Abstract
To evaluate a decentralised testing model and simplified treatment protocol of hepatitis C virus (HCV) infection to facilitate treatment scale-up in Myanmar, this prospective, observational study recruited HIV–HCV co-infected outpatients receiving sofosbuvir/daclatasvir in Yangon, Myanmar. The study examined the outcomes and factors associated with a sustained virological response (SVR). A decentralised “hub-and-spoke” testing model was evaluated where fingerstick capillary specimens were transported by taxi and processed centrally. The performance of the Xpert HCV VL Fingerstick Assay in detecting HCV RNA was compared to the local standard of care ( plasma HCV RNA collected by venepuncture). Between January 2019 and February 2020, 162 HCV RNA-positive individuals were identified; 154/162 (95%) initiated treatment, and 128/154 (84%) returned for their SVR12 visit. A SVR was achieved in 119/154 (77%) participants in the intent-to-treat population and 119/128 (93%) participants in the modified-intent-to-treat population. Individuals receiving an antiretroviral therapy were more likely to achieve a SVR (with an odds ratio (OR) of 7.16, 95% CI 1.03–49.50), while those with cirrhosis were less likely (OR: 0.26, 95% CI 0.07–0.88). The sensitivity of the Xpert HCV VL Fingerstick Assay was 99.4% (95% CI 96.7–100.0), and the specificity was 99.2% (95% CI 95.9–99.9). A simplified treatment protocol using a hub-and-spoke testing model of fingerstick capillary specimens can achieve an SVR rate in LMIC comparable to well-resourced high-income settings.
1. Introduction
The introduction of direct acting antiviral (DAA) regimens has revolutionised the management of hepatitis C virus (HCV) infection. The high efficacy and low toxicity of these regimens mean that on-treatment virological monitoring is no longer required and that the monitoring for toxicity is minimised [1]. DAA therapy has improved HCV management in low- and middle-income countries (LMIC), which have the highest global HCV burden [2]. Although Myanmar has set national targets to diagnose and treat 50% of people living with HCV by 2030, the current HCV testing and treatment levels are inadequate [3]. The cost of diagnostics and DAA therapy [4], and the accessibility to testing and treatment remain barriers to HCV treatment in Myanmar [5], similar to other LMIC [6,7].
In Myanmar, the prevalence of HCV infection is estimated to be 2.7%; this equates to at least 1.1 million people living with chronic HCV [8]. In people living with Human Immunodeficiency Virus, estimates of HCV co-infection range from 5% to 23% [5,9]. Without a treatment scale-up, it is estimated that 333,000 new HCV infections and 97,000 HCV-related deaths will occur in Myanmar between 2020 and 2030 [3]. Standard of care HCV RNA testing is available throughout Myanmar as part of the National Hepatitis Control program that was launched in 2017, and the national HCV treatment guidelines—issued in the same year—provide a comprehensive treatment pathway: antibody screening by immunoassay, a confirmation by viral load testing, a series of pre-treatment assessments ( HIV testing, liver staging and renal function) and a confirmation of SVR 12 weeks post-treatment [10]. While the program provided testing and treatment for more than 8000 people from 2017–2020 [11], national data on the SVR rates are not available. Furthermore, the overall time between the standard of care HCV RNA testing, pre-treatment assessments, DAA initiation and laboratory confirmation of a cure can vary across the provinces of Myanmar, as accessibility to testing and treatment is limited [5]. Public sector treatment is only possible in secondary and tertiary hospitals and is informed by testing that is performed at reference laboratories in Yangon and Mandalay, Myanmar’s largest cities [3]. Simplification and decentralisation of the current HCV management algorithms are crucial for a treatment scale-up.
Point-of-care (POC) HCV RNA testing with the Xpert HCV VL Fingerstick test has a good diagnostic accuracy compared to laboratory-based testing following a venepuncture sampling [12,13]. POC HCV RNA testing reduces the transport and laboratory costs [14,15] and it shortens the time to treatment initiation [16,17,18]. While molecular POC testing has been implemented in some resource-limited settings, few studies have evaluated its efficacy. Furthermore, POC molecular testing may not be viable in resource-limited settings due to the cost and accessibility, particularly in rural and remote regions [19,20].
Decentralised hub-and-spoke models where POC fingerstick specimens are collected at clinical sites (spokes), and then transported using a fingerstick EDTA collection tube to the nearest GeneXpert machine, possibly located some distance away (i.e., a hub), may be more successful. This innovative model has been demonstrated for other infectious diseases [21,22,23], but not for HCV.
This prospective, observational study evaluated virological outcomes using a simplified model of care in HIV–HCV co-infected individuals undergoing DAA therapy in hospital outpatient settings in Yangon, Myanmar. This study compared the sensitivity and specificity of the Xpert HCV Viral Load VL Fingerstick Assay for HCV RNA detection (i.e., fingerstick capillary whole blood samples transferred in a microvette EDTA collection tube), to the Xpert HCV Viral Load assay (plasma collected by venepuncture) before and following the DAA therapy in the context of the novel hub-and-spoke model.
2. Materials and Methods
2.1. Study Design and Participants
From 22 January 2019 to 3 February 2020, the participants were screened in the HIV outpatient departments of the Specialist Hospital Mingaladon and Specialist Hospital Waibargi. All the enrolled participants were HIV-1 antibody-positive and HCV antibody-positive. The national guidelines for simplified HCV treatment were used to determine treatment eligibility [10]. Individuals who were unwilling or unable to provide informed consent, had an impaired renal function, were pregnant, were taking prohibited concomitant medication, or had a significant illness that would interfere with the treatment, assessment, or adherence to the study protocol were excluded.
2.2. Study Assessments
Study assessments were conducted at two on-site visits: pre-treatment screening and 12 weeks post-treatment (SVR12). The participants completed an interviewer-administered questionnaire on a tablet computer at both visits, collecting their demographic data, history of drug/alcohol use, and previous incarceration. A standardised measure of the quality of life (EQ-5D-5L), including a visual analogue scale (VAS) measuring the overall perceived health status (out of 100), was collected (Table S1) [24]. Current alcohol consumption was evaluated using the Hazard Alcohol Use Identification Test (AUDIT-c), with a hazardous consumption indicated by scores of >3 (for women) and >4 (for men) [25].
Within one week of screening, participants with detectable HCV RNA (>10 IU/mL) were offered sofosbuvir + dose-adjusted daclatasvir therapy (without cirrhosis—12 weeks therapy, and with cirrhosis—24 weeks therapy). During treatment, the participants attended the outpatient department monthly to collect their medication. A post-treatment clinical assessment was performed for the participants who returned for their SVR12 visit.
2.3. Diagnostic Testing Methodology
HIV Antibody testing: Confirmation of the HIV status was performed in all participants using three approved rapid HIV antibody tests: Determine® HIV-1/2 (Abbott, Chicago, IL, USA), Uni-Gold™ HIV-1/2 (Trinity Biotech, Co Wicklow, Ireland), and HIV1/2 Stat-Pak Dipstick (Chembio Diagnostic System, Inc, Medford, NY, USA) [26].
HCV Antibody testing: A positive HCV antibody was confirmed in all the participants at screening using a World Health Organization (WHO) prequalified POC HCV antibody test (SD, Abbott, Chicago, IL, USA) [26].
Standard of care HCV RNA testing: The presence of HCV RNA in plasma (derived from 6mL whole-blood) collected by standard venepuncture was determined at both scheduled visits as per the national Myanmar HCV treatment guidelines (Myanmar Ministry of Health and Sports; 2019), using an Xpert HCV Viral Load assay on the GeneXpert R2 6-colour, 4 module machine (GXIV-4-L System, 900-0513, GeneXpert Dx software v4.6a, lower limit of quantification: (LLoQ) 10 IU/mL; Cepheid, Sunnyvale, CA, USA) [27]. HCV RNA genotyping is not a prerequisite for HCV treatment in Myanmar and was not performed.
Fingerstick HCV RNA testing: A fingerstick capillary whole-blood sample (100 µL) was collected into a microvette collection tube (Microvette® 100 μL K3EDTA, Sarstedt, Nümbrecht, Germany) at both visits using a MiniCollect® Safety Lancet (Greiner Bio-One, Kremsmünster, Austria), as per the manufacturer’s directions and WHO guidelines [28]. The samples were transported at ambient temperature by a same-day taxi (or motorbike when a taxi was not available) to the Myanmar–Australia Research Collaboration for Health (MARCH) laboratory in Yangon. The transit time between the collection and delivery of the samples ranged between 15 and 30 min. The majority (87%) of the POC HCV RNA testing was performed on the same day of the sample collection, with the remainder of the samples tested 1–4 days later. The HCV RNA testing was performed using the Xpert HCV VL Fingerstick Assay (Cepheid, Sunnyvale, CA, USA; (LLOQ): 100 IU/mL, upper limit of quantification: 108 log10 IU/mL; 100% sensitivity, 100% specificity) on a GeneXpert R2 6-colour, 2 module machine (GXII-2-L System, GeneXpert Dx software v4.6a; Cepheid, Sunnyvale, CA, USA) (Cepheid, 2019). The GeneXpert enables the quantification of HCV RNA through real-time PCR technology, with the time to result taking approximately one hour [29].
2.4. Study Definitions
Cirrhosis was defined clinically or using the aspartate aminotransferase to platelet ratio index (APRI) ≥ 2.0.
Current injecting drug use (IDU) was defined as injecting in the last month.
HCV virological suppression was defined as a HCV RNA below the lower limit of quantitation (LLoQ) (target not detected (TND), or target detected, not quantifiable (TD, nq)).
Sustained Virologic Response (SVR) was defined as a HCV RNA target not detected (TND) or target detected, not quantifiable (TD, nq) at the post-treatment week 12.
HCV virologic failure was defined as a non-response or failure of virological suppression with quantifiable HCV RNA at the end of treatment.
Invalid results from the Xpert HCV VL Fingerstick Assay occur when no HCV RNA result is obtainable as a result of samples not being properly processed, a reverse transcription PCR (RT-PCR) inhibition is encountered due to sample integrity, or the sample is improperly collected and transported [29].
Error results from the Xpert HCV VL Fingerstick Assay occur when no HCV RNA result is available and the GeneXpert encounters a system component failure, a reagent check fails, or a PCR probe check fail that terminates the assay [29].
First-pass testing was defined as testing performed without the repeat testing of invalid or error results.
2.5. Study Endpoints
The primary endpoint of the study was SVR12 (undetectable HCV RNA at >12 weeks following the treatment completion) by an intention-to-treat (ITT) analysis (for all participants who received ≥1 dose of the study drug). A modified intention-to-treat (mITT) analysis was also conducted, including only those patients with available SVR12 HCV RNA results. A secondary analysis was performed which evaluated the sensitivity and specificity of the Xpert HCV VL Fingerstick Assay using capillary blood collected via fingerstick testing, and compared it with the standard of care plasma HCV RNA collected via venepuncture (Xpert HCV Viral Load Assay).
2.6. Statistical Analysis
The data were collected electronically and analysed using statistical software (STATA version 14.0; Stata Corporation, College Station, TX, USA). The proportion of the participants achieving SVR confirmed through the standard of care HCV RNA testing was calculated in both the ITT and mITT populations.
Logistic regression analysis was used to identify the factors associated with SVR (in the ITT and mITT populations). All variables were considered in multivariate logistic regression models using a Firth-type logistic regression to reduce small sample biases [30]. The final models included factors remaining significant at the 0.05 level.
The sensitivity and specificity of HCV RNA on the GeneXpert machine using capillary blood collected via fingerstick was compared using the standard of care HCV RNA plasma testing as the gold standard. This included both positive and negative samples at screening, following the completion of treatment among the participants who were enrolled in this study. Any discordant results were included in all calculations of the sensitivity and specificity. Bland–Altman difference plots were generated to assess the bias and agreement measurements, including the limits of agreement between the quantification of HCV by the Xpert HCV VL Fingerstick Assay compared to the standard of care. The data were analysed with GraphPad Prism (version 7.03).
3. Results
3.1. Participant Characteristics
Of the 205 participants screened, 194/205 (95%) were tested for HCV RNA, of whom 162 (84%) were HCV RNA-positive (Figure 1).
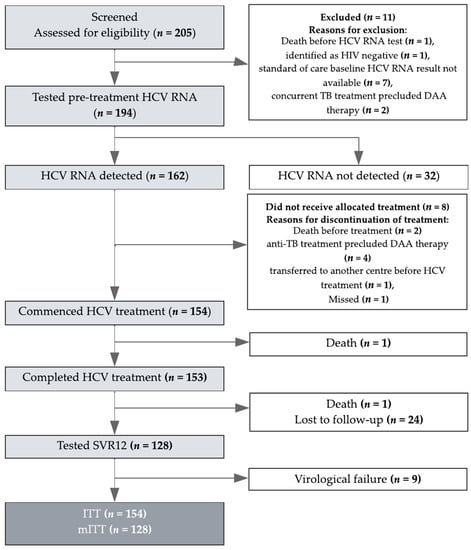
Figure 1.
Patient Disposition. HCV: hepatitis C virus; ITT: intention-to-treat; mITT: modified intention-to-treat. The number of participants screened (194/205; 95%), with detectable HCV RNA (162/194; 84%) and who commenced treatment (154/162; 95%) is depicted. Of the 11 patients who were excluded at screening, 7/11 (64%) did not have a standard of care HCV RNA result available at the time of treatment assessment, 2/11 (18%) had concurrent treatment, 1/11 (9%) died prior to HCV RNA testing, and 1/11 (9%) was identified as HIV-negative. Among those 8/162 (5%) who did not commence treatment, 2/8 (25%) died before treatment, 4/8 (50%) were receiving anti-TB treatment precluding DAA therapy, 1 was transferred to another centre before treatment and 1 missed the treatment. One death occurred during treatment due to hepatic decompensation. This participant had underlying cirrhosis and died 15 weeks after commencing therapy.
The median (interquartile (IQR)) age was 39 (29–47) years, 54/194 (28%) were female, and 165/194 (85%) were receiving antiretroviral therapy (ART), predominantly tenofovir, lamivudine and efavirenz combination therapy (Table 1).

Table 1.
Baseline socio-demographic and clinical characteristics.
At screening, the CD4 cell count was ≥200 cells/µL in 129/194 (67%) and <100 cells/µL in 26/194 (14%) (Table 1). The median (IQR) HCV RNA viral load was 6.2 log10 (5.4–6.6) IU/mL. Cirrhosis was present in 15/194 (8%). The median (IQR) score for the self-reported perception of health was 85 (80–90) (Table S1).
3.2. Treatment Initiation, Uptake, and Completion
Of the 162 participants with detectable HCV RNA at screening, 154 (95%) initiated treatment and 153 (99%) completed treatment (Figure 1). Of these 153, 128 (84%) returned for their SVR12 visit and were tested for HCV RNA. Of the 24/153 (16%) lost to follow-up, 18/24 (75%) occurred in 2020 after the onset of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic and the resulting stay-at-home orders in Yangon. One death occurred during treatment due to hepatic decompensation. This participant had underlying cirrhosis and died 15 weeks after commencing therapy.
3.3. Treatment Efficacy and Factors Associated with SVR
A SVR was achieved in 119/154 (77%) in the ITT and 119/128 (93%) in the mITT populations (Figure 2).
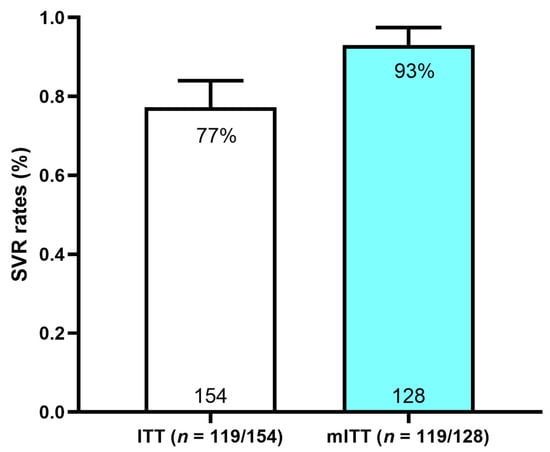
Figure 2.
SVR12 rates in ITT and mITT populations. SVR: sustained virologic response; ITT: intention-to-treat; mITT: modified intention-to-treat.
Baseline characteristics that were associated with the SVR are presented in Table 2 (for the ITT and mITT populations). In a multivariate analysis for the ITT population, the employment (with an odds ratio (OR): 2.79, 95% confidence interval (CI): (1.15–6.79), p = 0.02) and APRI ≥ 2 (OR 0.26, (0.07–0.88), p = 0.03) were independently associated with SVR.

Table 2.
Predictors of SVR12 (ITT and mITT); adjusted odds ratio presented.
In the multivariate analysis for the mITT population, only the receipt of ART was independently associated with SVR (OR 7.16 (95% CI: 1.03–49.50) p = 0.04).
3.4. Virological Failure
Virological failure was observed in 9/128 (7%) participants (Table 3).

Table 3.
Virological failure characteristics by participant.
All nine of these participants had no evidence of cirrhosis and a CD4 ≥ 200 cells/µL, but only 4/9 (44%) were on ART. The median (IQR) baseline for the HCV RNA viral load was 6.4 (5.8–6.6) log10 IU/mL.
3.5. Diagnostic Performance of Fingerstick Testing
Of the 205 participants, 194 had available samples for testing (Figure S1). In total, 343 Xpert HCV VL Fingerstick Assay test runs were performed during the study; however, 35/343 (10%) resulted in an invalid result reading, and 9/343 (3%) had an error result reading. Where possible, subsequent testing on a recollected sample was attempted resulting in an additional 27 (27/44; 61%) valid results, and 4 (4/44; 9%) error and 13 (13/44; 30%) invalid result tests that were unable to be retested (Figure S1). There was a total of 299 instances where both a valid Xpert HCV VL Fingerstick test and a standard of care HCV RNA test result were available.
3.6. Sensitivity & Specificity Analysis
Among the samples with a valid test result, the sensitivity of the Xpert HCV VL Fingerstick Assay for HCV RNA detection in the samples collected by fingerstick capillary whole blood was 99.4% (95% CI 96.7–100.0), and the specificity was 99.2% (95% CI 95.9–99.9; Table 4). The positivity predictive value (PPV) was 99.3% (95% CI 95.8–99.9%) and negative predictive value (NPV) was 99.2% (95.0–99.8%). As the study was conducted in a clinical setting, samples that produced an invalid or error result were recollected where possible, and a sensitivity and specificity analysis was also performed as a first-pass (testing performed without the repeat testing of invalid or error results) to compare the estimates (Table S2).

Table 4.
Sensitivity and specificity of the Xpert HCV VL Fingerstick Assay for HCV RNA detection compared with the standard of care.
As shown by the Bland–Altman plot analysis (Figure 3), the HCV RNA concentrations detected by the Xpert HCV Viral Load Assay using fingerstick capillary whole blood were a mean 0.03 (SD 0.24) log10 IU/mL lower than those measured by the standard of care, with 95% of the differences between −0.51 and 0.44 log10 IU/mL. Two screening visit samples had discordant results for the HCV RNA quantification with fingerstick capillary whole blood and were excluded in the Bland–Altman plot. In the first sample with a discordant result, the HCV RNA concentration was 7.6 log10 IU/mL when tested by the Xpert HCV VL Fingerstick Assay and was undetectable when tested by the standard of care. An Xpert HCV VL Fingerstick result confirmation was not performed due to an insufficient sample; however, confirmation testing by the standard of care was performed, and this participant subsequently did not receive treatment. In the second sample with a discordant result, the HCV RNA concentration was undetectable when tested by the Xpert HCV VL Fingerstick Assay and 3.7 log10 IU/mL when tested by the standard of care. As per the standard of care, this participant was initiated onto treatment.
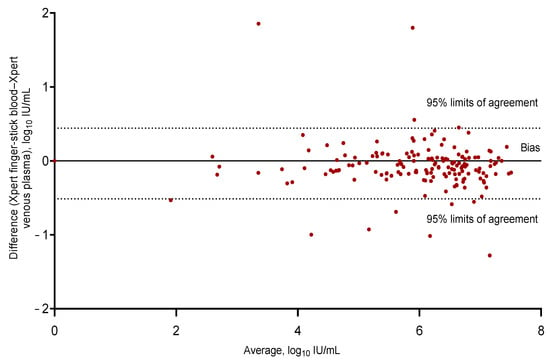
Figure 3.
Bland–Altman bias plot of differences for the Xpert HCV VL Fingerstick Assay for HCV RNA detection in fingerstick capillary whole-blood samples compared with the Xpert HCV Viral Load Assay for HCV RNA detection in venous plasma (with two discrepant results excluded from the analysis); n = 297, bias −0.03643, 95% limits of agreement −0.51 to 0.44. HCV: hepatitis C virus.
4. Discussion
This study performed amongst HIV/HCV co-infected participants at two outpatient clinics in Yangon, Myanmar, demonstrates that participants managed using a simplified DAA treatment protocol can achieve excellent SVR rates, comparable to those achieved in resource-rich settings. It also highlights the excellent sensitivity and specificity of the Xpert HCV VL Fingerstick Assay for HCV RNA detection, suggesting that it may have a role in resource-limited settings in optimising access to care. Finally, the hub-and-spoke testing model—using a fingerstick EDTA collection tube—facilitated the remote sample collection with centralised testing. This provided the clinicians and patients with prompt clinical information, expediting the initiation of HCV therapy. The SVR of 93% in the mITT population is consistent with trials among HIV/HCV co-infected participants receiving DAA therapy in well-resourced settings [31,32,33]. This is particularly notable given the use of a first-generation regimen (i.e., sofosbuvir/daclatasvir) which is more complicated to administer and has greater drug–drug interactions than the newer pan-genotypic regimens.
Employment was associated with SVR, suggesting a better treatment access and increased adherence in these individuals [34]. Cirrhosis was associated with a lack of SVR, further highlighting that these individuals are more difficult to cure [35]. ART was associated with SVR, suggesting a higher health engagement in individuals on ART and/or increased health provision for this population. Notably, in the participants with virological failure, less than half of the individuals were on ART, which might be explained by a greater geographical distance from health services, the presence of social stressors affecting the access to care, or poorer health literacy [36].
Although the SVR was lower in the ITT analysis (Figure 2), 75% of the participants that were lost to follow-up were unable to attend their SVR12 visit due to their expected clinic visit coinciding with a city-wide lockdown in Yangon in response to the SARS-CoV2 pandemic [37,38]. The participants were unable to access hospital services during the follow-up period, and clinicians were unable to transport specimens into Yangon for testing. Similar rates of loss to follow-up in 2020 have been reported in several studies [39,40,41]. Many of these participants completed their full course of therapy as directed, and most would, therefore, be anticipated to have achieved a cure. The inclusion of interventions designed to enhance the HCV follow-up are clearly vital in vulnerable populations with a high risk of HCV infection [42], to mitigate the loss to follow-up of patients especially during the ongoing global SARS-CoV2 pandemic [41].
The high sensitivity and specificity of the Xpert HCV VL Fingerstick Assay for HCV RNA detection by fingerstick with the use of a novel collection vessel is an important finding of this study. The Xpert HCV VL Fingerstick Assay demonstrated a strong agreement with the local standard of care assay (Xpert HCV VL Assay via venous plasma) with a 0.3 log10 IU/mL or lower difference between 95% limits of agreement of all the measurements across all the HCV RNA concentrations tested. High sensitivity and specificity estimates were also observed when the analysis was performed as a first-pass testing (Table S2). Although there were two discrepant results when comparing the Xpert HCV VL Fingerstick Assay for HCV RNA detection by fingerstick with the standard of care, these discrepancies at the screening visit were likely to be explained by human error and would be less likely to occur with more experience and improved quality control processes.
Repeat testing of 44 (13%) fingerstick tests were required due to invalid and error results on the first-pass testing and are likely in part explained by logistical and environmental factors experienced during the study. The invalid results on the GeneXpert can be attributed to the sample condition level prior to testing (e.g., problems during the collection, transport, or sample environment). A small number of samples that missed taxi transport were transported by motorbike which may have resulted in conditions that may have affected the sample integrity. While the sample testing was performed on the same day of collection, there was a delay in testing in some samples (13% of all samples tested; range of 1–4 days post-sample collection), which may also have adversely affected sample quality. The effect of lag times between the blood draw and HCV RNA testing on the sample integrity has been reported previously [43]. Additionally, while the samples were transported at ambient temperature, the impact of the Yangon’s tropical climate meant that these temperatures approached 40 °C at some times and this may also have impacted the sample integrity. Our study has highlighted the potential for the hub-and-spoke model but also the requirement for temperature and transport safeguards to be firmly established to minimize retesting.
HCV POC tests that resulted in an error on the GeneXpert can be attributed to machine or user errors encountered during the loading and testing of the sample and were low in our study (3%) and decreased in frequency as the study progressed, thus highlighting the importance of operator ongoing education, training, and competency assessment. A learning curve for laboratory scientists and clinicians can be expected when implementing new models of care and novel molecular techniques [44].
The hub-and-spoke model of testing with the GeneXpert platform with an EDTA microvette sample collection is novel. Decentralisation of the specimen collection and testing at POC is increasingly recognised in many infectious diseases as an ideal way to ensure prompt access to treatment [45], particularly in remote or difficult to access locations [46,47,48]. This model is particularly suited to infections where a diagnosis can be made immediately (through a lateral flow assay or rapid test) [49]; however, where testing involves an element of diagnostic infrastructure such as the GeneXpert, which, although widespread is not available in every clinic, a hub-and-spoke model may be appealing. This model of delivery of diagnostic services has been shown to be highly cost-effective in the provision of care for other infectious diseases—such as Tuberculosis [21,23] and Malaria [50], as well as HCV [51]. Demonstration of its success will add another option to facilitate treatment commencement; however, as this study has shown, careful planning and optimisation of the laboratory quality control processes are essential if this model is to be implemented successfully.
The study has several limitations. Despite the relatively small sample size, the overall sensitivity and specificity of the Xpert HCV Viral Load test for HCV RNA detection by fingerstick was excellent; however, further evaluation of this assay is crucial and should be conducted in similar settings to confirm the findings’ reproducibility. While the losses-to-follow up in the ITT population due to the events of the SARS-CoV2 pandemic and subsequent lockdowns in 2020 were unanticipated, almost all patients who commenced treatment completed their regimen (99%; 153/154). Considering the high efficacy of sofusbuvir/daclatasvir, the patients lost to follow-up would be expected to have achieved a SVR [52]. The inclusion of interventions to improve follow-up, however, would further facilitate patients achieving a SVR [40,41]. Finally, a selection bias may have contributed to the findings as the participants were already engaged in HIV care at the clinics. Reaching those at higher risk and who are less engaged with healthcare may be challenging, particularly given the recent changes in healthcare delivery in Myanmar [53].
5. Conclusions
In conclusion, the study shows excellent SVR rates among those who returned for post-treatment HCV RNA testing, and a high sensitivity and specificity of the Xpert HCV VL Fingerstick Assay for HCV RNA detection by fingerstick capillary whole-blood compared to the local gold standard method of testing in HIV/HCV co-infected people in Yangon, Myanmar. The study also demonstrated the feasibility and promise of the hub-and-spoke model in a resource-limited setting, with the microvette collection vessel addressing delays in testing and preventing a loss of samples or the opportunity to collect from patients. This highlights the feasibility of this approach, but also suggests that optimal sample handling and laboratory quality control is essential. This study highlights the potential of a simplified treatment protocol with a hub-and-spoke testing model to improve the testing, diagnosis, linkage to care and DAA therapy initiation in HIV/HCV co-infected individuals and potentially other vulnerable populations living with HCV globally.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15020521/s1, Figure S1: Study sampling profile; Table S1: EQ-5D-EL Utility test: Performance status of participants, Table S2: Sensitivity and specificity of the Xpert HCV VL Fingerstick Assay for HCV detection compared with standard of care, performed as first-pass.
Author Contributions
Conceptualization, K.S.L., G.M., J.H. and T.A.; methodology, K.S.L., G.M., J.H. and T.A.; validation, S.T. and P.P.N.; formal analysis, S.T. and P.P.N.; investigation, K.S.L., G.M., J.H., P.P.N., K.S.L., S.P., M.M.Y., K.L.H., M.T.A., M.M.L., S.T. and P.P.N.; data curation, S.T. and P.P.N.; writing—original draft preparation, S.T. and P.P.N.; writing—review and editing, K.S.L., G.M, J.H., J.G. and T.A.; visualization, S.T. and P.P.N.; supervision, K.S.L., G.M., J.H. and T.A. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support provided by The Kirby Institute, UNSW. The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government. This study was also supported by a research grant from Cepheid (provided tests).
Institutional Review Board Statement
The study has been reviewed and approved by the Human Research Ethics Committee of University of New South Wales (protocol code: HC180511 and date of approval: 8 August 2018) and the Institutional Technical and Ethical Review Board of University of Public Health (Myanmar) (protocol code: 2018/Research/21 and date of approval: 6 June 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the sensitive nature of some of the data, including that related to injection drug use.
Acknowledgments
Amanda Erratt (AE) provided project administration.
Conflicts of Interest
G.M. reports grants from Gilead Sciences and grants from AbbVie. J.G. reports grants from Merck, grants from Cepheid, during the conduct of the study; grants and personal fees from AbbVie, grants and personal fees from Gilead Sciences, grants and personal fees from Merck, grants and personal fees from Cepheid, grants from Hologic, and grants from Indivior, outside the submitted work. All others declare no potential competing interests.
References
- Davis, J.S.; Young, M.; Marshall, C.; Tate-Baker, J.; Madison, M.; Sharma, S.; Silva, C.; Jones, T.; Davies, J. Minimal Compared With Standard Monitoring During Sofosbuvir-Based Hepatitis C Treatment: A Randomized Controlled Trial. Open Forum Infect. Dis. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.; Barber, E.; Cook, N.A.; Gomaa, A.; Harley, Y.X.; Jones, C.; Lim, A.G.; Mohamed, Z.; Nayagam, A.S.; Ndow, G.; et al. Aiming at the Global Elimination of Viral Hepatitis: Challenges Along the Care Continuum. Open Forum Infect. Dis. 2018, 5, 252. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.; Win, T.M.; Tidhar, T.; Htay, H.; Draper, B.; Aung, P.T.Z.; Xiao, Y.; Bowring, A.; Kuschel, C.; Shilton, S.; et al. Hepatitis C elimination in Myanmar: Modelling the impact, cost, cost-effectiveness and economic benefits. Lancet Reg. Health West. Pac. 2021, 10, 100129. [Google Scholar] [CrossRef] [PubMed]
- Marquez, L.K.; Chaillon, A.; Soe, K.P.; Johnson, D.C.; Zosso, J.M.; Incerti, A.; Loarec, A.; Nguyen, A.; Walker, J.G.; Mafirakureva, N.; et al. Cost and cost-effectiveness of a real-world HCV treatment program among HIV-infected individuals in Myanmar. BMJ Glob. Health 2021, 6, 4181. [Google Scholar] [CrossRef]
- Min Thaung, Y.; Chasela, C.S.; Chew, K.W.; Minior, T.; Lwin, A.A.; Sein, Y.Y.; Drame, N.; Marange, F.; van Der Horst, C.; Thwin, H.T.; et al. Treatment outcomes and costs of a simplified antiviral treatment strategy for hepatitis C among monoinfected and HIV and/or hepatitis B virus-co-infected patients in Myanmar. J. Viral Hepat. 2021, 28, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Boeke, C.E.; Adesigbin, C.; Agwuocha, C.; Anartati, A.; Aung, H.T.; Aung, K.S.; Grover, G.S.; Ngo, D.; Okamoto, E.; Ngwije, A.; et al. Initial success from a public health approach to hepatitis C testing, treatment and cure in seven countries: The road to elimination. BMJ Glob. Health 2020, 5, e003767. [Google Scholar] [CrossRef]
- Martin, N.K.; Vickerman, P.; Grebely, J.; Hellard, M.; Hutchinson, S.J.; Lima, V.D.; Foster, G.R.; Dillon, J.F.; Goldberg, D.J.; Dore, G.J.; et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013, 58, 1598–1609. [Google Scholar] [CrossRef]
- Aung, K.S. Viral Hepatitis in Myanmar: Current situation and response 2016. In National Hepatitis Program; Ministry of Health and Family Welfare: New Delhi, India, 2016. [Google Scholar]
- Zaw, S.K.K.; Tun, S.T.T.; Thida, A.; Aung, T.K.; Maung, W.; Shwe, M.; Aye, M.M.; Clevenbergh, P. Prevalence of hepatitis C and B virus among patients infected with HIV: A cross-sectional analysis of a large HIV care programme in Myanmar. Trop. Dr. 2013, 43, 113–115. [Google Scholar] [CrossRef]
- Ministry of Health and Sports. Myanmar National Simplified Treatment Guidelines for Hepatitis C Infection, 2nd ed.; Ministry of Health and Sports: Naypyidaw, Myanmar, 2019. Available online: http://mohs.gov.mm/Main/content/publication/hepatitis-national-simplified-treatment-guidelines-of-viral-hepatitis-c-infection-second-edition-july-2019 (accessed on 11 March 2022).
- CT2 Study Myanmar Hepatitis C: Community-Based Testing and Treatment Summary Report. 2020. Available online: https://burnet.edu.au/system/asset/file/4513/Summary-Report_Hep_C_Myanmar_DigitalFinal.pdf (accessed on 24 January 2023).
- Catlett, B.; Hajarizadeh, B.; Cunningham, E.; Wolfson-Stofko, B.; Wheeler, A.; Khandaker-Hussain, B.; Feld, J.J.; Martró, E.; Chevaliez, S.; Pawlotsky, J.M.; et al. Diagnostic Accuracy of Assays Using Point-of-Care Testing or Dried Blood Spot Samples for the Determination of Hepatitis C Virus RNA: A Systematic Review. J. Infect. Dis. 2022, 226, 1005–1021. [Google Scholar] [CrossRef]
- Lamoury, F.M.J.; Bajis, S.; Hajarizadeh, B.; Marshall, A.D.; Martinello, M.; Ivanova, E.; Catlett, B.; Mowat, Y.; Marks, P.; Amin, J.; et al. Evaluation of the Xpert HCV Viral Load Finger-Stick Point-of-Care Assay. J. Infect. Dis. 2018, 217, 1889–1896. [Google Scholar] [CrossRef]
- Koo, V.; Tian, F.; Wong, W.W. Cost-effectiveness analysis of hepatitis C virus (HCV) point-of-care assay for HCV screening. Liver Int. 2022, 42, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.; Scott, N.; Nayagam, S.; Rwegasha, J.; Mbwambo, J.; Thursz, M.R.; Brown, A.S.; Hellard, M.; Lemoine, M. Cost effectiveness of simplified HCV screening-and-treatment interventions for people who inject drugs in Dar-es-Salaam, Tanzania. Int. J. Drug Policy 2022, 99, 103458. [Google Scholar] [CrossRef] [PubMed]
- Midgard, H.; Bjørnestad, R.; Egeland, M.; Dahl, E.; Finbråten, A.; Kielland, K.B.; Blindheim, M.; Dalgard, O. Peer support in small towns: A decentralized mobile Hepatitis C virus clinic for people who inject drugs. Liver Int. 2022, 42, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Tao, Y.; Fajardo, E.; Reipold, E.I.; Chou, R.; Tucker, J.D.; Easterbrook, P. Diagnostic Accuracy of Point-of-Care HCV Viral Load Assays for HCV Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Valencia, J.; Lazarus, J.V.; Ceballos, F.C.; Troya, J.; Cuevas, G.; Resino, S.; Torres-Macho, J.; Ryan, P. Differences in the hepatitis C virus cascade of care and time to initiation of therapy among vulnerable subpopulations using a mobile unit as point-of-care. Liver Int. 2022, 42, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Grebely, J.; Applegate, T.; Cunningham, P.; Feld, J.J. Hepatitis C point-of-care diagnostics: In search of a single visit diagnosis. Expert Rev. Mol. Diagn. 2017, 17, 1109–1115. [Google Scholar] [CrossRef]
- Brown, S.; Leavy, J.E.; Jancey, J. Implementation of GeneXpert for TB Testing in Low- and Middle-Income Countries: A Systematic Review. Glob. Health Sci. Pr. 2021, 9, 698–710. [Google Scholar] [CrossRef]
- Casiraghi, A.; Domenicucci, M.; Cattaneo, S.; Maggini, E.; Albertini, F.; Avanzini, S.; Marini, M.P.; Galante, C.; Guizzi, P.; Milano, G. Operational strategies of a trauma hub in early coronavirus disease 2019 pandemic. Int. Orthop. 2020, 44, 1511–1518. [Google Scholar] [CrossRef]
- Nalugwa, T.; Shete, P.B.; Nantale, M.; Farr, K.; Ojok, C.; Ochom, E.; Mugabe, F.; Joloba, M.; Dowdy, D.W.; Moore, D.A.J.; et al. Challenges with scale-up of GeneXpert MTB/RIF® in Uganda: A health systems perspective. BMC Health Serv. Res. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Euroqol Research Foundation. EQ-5D-5L User Guide. 2019. Available online: https://euroqol.org/publications/user-guides (accessed on 9 February 2021).
- Bush, K.; Kivlahan, D.R.; McDonell, M.B.; Fihn, S.D.; Bradley, K.A. The AUDIT Alcohol Consumption Questions (AUDIT-C). An Effective Brief Screening Test for Problem Drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998, 158, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO List of Prequalified In Vitro Diagnostic Products; World Health Organization: Geneva, Switzerland, 2022; Available online: https://extranet.who.int/pqweb/vitro-diagnostics/vitro-diagnostics-lists (accessed on 11 March 2022).
- Xpert® HCV Viral Load [Package Insert]; Cepheid: Solna, Sweden, 2021.
- World Health Organization. WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy; World Health Organization: Geneva, Switzerland, 2010; Available online: https://www.who.int/publications/i/item/9789241599221 (accessed on 11 March 2022).
- Xpert® HCV VL Fingerstick [Package Insert]; Cepheid: Solna, Sweden, 2019.
- Rahman, M.S.; Sultana, M. Performance of Firth-and logF-type penalized methods in risk prediction for small or sparse binary data. BMC Med Res. Methodol. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Alessio, L.; Onorato, L.; Sangiovanni, V.; Borrelli, F.; Manzillo, E.; Esposito, V.; Simeone, F.; Martini, S.; Capoluongo, N.; Leone, S.; et al. DAA-Based Treatment for HIV–HCV-Coinfected Patients: Analysis of Factors of Sustained Virological Response in a Real-Life Study. Antivir. Ther. 2020, 25, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, L.; Lai, A.; Calvi, E.; Ronzi, P.; Micheli, V.; Binda, F.; Ridolfo, A.; Gervasoni, C.; Galli, M.; Antinori, S.; et al. Direct-acting antivirals in hepatitis C virus (HCV)-infected and HCV/HIV-coinfected patients: Real-life safety and efficacy. HIV Med. 2017, 18, 284–291. [Google Scholar] [CrossRef]
- Patel, S.V.; Jayaweera, D.T.; Althoff, K.N.; Eron, J.J.; Radtchenko, J.; Mills, A.; Moyle, G.; Santiago, S.; Sax, P.E.; Gillman, J.; et al. Real-world efficacy of direct acting antiviral therapies in patients with HIV/HCV. PLoS ONE 2020, 15, e0228847. [Google Scholar] [CrossRef]
- Ward, K.M.; Falade-Nwulia, O.; Moon, J.; Sutcliffe, C.G.; Brinkley, S.; Haselhuhn, T.; Katz, S.; Herne, K.; Arteaga, L.; Mehta, S.H.; et al. A Randomized Controlled Trial of Cash Incentives or Peer Support to Increase HCV Treatment for Persons With HIV Who Use Drugs: The CHAMPS Study. Open Forum Infect. Dis. 2019, 6, 166. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Pineda, J.A.; Calleja, J.L.; Casado, M.; Rodríguez, M.; Turnes, J.; Amado, L.E.; Morillas, R.M.; Forns, X.; Acevedo, J.M.; et al. Efficacy of sofosbuvir and velpatasvir, with and without ribavirin, in with hepatitis C virus genotype 3 infection and cirrhosis. Gastroenterology 2018, 155, 1120–1127. [Google Scholar] [CrossRef]
- Ousley, J.; Nesbitt, R.; Kyaw, N.T.T.; Bermudez, E.; Soe, K.P.; Anicete, R.; Mon, P.E.; Ei, W.L.S.S.; Christofani, S.; Fernandez, M.; et al. Increased hepatitis C virus co-infection and injection drug use in HIV-infected fishermen in Myanmar. BMC Infect. Dis. 2018, 18, 657. [Google Scholar] [CrossRef]
- San Lin, K.; Nay Win, K.H.; Khine, T.; Lei, S.L.; Zaw, K.K.; Oo, W.M. The characteristics and trend of COVID-19 outbreak in Myanmar: Lessons from a developing country. Asia Pac. J. Public Health 2021, 33, 311–313. [Google Scholar] [CrossRef]
- Win, A. Rapid rise of COVID-19 second wave in Myanmar and implications for the Western Pacific region. Qjm: Int. J. Med. 2020, 113, 856–857. [Google Scholar] [CrossRef]
- Magro, P.; Formenti, B.; Marchese, V.; Gulletta, M.; Tomasoni, L.R.; Caligaris, S.; Castelli, F.; Matteelli, A. Impact of the SARS-CoV-2 epidemic on tuberculosis treatment outcome in Northern Italy. Eur. Respir. J. 2020, 56, 2002665. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Xu, L.; Qin, J.; Hahn, E.E.; Ngo-Metzger, Q.; Mittman, B.; Tewari, D.; Hodeib, M.; Wride, P.; Saraiya, M.; et al. Impact of COVID-19 on cervical cancer screening rates among women aged 21–65 years in a large integrated health care system—Southern California, January 1–September 30, 2019, and January 1–September 30, 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 109. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Accarino, E.; Camprecios, J.M.; Domínguez-Hernández, R.; Rando-Segura, A.; Riveiro-Barciela, M.; Rodríguez-Frías, F.; Barreira-Diaz, A.; Palom, A.; Casado, M.A.; Esteban-Mur, R.; et al. Impact of COVID-19 pandemic in the relink-c strategy to search and retrieve lost-to follow-up hcv patients. Hepatology 2021, 74, 548A. [Google Scholar]
- Cunningham, E.B.; Wheeler, A.; Hajarizadeh, B.; French, C.E.; Roche, R.; Marshall, A.D.; Fontaine, G.; Conway, A.; Valencia, B.M.; Bajis, S.; et al. Interventions to enhance testing, linkage to care, and treatment initiation for hepatitis C virus infection: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Calzia, A.; Dublineau, A.; Rouet, F.; Nouhin, J.; Yann, S.; Pin, S.; Sun, C.; Sann, K.; Dimanche, C.; et al. Field evaluation of GeneXpert® (Cepheid) HCV performance for RNA quantification in a genotype 1 and 6 predominant patient population in Cambodia. J. Viral Hepat. 2019, 26, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Baudhuin, L.M.; Donato, L.J.; Uphoff, T.S. How novel molecular diagnostic technologies and biomarkers are revolutionizing genetic testing and patient care. Expert Rev. Mol. Diagn. 2012, 12, 25–37. [Google Scholar] [CrossRef]
- Draper, B.; Yee, W.L.; Pedrana, A.; Kyi, K.P.; Qureshi, H.; Htay, H.; Naing, W.; Thompson, A.J.; Hellard, M.; Howell, J. Reducing liver disease-related deaths in the Asia-Pacific: The important role of decentralised and non-specialist led hepatitis C treatment for cirrhotic patients. Lancet Reg. Health West. Pac. 2022, 20, 100359. [Google Scholar] [CrossRef]
- Draper, B.L.; Htay, H.; Pedrana, A.; Yee, W.L.; Howell, J.; Pyone Kyi, K.; Naing, W.; Sanda Aung, K.; Markby, J.; Easterbrook, P.; et al. Outcomes of the CT2 study: A ‘one-stop-shop’for community-based hepatitis C testing and treatment in Yangon, Myanmar. Liver Int. 2021, 41, 2578–2589. [Google Scholar] [CrossRef]
- Hengel, B.; Causer, L.; Matthews, S.; Smith, K.; Andrewartha, K.; Badman, S.; Spaeth, B.; Tangey, A.; Cunningham, P.; Saha, A.; et al. A decentralised point-of-care testing model to address inequities in the COVID-19 response. Lancet Infect. Dis. 2021, 21, e183–e190. [Google Scholar] [CrossRef]
- Opollo, V.S.; Nikuze, A.; Ben-Farhat, J.; Anyango, E.; Humwa, F.; Oyaro, B.; Wanjala, S.; Omwoyo, W.; Majiwa, M.; Akelo, V.; et al. Field evaluation of near point of care Cepheid GeneXpert HIV-1 Qual for early infant diagnosis. PLoS ONE 2018, 13, e0209778. [Google Scholar] [CrossRef]
- Myint, N.P.S.T.; Zaw, T.T.; Sain, K.; Waiyan, S.; Danta, M.; Cooper, D.; Aung, N.M.; Kyi, M.M.; Hanson, J. Sequential Helicobacter pylori eradication therapy in Myanmar; a randomized clinical trial of efficacy and tolerability. J. Gastroenterol. Hepatol. 2020, 35, 617–623. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, E.; Chan, G.; Hudoba, M.; Markin, T.; Yakimec, J.; Roland, K. Malaria screening using front-line loop-mediated isothermal amplification: Fourteen-month experience in a nonendemic regional hub-and-spoke laboratory setting. Am. J. Clin. Pathol. 2021, 155, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.; Mbwambo, J.; Rwegasha, J.; Mgina, N.; Doulla, B.; Mwakale, P.; Tuaillon, E.; Chevaliez, S.; Shimakawa, Y.; Taylor-Robinson, S.D.; et al. In-field evaluation of Xpert® HCV viral load Fingerstick assay in people who inject drugs in Tanzania. Liver Int. 2020, 40, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, M.S.; Gardiner, D.F.; Rodriguez-Torres, M.; Reddy, K.R.; Hassanein, T.; Jacobson, I.; Lawitz, E.; Lok, A.S.; Hinestrosa, F.; Thuluvath, P.J.; et al. Daclatasvir plus Sofosbuvir for Previously Treated or Untreated Chronic HCV Infection. New Engl. J. Med. 2014, 370, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Low, S.; Tun, K.T.; Mhote, N.P.P.; Htoo, S.N.; Maung, C.; Kyaw, S.W.; Oo, S.E.K.S.; Pocock, N.S. Human resources for health: Task shifting to promote basic health service delivery among internally displaced people in ethnic health program service areas in eastern Burma/Myanmar. Glob. Health Action 2014, 7, 24937. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).