents
Abstract
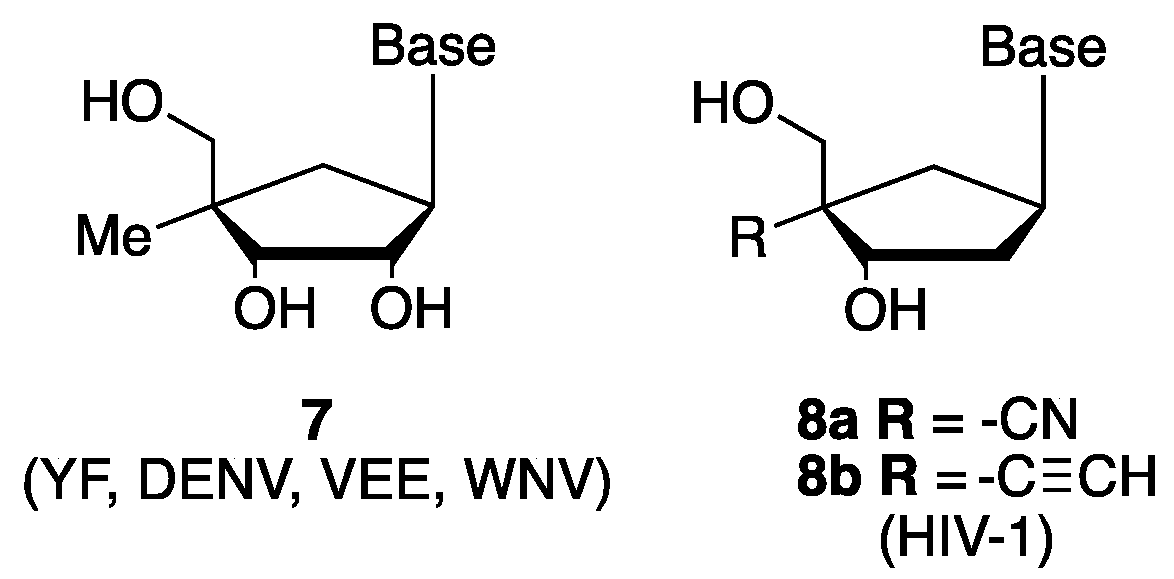
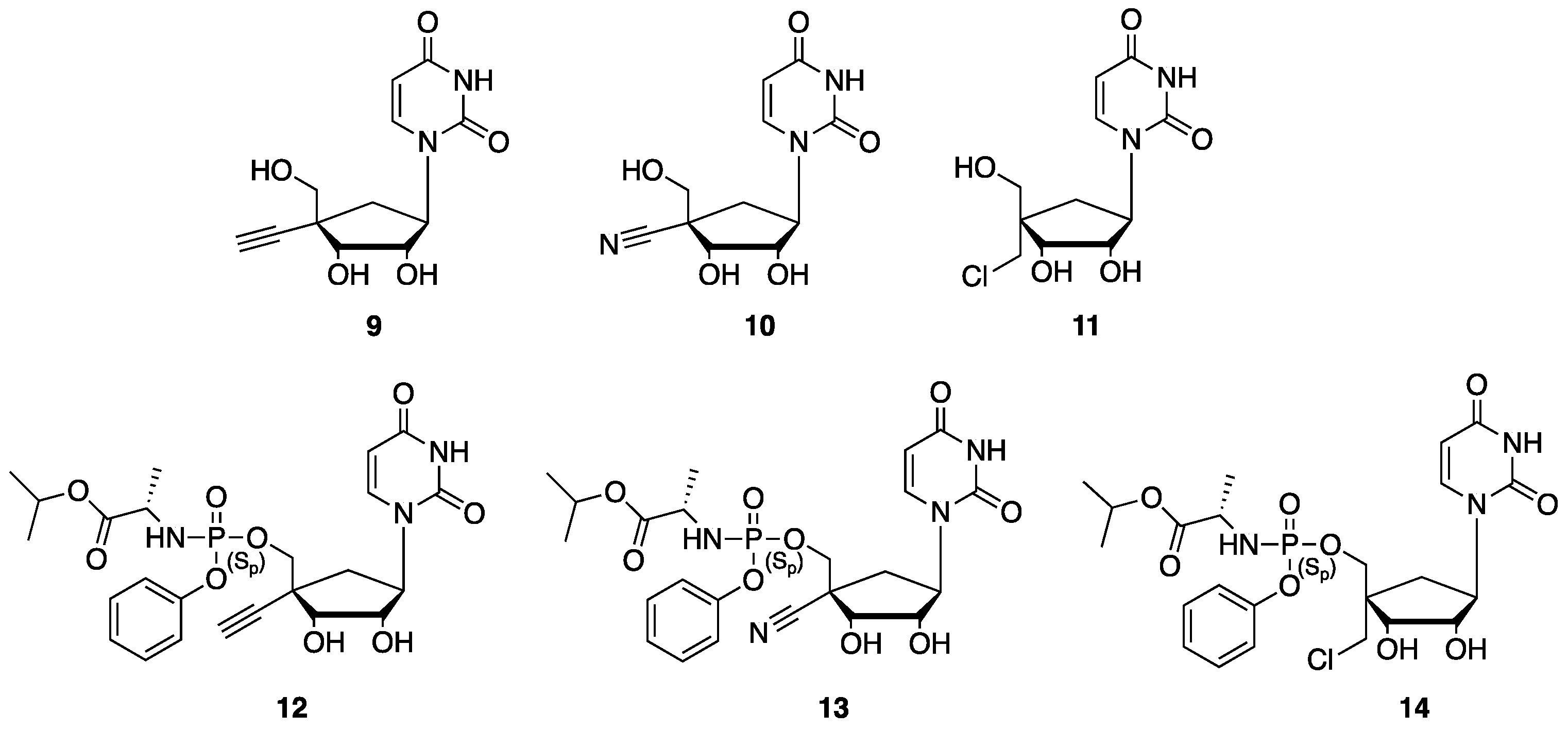
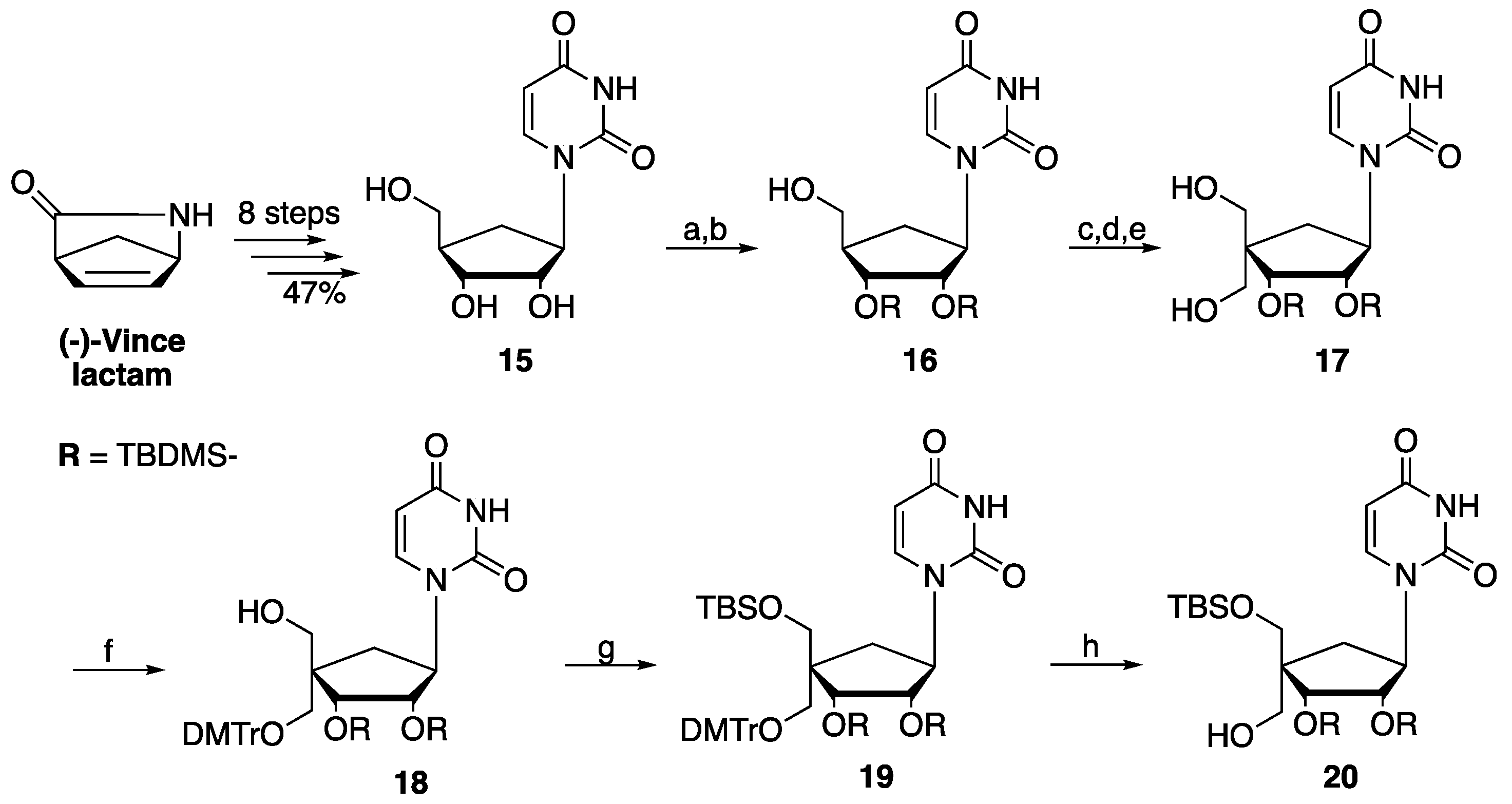
1. Introduction
2. Material and Methods
2.1. Antiviral Assays
2.1.1. SARS-CoV-2 Antiviral Assays
2.1.2. Norovirus Antiviral Assays
2.1.3. Influenza A/B Antiviral Assays
2.2. Cytotoxicity Assays
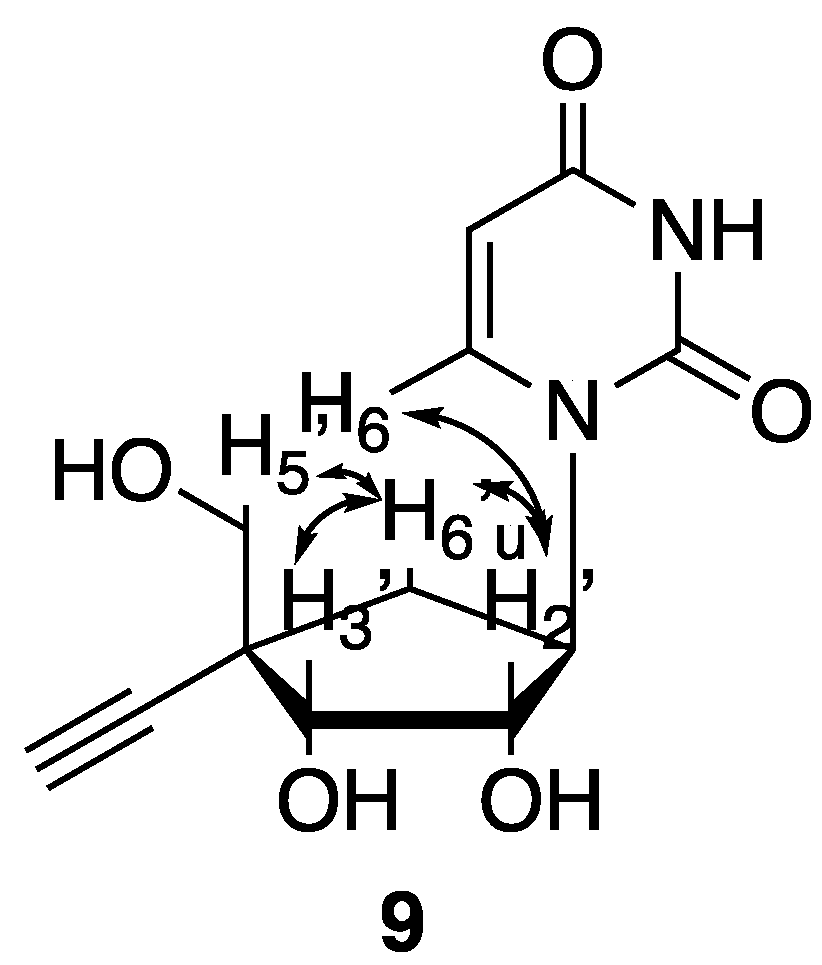
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seley-Radtke, K.L.; Yates, M.K. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antivir. Res. 2018, 154, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liang, C.; Zou, L.; Yin, Y.; Wang, J.; Chen, D.; Lan, W. Advance of structural modification of nucleosides scaffold. Eur. J. Med. Chem. 2021, 214, 113233. [Google Scholar] [CrossRef] [PubMed]
- Betson, M.; Allanson, N.; Wainwright, P. A review of methods to synthesise 4′-substituted nucleosides. Org. Biomol. Chem. 2014, 12, 9291–9306. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Martin, J.A.; Klumpp, K.; Baker, S.J.; Blomgren, P.A.; Devos, R.; Granycome, C.; Hang, J.; Hobbs, C.J.; Jiang, W.-R.; et al. Design, synthesis, and antiviral properties of 4′-substituted ribonucleosides as inhibitors of hepatitis C virus replication: The discovery of R1479. Bioorg. Med. Chem. Lett. 2007, 17, 2570–2576. [Google Scholar] [CrossRef]
- Coats, S.J.; Garnier-Amblard, E.C.; Amblard, F.; Ehteshami, M.; Amiralaei, S.; Zhang, H.; Zhou, L.; Boucle, S.R.; Lu, X.; Bondada, L.; et al. Chutes and ladders in hepatitis C nucleoside drug development. Antivir. Res. 2014, 102, 119–147. [Google Scholar] [CrossRef]
- Wang, G.; Deval, J.; Hong, J.; Dyatkina, N.; Prhavc, M.; Taylor, J.; Fung, A.; Jin, Z.; Stevens, S.K.; Serebryany, V.; et al. Discovery of 4′-chloromethyl-2′-deoxy-3′,5′-di-O-isobutyryl-2′-fluorocytidine (ALS-8176), a first-in-class RSV polymerase inhibitor for treatment of human respiratory syncytial virus infection. J. Med. Chem. 2015, 58, 1862–1878. [Google Scholar] [CrossRef]
- Patel, K.; Kirkpatrick, C.M.; Nieforth, K.A.; Chanda, S.; Zhang, Q.; McClure, M.; Fry, J.; Symons, J.A.; Blatt, L.M.; Beigelman, L.; et al. Respiratory syncytial virus-A dynamics and the effects of lumicitabine, a nucleoside viral replication inhibitor, in experimentally infected humans. J. Antimicrob. Chemother. 2019, 74, 442–452. [Google Scholar] [CrossRef]
- Markowitz, M.; Sarafianos, S.G. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine, MK-8591: A novel HIV-1 reverse transcriptase translocation inhibitor. Curr. Opin. HIV AIDS 2018, 4, 294–299. [Google Scholar] [CrossRef]
- Menéndez-Arias, L.; Delgad, R. Update and latest advances in antiretroviral therapy. Trends Pharmacol. Sci. 2022, 43, 16–29. [Google Scholar] [CrossRef]
- Derbalah, A.; Karpick, H.; Maize, H.; Skersick, P.; Cottrell, M.; Rao, G. Role of islatravir in HIV treatment and prevention: An update. Curr. Opin. HIV AIDS 2022, 17, 240–246. [Google Scholar] [CrossRef]
- Masone, M.C. Islatravir implant as HIV-1 pre-exposure treatment. Nat. Rev. Urol. 2021, 18, 706. [Google Scholar] [CrossRef] [PubMed]
- Painter, G.R.; Perryman, D.; Bluemling, G.R. Preparation of 4′-halogen containing nucleotide and nucleoside therapeutics and uses related thereto. WO2019173602 A1, 12 September 2019. [Google Scholar]
- Sourimant, J.; Lieber, C.M.; Aggarwal, M.; Cox, R.M.; Wolf, J.D.; Yoon, J.-J.; Toots, M.; Ye, C.; Sticher, Z.; Kolykhalov, A.A.; et al. 4′-Fluorouridine is an oral antiviral that blocks respiratory syncytial virus and SARS-CoV-2 replication. Science 2022, 375, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Amblard, F.; Nolan, S.P.; Agrofoglio, L.A. Metathesis strategy in nucleoside chemistry. Tetrahedron 2005, 61, 7067–7080. [Google Scholar] [CrossRef]
- Bessières, M.; Chevrier, F.; Roy, V.; Agrofoglio, L.A. Recent progress for the synthesis of selected carbocyclic nucleosides. Fut. Med. Chem. 2015, 13, 1809–1828. [Google Scholar] [CrossRef]
- Ojeda-Porras, A.C.; Roy, V.; Agrofoglio, L.A. Chemical approaches to carbocyclic nucleosides. Chem. Rec. 2022, 22, e202100307. [Google Scholar] [CrossRef] [PubMed]
- Boutureira, O.; Matheu, M.I.; Díaz, Y.; Castillón, S. Advances in the enantioselective synthesis of carbocyclic nucleosides. Chem. Soc. Rev. 2013, 42, 5056–5072. [Google Scholar] [CrossRef]
- Liu, P.; Sharon, A.; Chu, C.K. Enantiomeric synthesis of carbocyclic D-4′-C-methylribonucleosides as potential antiviral agents. Tet. Asymm. 2006, 17, 3304–3314. [Google Scholar] [CrossRef]
- Alexandre, F.-R.; Rahali, R.; Rahali, H.; Guillon, S.; Convard, T.; Fillgrove, K.; Lai, M.-T.; Meillon, J.-C.; Xu, M.; Small, J.; et al. Synthesis and antiviral evaluation of carbocyclic nucleoside analogs of nucleoside reverse transcriptase translocation inhibitor MK-8591 (4′-ethynyl-2-fluoro-2′-deoxyadenosine). J. Med. Chem. 2018, 61, 9218–9228. [Google Scholar] [CrossRef]
- Zandi, K.; Amblard, F.; Musall, K.; Downs-Bowen, J.; Kleinbard, R.; Oo, A.; Cao, D.; Liang, B.; Russell, O.O.; McBrayer, T.; et al. Repurposing nucleoside analogs for human coronaviruses. Antimicrob. Agents Chemother. 2020, 6, e01652-20. [Google Scholar] [CrossRef]
- Costantini, V.P.; Whitaker, T.; Barclay, L.; Lee, D.; McBrayer, T.R.; Schinazi, R.F.; Vinjé, J. Antiviral activity of nucleoside analogues against norovirus. Antivir. Ther. 2012, 17, 981–991. [Google Scholar] [CrossRef]
- Muzzarelli, K.M.; Kuiper, B.; Spellmon, N.; Brunzelle, J.; Hackett, J.; Amblard, F.; Zhou, S.; Liu, P.; Kovari, I.A.; Yang, Z.; et al. Structural and antiviral studies of the human norovirus GII.4 protease. Biochemistry 2019, 58, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, L.; Amichai, S.; Zandi, K.; Cox, B.; Schinazi, R.; Amblard, F. Novel influenza polymerase PB2 inhibitors for the treatment of influenza A infection. Bioorg. Med. Chem. Lett. 2019, 29, 126639. [Google Scholar] [CrossRef] [PubMed]
- Mengshetti, S.; Zhou, L.; Sari, O.; De Schutter, C.; Zhang, H.; Cho, J.H.; Tao, S.; Bassit, L.C.; Verma, K.; Domaoal, R.A.; et al. Discovery of a series of 2′-α-fluoro,2′-β-bromo-ribonucleosides and their phosphoramidate prodrugs as potent pan-genotypic inhibitors of hepatitis C virus. J. Med. Chem. 2019, 62, 1859–1874. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, J.; Wang, P.; Nagarathnam, D.; Espiritu, C.L.; Bao, H.; Murakami, E.; Furman, P.A.; Sofia, M.J. A 2′-deoxy-2′-fluoro-2′-C-methyl uridine cyclopentyl carbocyclic analog and its phosphoramidate prodrug as inhibitors of HCV NS5B polymerase. Nucleosides Nucleotides Nucleic Acids 2012, 31, 277–285. [Google Scholar] [CrossRef]
- Sato, T.; Tsuzuki, T.; Takano, S.; Kohtaro, K.; Fukuda, H.; Arisawa, M.; Shuto, S. Construction of a chiral quaternary carbon center by a radical cyclization/ring-enlargement reaction: Synthesis of 4α-azidoethyl carbocyclic ribose, a key unit for the synthesis of cyclic ADP-ribose derivatives of biological importance. Tetrahedron 2015, 71, 5407–5413. [Google Scholar] [CrossRef]
- Akabane-Nakata, M.; Chickering, T.; Harp, J.M.; Schlegel, M.K.; Matsuda, S.; Egli, M.; Manoharan, M. RNAs containing carbocyclic ribonucleotides. Org. Lett. 2022, 24, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Pradere, U.; Garnier-Amblard, E.C.; Coast, S.J.; Amblard, F.; Schinazi, R.F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014, 114, 9154–9218. [Google Scholar] [CrossRef]
- Ross, B.S.; Reddy, P.G.; Zhang, H.R.; Rachakonda, S.; Sofia, M. Synthesis of diastereomerically pure nucleotide phosphoramidates. J. Org. Chem. 2011, 76, 8311–8319. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biteau, N.G.; Amichai, S.A.; Azadi, N.; De, R.; Downs-Bowen, J.; Lecher, J.C.; MacBrayer, T.; Schinazi, R.F.; Amblard, F. Synthesis of 4′-Substituted Carbocyclic Uracil Derivatives and Their Monophosphate Prodrugs as Potential Antiviral Agents. Viruses 2023, 15, 544. https://doi.org/10.3390/v15020544
Biteau NG, Amichai SA, Azadi N, De R, Downs-Bowen J, Lecher JC, MacBrayer T, Schinazi RF, Amblard F. Synthesis of 4′-Substituted Carbocyclic Uracil Derivatives and Their Monophosphate Prodrugs as Potential Antiviral Agents. Viruses. 2023; 15(2):544. https://doi.org/10.3390/v15020544
Chicago/Turabian StyleBiteau, Nicolas G., Sarah A. Amichai, Niloufar Azadi, Ramyani De, Jessica Downs-Bowen, Julia C. Lecher, Tamara MacBrayer, Raymond F. Schinazi, and Franck Amblard. 2023. "Synthesis of 4′-Substituted Carbocyclic Uracil Derivatives and Their Monophosphate Prodrugs as Potential Antiviral Agents" Viruses 15, no. 2: 544. https://doi.org/10.3390/v15020544
APA StyleBiteau, N. G., Amichai, S. A., Azadi, N., De, R., Downs-Bowen, J., Lecher, J. C., MacBrayer, T., Schinazi, R. F., & Amblard, F. (2023). Synthesis of 4′-Substituted Carbocyclic Uracil Derivatives and Their Monophosphate Prodrugs as Potential Antiviral Agents. Viruses, 15(2), 544. https://doi.org/10.3390/v15020544




