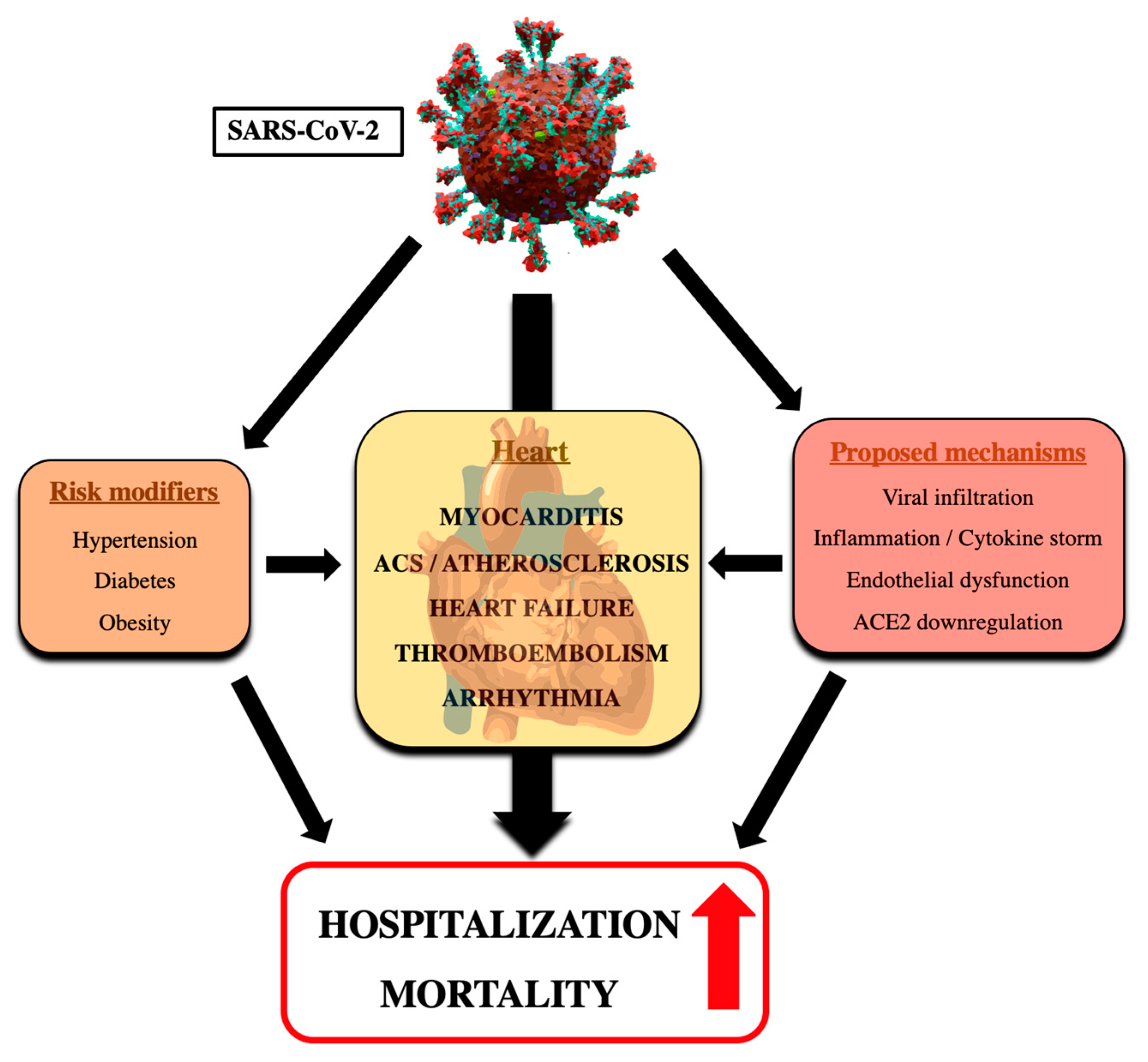
Impact of COVID-19 on Cardiovascular Disease
Abstract
1. Introduction
2. Pathogenesis of COVID-19
3. Acute COVID-19 as a Risk Modifier for CVD
3.1. Diabetes Mellitus
3.2. Hypertension
3.3. Obesity
4. Cardiovascular Sequelae of COVID-19 and Long-Term CVD Risk Modification
4.1. COVID-19-Related Myocarditis
4.2. Acute Coronary Syndrome
4.3. Heart Failure
4.4. Thromboembolic Complications
4.5. Arrhythmias
4.6. COVID-19 as CV Risk Factor
4.7. Long COVID and CVD
5. COVID-19 Vaccines
5.1. COVID-19 Vaccines and Myocarditis
5.1.1. Epidemiological Data
5.1.2. Clinical Course and Pathogenesis of Vaccine-Related Myocarditis
5.1.3. Myocarditis Risk in Young Individuals
5.2. Vaccine-Induced Thrombocytopenia and Thrombosis (VITT)
6. COVID-19 Treatment Strategies and Impact on CV Disease
6.1. Immunomodulatory Drugs
6.1.1. Corticosteroids
6.1.2. Interleukin-6 Inhibition
6.1.3. Janus Kinase Inhibitors
6.1.4. Interleukin-1 Inhibition
6.2. Antiviral Agents
6.2.1. Remdesivir
6.2.2. Nirmatrelvir/Ritonavir
6.2.3. Molnupiravir
| Substance | Mechanism | Impact on CV System/Disease in COVID-19 | |
|---|---|---|---|
| General CV Effects | CV Effects in COVID-19 Patients | ||
| Immunomodulatory drugs | |||
| Corticosteroids | Immunosuppression | Metabolic disease (diabetes, obesity, hyperglycemia) and hypertension [178] Risk for atrial flutter/fibrillation ↑ [179] | In-hospital mortality, decompensation, complications in heart failure patients with COVID-19 ↑ [180] Myocardial inflammation ↓ and recovery of LV function in patients with COVID-19 associated myocarditis ↑ [181] |
| Baricitinib | JAK1/2 and TYK2 inhibition | No increase in MACE, ATE, and CHF [203] | No increase in MACE [200,201,202] |
| Tofacitinib | Mainly JAK1/3 inhibition | Controversial data regarding MACE [205,206,207] CIMT in patients with pre-existing atherosclerosis ↓ [208] | Deep-vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis occurred in 1 patient each in the tofacitinib group; hemorrhagic stroke and cardiogenic shock occurred in 1 patient each in the placebo group [204] Ameliorated macrophage-induced myocardial injury in vitro [209] |
| Anakinra | IL-1R1 inhibition | Hospitalization rate and new onset heart failure after STEMI ↓ [237,238,239] PeakVO2 and exercise time in HFpEF and HFrEF ↑ [240,241,242] Risk of recurrence of pericarditis in therapy-refractory pericarditis patients ↓ [243] | Improvement in CMR markers, LV function, and inflammation in COVID-19 associated myocarditis (case report) [214] |
| Tocilizumab | IL-6 antagonism | hsCRP and troponin T release in NSTEMI patients ↓ [190] QT-prolongation (?) [193] | Cardiac function and clinical outcomes of COVID-19-associated cardiomyopathy ↑ [191,192] |
| Antiviral drugs | |||
| Remdesivir | Inhibitor of viral RNA polymerase | In vitro cardiac toxicity [227] | QT-prolongation/torsade de pointes tachycardia, bradycardia, hypotension, AV-block [223,224,225,226] |
| Ritonavir/Nirmatrelvir | Mpro-Inhibitor | Drug-to-drug interactions via a strong inhibition of CYP3A4 [229] | Drug-to-drug interactions via a strong inhibition of CYP3A4 [229] |
| Molnupiravir | Antivairal effect via the RNA-dependent RNA polymerase (RdRp) | No reports of cardiac side effects so far [231,233,234,235,236] | No reports of cardiac side effects so far [231,233,234,235,236] |
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Johns Hopkins University & Medicine Coronavirus Resource Center. COVID-19 Dashboard. Available online: https://coronavirus.jhu.edu/map.html (accessed on 29 January 2023).
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, Y.; Wang, F.; Chen, Y.; Kaminga, A.C.; Xu, H. Comorbidities’ potential impacts on severe and non-severe patients with COVID-19: A systematic review and meta-analysis. Medicine 2021, 100, e24971. [Google Scholar] [CrossRef] [PubMed]
- Mitrani, R.D.; Dabas, N.; Goldberger, J.J. COVID-19 cardiac injury: Implications for long-term surveillance and outcomes in survivors. Heart Rhythm 2020, 17, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K., 3rd; Harmon, K.G.; et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 79, 1717–1756. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e286. [Google Scholar] [CrossRef]
- Yang, H.; Rao, Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Nguyen, L.; McCord, K.A.; Bui, D.T.; Bouwman, K.M.; Kitova, E.N.; Elaish, M.; Kumawat, D.; Daskhan, G.C.; Tomris, I.; Han, L.; et al. Sialic acid-containing glycolipids mediate binding and viral entry of SARS-CoV-2. Nat. Chem. Biol. 2022, 18, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 11 August 2022).
- Maslo, C.; Friedland, R.; Toubkin, M.; Laubscher, A.; Akaloo, T.; Kama, B. Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared with Previous Waves. JAMA 2022, 327, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, D.; Visseaux, B.; Kassasseya, C.; Daoud, A.; Femy, F.; Hermand, C.; Truchot, J.; Beaune, S.; Javaud, N.; Peyrony, O.; et al. Comparison of Patients Infected with Delta Versus Omicron COVID-19 Variants Presenting to Paris Emergency Departments: A Retrospective Cohort Study. Ann. Intern. Med. 2022, 175, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Mauer, N.; Chiecca, G.; Carioli, G.; Gianfredi, V.; Iacoviello, L.; Bertagnolio, S.; Guerra, R.; Odone, A.; Signorelli, C. The First 110,593 COVID-19 Patients Hospitalised in Lombardy: A Regionwide Analysis of Case Characteristics, Risk Factors and Clinical Outcomes. Int. J. Public Health 2022, 67, 1604427. [Google Scholar] [CrossRef] [PubMed]
- Calvisi, S.L.; Ramirez, G.A.; Scavini, M.; Da Prat, V.; Di Lucca, G.; Laurenzi, A.; Gallina, G.; Cavallo, L.; Borio, G.; Farolfi, F.; et al. Thromboembolism risk among patients with diabetes/stress hyperglycemia and COVID-19. Metabolism 2021, 123, 154845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Zuliani, G.; Rigatelli, A.; Mazza, A.; Roncon, L. Arterial hypertension and risk of death in patients with COVID-19 infection: Systematic review and meta-analysis. J. Infect. 2020, 81, e84–e86. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.M.; Jessup, J.; Chappell, M.C.; Averill, D.B.; Brosnihan, K.B.; Tallant, E.A.; Diz, D.I.; Gallagher, P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005, 111, 2605–2610. [Google Scholar] [CrossRef]
- Pan, M.; Vasbinder, A.; Anderson, E.; Catalan, T.; Shadid, H.R.; Berlin, H.; Padalia, K.; O’Hayer, P.; Meloche, C.; Azam, T.U.; et al. Angiotensin-Converting Enzyme Inhibitors, Angiotensin II Receptor Blockers, and Outcomes in Patients Hospitalized for COVID-19. J. Am. Heart Assoc. 2021, 10, e023535. [Google Scholar] [CrossRef]
- Bauer, A.; Schreinlechner, M.; Sappler, N.; Dolejsi, T.; Tilg, H.; Aulinger, B.A.; Weiss, G.; Bellmann-Weiler, R.; Adolf, C.; Wolf, D.; et al. Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): A prospective, parallel group, randomised, controlled, open-label trial. Lancet Respir. Med. 2021, 9, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, S.; Jeong, H.W.; Ahn, J.Y.; Choi, S.J.; Lee, H.; Choi, B.; Nam, S.K.; Sa, M.; Kwon, J.S.; et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. 2020, 5, eabd1554. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Reidy, L.; Romero, M.; Diaz, A.; Cray, C.; Kahl, K.; Blomberg, B.B. The majority of SARS-CoV-2-specific antibodies in COVID-19 patients with obesity are autoimmune and not neutralizing. Int. J. Obes. 2022, 46, 427–432. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, N.R.; Jethani, N.; Chen, J.; Aphinyanaphongs, Y.; Zhang, R.; Dogra, S.; Alviar, C.L.; Keller, N.; Razzouk, L.; Quinones-Camacho, A.; et al. Myocardial Injury in Adults Hospitalized with COVID-19. Circulation 2020, 142, 2393–2395. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized with COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef]
- Ogungbe, O.; Kumbe, B.; Fadodun, O.A.; Latha, T.; Meyer, D.; Asala, A.F.; Davidson, P.M.; Dennison Himmelfarb, C.R.; Post, W.S.; Commodore-Mensah, Y. Subclinical myocardial injury, coagulopathy, and inflammation in COVID-19: A meta-analysis of 41,013 hospitalized patients. Int. J. Cardiol. Heart Vasc. 2022, 40, 100950. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Croft, L.B.; Stefanini, G.G.; Bragato, R.; Silbiger, J.J.; Vicenzi, M.; Danilov, T.; Kukar, N.; Shaban, N.; Kini, A.; et al. Characterization of Myocardial Injury in Patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2043–2055. [Google Scholar] [CrossRef]
- Farshidfar, F.; Koleini, N.; Ardehali, H. Cardiovascular complications of COVID-19. JCI Insight 2021, 6, e148980. [Google Scholar] [CrossRef]
- Mele, D.; Flamigni, F.; Rapezzi, C.; Ferrari, R. Myocarditis in COVID-19 patients: Current problems. Intern. Emerg. Med. 2021, 16, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Shiwani, H.A.; Elfaki, M.Y.; Hamid, M.; Pharithi, R.; Kamgang, R.; Egom, C.B.; Oyono, J.L.E.; Egom, E.E. COVID-19 and myocarditis: A review of literature. Egypt Heart J. 2022, 74, 23. [Google Scholar] [CrossRef]
- Doyen, D.; Moceri, P.; Ducreux, D.; Dellamonica, J. Myocarditis in a patient with COVID-19: A cause of raised troponin and ECG changes. Lancet 2020, 395, s0140–s6736. [Google Scholar] [CrossRef]
- Zeng, J.H.; Liu, Y.X.; Yuan, J.; Wang, F.X.; Wu, W.B.; Li, J.X.; Wang, L.F.; Gao, H.; Wang, Y.; Dong, C.F.; et al. First case of COVID-19 complicated with fulminant myocarditis: A case report and insights. Infection 2020, 48, 773–777. [Google Scholar] [CrossRef]
- Basso, C.; Leone, O.; Rizzo, S.; De Gaspari, M.; van der Wal, A.C.; Aubry, M.C.; Bois, M.C.; Lin, P.T.; Maleszewski, J.J.; Stone, J.R. Pathological features of COVID-19-associated myocardial injury: A multicentre cardiovascular pathology study. Eur. Heart J. 2020, 41, 3827–3835. [Google Scholar] [CrossRef]
- Irabien-Ortiz, A.; Carreras-Mora, J.; Sionis, A.; Pamies, J.; Montiel, J.; Tauron, M. Fulminant myocarditis due to COVID-19. Rev. Esp. Cardiol. 2020, 73, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Spano, G.; Fischer, K.; Maillat, C.; Vicario, G.; Huber, A.T.; Grani, C. Delayed isolated peri-myocarditis in a COVID-19 patient with respiratory symptoms but without lung involvement. Int. J. Cardiovasc. Imaging 2020, 36, 2279–2280. [Google Scholar] [CrossRef]
- Bajaj, R.; Sinclair, H.C.; Patel, K.; Low, B.; Pericao, A.; Manisty, C.; Guttmann, O.; Zemrak, F.; Miller, O.; Longhi, P.; et al. Delayed-onset myocarditis following COVID-19. Lancet Respir. Med. 2021, 9, e32–e34. [Google Scholar] [CrossRef]
- Ammirati, E.; Lupi, L.; Palazzini, M.; Hendren, N.S.; Grodin, J.L.; Cannistraci, C.V.; Schmidt, M.; Hekimian, G.; Peretto, G.; Bochaton, T.; et al. Prevalence, Characteristics, and Outcomes of COVID-19-Associated Acute Myocarditis. Circulation 2022, 145, 1123–1139. [Google Scholar] [CrossRef]
- Tuvali, O.; Tshori, S.; Derazne, E.; Hannuna, R.R.; Afek, A.; Haberman, D.; Sella, G.; George, J. The Incidence of Myocarditis and Pericarditis in Post COVID-19 Unvaccinated Patients-A Large Population-Based Study. J. Clin. Med. 2022, 11, 2219. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, K.; Abozenah, M.; Kadado, A.J.; Battisha, A.; Al-Akchar, M.; Salerno, C.; Hernandez-Montfort, J.; Islam, A.M. Systematic Review of COVID-19 Related Myocarditis: Insights on Management and Outcome. Cardiovasc. Revasc. Med. 2021, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Theetha Kariyanna, P.; Sutarjono, B.; Grewal, E.; Preet Singh, K.; Aurora, L.; Smith, L.; Priyan Chandrakumar, H.; Jayarangaiah, A.; Goldman, S.A.; Salifu, M.O.; et al. A Systematic Review of COVID-19 and Myocarditis. Am. J. Med. Case Rep. 2020, 8, 299–305. [Google Scholar] [CrossRef]
- Esposito, A.; Palmisano, A.; Natale, L.; Ligabue, G.; Peretto, G.; Lovato, L.; Vignale, D.; Fiocchi, F.; Marano, R.; Russo, V. Cardiac Magnetic Resonance Characterization of Myocarditis-Like Acute Cardiac Syndrome in COVID-19. JACC Cardiovasc. Imaging 2020, 13, 2462–2465. [Google Scholar] [CrossRef]
- Daniels, C.J.; Rajpal, S.; Greenshields, J.T.; Rosenthal, G.L.; Chung, E.H.; Terrin, M.; Jeudy, J.; Mattson, S.E.; Law, I.H.; Borchers, J.; et al. Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes with Recent SARS-CoV-2 Infection: Results From the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021, 6, 1078–1087. [Google Scholar] [CrossRef]
- Aengevaeren, V.L.; Baggish, A.L.; Chung, E.H.; George, K.; Kleiven, O.; Mingels, A.M.A.; Orn, S.; Shave, R.E.; Thompson, P.D.; Eijsvogels, T.M.H. Exercise-Induced Cardiac Troponin Elevations: From Underlying Mechanisms to Clinical Relevance. Circulation 2021, 144, 1955–1972. [Google Scholar] [CrossRef]
- Domenech-Ximenos, B.; Sanz-de la Garza, M.; Prat-Gonzalez, S.; Sepulveda-Martinez, A.; Crispi, F.; Duran-Fernandez, K.; Perea, R.J.; Bijnens, B.; Sitges, M. Prevalence and pattern of cardiovascular magnetic resonance late gadolinium enhancement in highly trained endurance athletes. J. Cardiovasc. Magn. Reson. 2020, 22, 62. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Brauninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.P.; et al. Association of Cardiac Infection with SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef]
- Escher, F.; Pietsch, H.; Aleshcheva, G.; Bock, T.; Baumeier, C.; Elsaesser, A.; Wenzel, P.; Hamm, C.; Westenfeld, R.; Schultheiss, M.; et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020, 7, 2440–2447. [Google Scholar] [CrossRef]
- Tavazzi, G.; Pellegrini, C.; Maurelli, M.; Belliato, M.; Sciutti, F.; Bottazzi, A.; Sepe, P.A.; Resasco, T.; Camporotondo, R.; Bruno, R.; et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020, 22, 911–915. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.E.; Li, G.; Akmatbekov, A.; Harbert, J.L.; Lameira, F.S.; Brown, J.Q.; Vander Heide, R.S. Unexpected Features of Cardiac Pathology in COVID-19 Infection. Circulation 2020, 142, 1123–1125. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef] [PubMed]
- Falasca, L.; Nardacci, R.; Colombo, D.; Lalle, E.; Di Caro, A.; Nicastri, E.; Antinori, A.; Petrosillo, N.; Marchioni, L.; Biava, G.; et al. Postmortem Findings in Italian Patients with COVID-19: A Descriptive Full Autopsy Study of Cases with and Without Comorbidities. J. Infect. Dis. 2020, 222, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Kawakami, R.; Sakamoto, A.; Kawai, K.; Gianatti, A.; Pellegrini, D.; Nasr, A.; Kutys, B.; Guo, L.; Cornelissen, A.; Mori, M.; et al. Pathological Evidence for SARS-CoV-2 as a Cause of Myocarditis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Talasaz, A.H.; Sadeghipour, P.; Kakavand, H.; Aghakouchakzadeh, M.; Kordzadeh-Kermani, E.; Van Tassell, B.W.; Gheymati, A.; Ariannejad, H.; Hosseini, S.H.; Jamalkhani, S.; et al. Recent Randomized Trials of Antithrombotic Therapy for Patients with COVID-19: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 1903–1921. [Google Scholar] [CrossRef] [PubMed]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T., Jr.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef]
- Bugger, H.; Gollmer, J.; Pregartner, G.; Wunsch, G.; Berghold, A.; Zirlik, A.; von Lewinski, D. Complications and mortality of cardiovascular emergency admissions during COVID-19 associated restrictive measures. PLoS ONE 2020, 15, e0239801. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lu, F.; Du, X.; Long, D.; Sang, C.; Tang, R.; Dong, J.; Guo, M.; Ma, C. Impact of COVID-19 Pandemic on Hospital Admissions of Acute Coronary Syndrome: A Beijing Inpatient Database Study. Lancet Reg. Health West. Pac. 2022, 19, 100335. [Google Scholar] [CrossRef]
- Metzler, B.; Siostrzonek, P.; Binder, R.K.; Bauer, A.; Reinstadler, S.J. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: The pandemic response causes cardiac collateral damage. Eur. Heart J. 2020, 41, 1852–1853. [Google Scholar] [CrossRef]
- Tam, C.F.; Cheung, K.S.; Lam, S.; Wong, A.; Yung, A.; Sze, M.; Lam, Y.M.; Chan, C.; Tsang, T.C.; Tsui, M.; et al. Impact of Coronavirus Disease 2019 (COVID-19) Outbreak on ST-Segment-Elevation Myocardial Infarction Care in Hong Kong, China. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006631. [Google Scholar] [CrossRef]
- Scholz, K.H.; Lengenfelder, B.; Thilo, C.; Jeron, A.; Stefanow, S.; Janssens, U.; Bauersachs, J.; Schulze, P.C.; Winter, K.D.; Schroder, J.; et al. Impact of COVID-19 outbreak on regional STEMI care in Germany. Clin. Res. Cardiol. 2020, 109, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Pastore, M.C.; Mandoli, G.E.; D’Ascenzi, F.; Focardi, M.; Biagioni, G.; Cameli, P.; Patti, G.; Franchi, F.; Mondillo, S.; et al. COVID-19 and Acute Coronary Syndromes: Current Data and Future Implications. Front. Cardiovasc. Med. 2020, 7, 593496. [Google Scholar] [CrossRef]
- Baldi, E.; Sechi, G.M.; Mare, C.; Canevari, F.; Brancaglione, A.; Primi, R.; Klersy, C.; Palo, A.; Contri, E.; Ronchi, V.; et al. Out-of-Hospital Cardiac Arrest during the COVID-19 Outbreak in Italy. N. Engl. J. Med. 2020, 383, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Aschieri, D.; Villani, M.; Lanati, M.; Didio, M.T.; Pellizzoni, V.; Villani, G.Q. Out-of-hospital cardiac arrest in COVID-19 era. A single center experience. Eur. Heart J. 2021, 42, ehab724.0658. [Google Scholar] [CrossRef]
- Pourasghari, H.; Tavolinejad, H.; Soleimanpour, S.; Abdi, Z.; Arabloo, J.; Bragazzi, N.L.; Behzadifar, M.; Rashedi, S.; Omidi, N.; Ayoubian, A.; et al. Hospitalization, major complications and mortality in acute myocardial infarction patients during the COVID-19 era: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2022, 41, 101058. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.; Fortuna, D.; Berti, E.; De Palma, R.; Pasquale, G.D.; Galvani, M.; Navazio, A.; Piovaccari, G.; Rubboli, A.; Guardigli, G.; et al. In- and out-of-hospital mortality for myocardial infarction during the first wave of the COVID-19 pandemic in Emilia-Romagna, Italy: A population-based observational study. Lancet Reg. Health Eur. 2021, 3, 100055. [Google Scholar] [CrossRef]
- Lazzerini, M.; Barbi, E.; Apicella, A.; Marchetti, F.; Cardinale, F.; Trobia, G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc. Health 2020, 4, e10–e11. [Google Scholar] [CrossRef]
- De Luca, G.; Verdoia, M.; Cercek, M.; Jensen, L.O.; Vavlukis, M.; Calmac, L.; Johnson, T.; Ferrer, G.R.; Ganyukov, V.; Wojakowski, W.; et al. Impact of COVID-19 Pandemic on Mechanical Reperfusion for Patients with STEMI. J. Am. Coll. Cardiol. 2020, 76, 2321–2330. [Google Scholar] [CrossRef]
- Einstein, A.J.; Shaw, L.J.; Hirschfeld, C.; Williams, M.C.; Villines, T.C.; Better, N.; Vitola, J.V.; Cerci, R.; Dorbala, S.; Raggi, P.; et al. International Impact of COVID-19 on the Diagnosis of Heart Disease. J. Am. Coll. Cardiol. 2021, 77, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Wu, J.; Timmis, A.; Curzen, N.; Clarke, S.; Zaman, A.; Nolan, J.; Shoaib, A.; Mohamed, M.O.; de Belder, M.A.; et al. Outcomes of COVID-19-positive acute coronary syndrome patients: A multisource electronic healthcare records study from England. J. Intern. Med. 2021, 290, 88–100. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Debel, N.; Cercek, M.; Jensen, L.O.; Vavlukis, M.; Calmac, L.; Johnson, T.; Ferrer, G.R.; Ganyukov, V.; Wojakowski, W.; et al. Impact of SARS-CoV-2 positivity on clinical outcome among STEMI patients undergoing mechanical reperfusion: Insights from the ISACS STEMI COVID 19 registry. Atherosclerosis 2021, 332, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Luscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef] [PubMed]
- Esposito, L.; Cancro, F.P.; Silverio, A.; Di Maio, M.; Iannece, P.; Damato, A.; Alfano, C.; De Luca, G.; Vecchione, C.; Galasso, G. COVID-19 and Acute Coronary Syndromes: From Pathophysiology to Clinical Perspectives. Oxid. Med. Cell. Longev. 2021, 2021, 4936571. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.C.; Rainger, G.E.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Bromage, D.I.; Cannata, A.; Rind, I.A.; Gregorio, C.; Piper, S.; Shah, A.M.; McDonagh, T.A. The impact of COVID-19 on heart failure hospitalization and management: Report from a Heart Failure Unit in London during the peak of the pandemic. Eur. J. Heart Fail. 2020, 22, 978–984. [Google Scholar] [CrossRef]
- Cannata, A.; Bromage, D.I.; Rind, I.A.; Gregorio, C.; Bannister, C.; Albarjas, M.; Piper, S.; Shah, A.M.; McDonagh, T.A. Temporal trends in decompensated heart failure and outcomes during COVID-19: A multisite report from heart failure referral centres in London. Eur. J. Heart Fail. 2020, 22, 2219–2224. [Google Scholar] [CrossRef]
- Konig, S.; Hohenstein, S.; Meier-Hellmann, A.; Kuhlen, R.; Hindricks, G.; Bollmann, A.; Helios Hospitals, G. In-hospital care in acute heart failure during the COVID-19 pandemic: Insights from the German-wide Helios hospital network. Eur. J. Heart Fail. 2020, 22, 2190–2201. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell, C.-R.C.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Inciardi, R.M.; Adamo, M.; Lupi, L.; Cani, D.S.; Di Pasquale, M.; Tomasoni, D.; Italia, L.; Zaccone, G.; Tedino, C.; Fabbricatore, D.; et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020, 41, 1821–1829. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Zuliani, G.; Roncon, L. Heart failure as a complication of COVID-19 infection: Systematic review and meta-analysis. Acta Cardiol. 2022, 77, 107–113. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Garcia, J.; Lee, S.; Gupta, A.; Cagliostro, M.; Joshi, A.A.; Rivas-Lasarte, M.; Contreras, J.; Mitter, S.S.; LaRocca, G.; Tlachi, P.; et al. Prognostic Impact of Prior Heart Failure in Patients Hospitalized with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2334–2348. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, D.; Inciardi, R.M.; Lombardi, C.M.; Tedino, C.; Agostoni, P.; Ameri, P.; Barbieri, L.; Bellasi, A.; Camporotondo, R.; Canale, C.; et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID-19. Results of the Cardio-COVID-Italy multicentre study. Eur. J. Heart Fail. 2020, 22, 2238–2247. [Google Scholar] [CrossRef]
- Italia, L.; Tomasoni, D.; Bisegna, S.; Pancaldi, E.; Stretti, L.; Adamo, M.; Metra, M. COVID-19 and Heart Failure: From Epidemiology During the Pandemic to Myocardial Injury, Myocarditis, and Heart Failure Sequelae. Front. Cardiovasc. Med. 2021, 8, 713560. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.; Zhang, D.; Jiang, C.; Mei, H.; Wang, J.; Zhang, C.; Li, H.; Xia, X.; Kong, S.; et al. Deep Vein Thrombosis in Hospitalized Patients with COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation 2020, 142, 114–128. [Google Scholar] [CrossRef]
- Middeldorp, S.; Coppens, M.; van Haaps, T.F.; Foppen, M.; Vlaar, A.P.; Muller, M.C.A.; Bouman, C.C.S.; Beenen, L.F.M.; Kootte, R.S.; Heijmans, J.; et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1995–2002. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef]
- Malas, M.B.; Naazie, I.N.; Elsayed, N.; Mathlouthi, A.; Marmor, R.; Clary, B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine 2020, 29, 100639. [Google Scholar] [CrossRef]
- Xiao, D.; Tang, F.; Chen, L.; Gao, H.; Li, X. Cumulative Evidence for the Association of Thrombosis and the Prognosis of COVID-19: Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 819318. [Google Scholar] [CrossRef]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.E.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.S.; Kunichoff, D.; Adhikari, S.; Ahuja, T.; Amoroso, N.; Aphinyanaphongs, Y.; Cao, M.; Goldenberg, R.; Hindenburg, A.; Horowitz, J.; et al. Prevalence and Outcomes of D-Dimer Elevation in Hospitalized Patients with COVID-19. Arter. Thromb. Vasc. Biol. 2020, 40, 2539–2547. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C.; et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef]
- Dettlaff-Pokora, A.; Swierczynski, J. Dysregulation of the Renin-Angiotensin-Aldosterone System (RAA) in Patients Infected with SARS-CoV-2-Possible Clinical Consequences. Int. J. Mol. Sci. 2021, 22, 4503. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Cho, J.H.; Namazi, A.; Shelton, R.; Ramireddy, A.; Ehdaie, A.; Shehata, M.; Wang, X.; Marban, E.; Chugh, S.S.; Cingolani, E. Cardiac arrhythmias in hospitalized patients with COVID-19: A prospective observational study in the western United States. PLoS ONE 2020, 15, e0244533. [Google Scholar] [CrossRef] [PubMed]
- Mountantonakis, S.E.; Saleh, M.; Fishbein, J.; Gandomi, A.; Lesser, M.; Chelico, J.; Gabriels, J.; Qiu, M.; Epstein, L.M.; Northwell, C.-R.C. Atrial fibrillation is an independent predictor for in-hospital mortality in patients admitted with SARS-CoV-2 infection. Heart Rhythm 2021, 18, 501–507. [Google Scholar] [CrossRef]
- Peltzer, B.; Manocha, K.K.; Ying, X.; Kirzner, J.; Ip, J.E.; Thomas, G.; Liu, C.F.; Markowitz, S.M.; Lerman, B.B.; Safford, M.M.; et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J. Cardiovasc. Electrophysiol. 2020, 31, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Ergun, B.; Ergan, B.; Sozmen, M.K.; Kucuk, M.; Yakar, M.N.; Comert, B.; Gokmen, A.N.; Yaka, E. New-onset atrial fibrillation in critically ill patients with coronavirus disease 2019 (COVID-19). J. Arrhythm. 2021, 37, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Musikantow, D.R.; Turagam, M.K.; Sartori, S.; Chu, E.; Kawamura, I.; Shivamurthy, P.; Bokhari, M.; Oates, C.; Zhang, C.; Pumill, C.; et al. Atrial Fibrillation in Patients Hospitalized with COVID-19: Incidence, Predictors, Outcomes, and Comparison to Influenza. JACC Clin. Electrophysiol. 2021, 7, 1120–1130. [Google Scholar] [CrossRef]
- Zareini, B.; Rajan, D.; El-Sheikh, M.; Jensen, M.H.; Hojbjerg Lassen, M.C.; Skaarup, K.; Hansen, M.L.; Biering-Sorensen, T.; Jabbari, R.; Kirk, O.; et al. Cardiac arrhythmias in patients hospitalized with COVID-19: The ACOVID study. Heart Rhythm O2 2021, 2, 304–308. [Google Scholar] [CrossRef]
- Rosenblatt, A.G.; Ayers, C.R.; Rao, A.; Howell, S.J.; Hendren, N.S.; Zadikany, R.H.; Ebinger, J.E.; Daniels, J.D.; Link, M.S.; de Lemos, J.A.; et al. New-Onset Atrial Fibrillation in Patients Hospitalized with COVID-19: Results From the American Heart Association COVID-19 Cardiovascular Registry. Circ. Arrhythm. Electrophysiol. 2022, 15, e010666. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Zanon, F.; Zuliani, G.; Roncon, L. Pre-existing atrial fibrillation is associated with increased mortality in COVID-19 Patients. J. Interv. Card. Electrophysiol. 2021, 62, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, A.; Mayer, M.M.; Adusumalli, S.; Hyman, M.C.; Oh, E.; Tierney, A.; Moss, J.; Chahal, A.A.; Anesi, G.; Denduluri, S.; et al. COVID-19 and cardiac arrhythmias. Heart Rhythm 2020, 17, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, E.; Mollazadeh, R.; Mansouri, P.; Keykhaei, M.; Mirshafiee, S.; Hedayat, B.; Salarifar, M.; Yuyun, M.F.; Yarmohammadi, H. Ventricular repolarization heterogeneity in patients with COVID-19: Original data, systematic review, and meta-analysis. Clin. Cardiol. 2022, 45, 110–118. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Mathur, S.; Zhang, R.; Yan, V.K.C.; Lai, F.T.T.; Chui, C.S.L.; Li, X.; Wong, C.K.H.; Chan, E.W.Y.; Yiu, K.H.; et al. Association of COVID-19 with short- and long-term risk of cardiovascular disease and mortality: A prospective cohort in UK Biobank. Cardiovasc. Res. 2023, cvac195. [Google Scholar] [CrossRef]
- Xie, Y.; Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2022, 10, 311–321. [Google Scholar] [CrossRef]
- Liu, F.; Long, X.; Zhang, B.; Zhang, W.; Chen, X.; Zhang, Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2128–2130.e2122. [Google Scholar] [CrossRef]
- Inamdar, S.; Benias, P.C.; Liu, Y.; Sejpal, D.V.; Satapathy, S.K.; Trindade, A.J.; Northwell, C.-R.C. Prevalence, Risk Factors, and Outcomes of Hospitalized Patients with Coronavirus Disease 2019 Presenting as Acute Pancreatitis. Gastroenterology 2020, 159, 2226–2228.e2222. [Google Scholar] [CrossRef]
- Laffin, L.J.; Kaufman, H.W.; Chen, Z.; Niles, J.K.; Arellano, A.R.; Bare, L.A.; Hazen, S.L. Rise in Blood Pressure Observed Among US Adults During the COVID-19 Pandemic. Circulation 2022, 145, 235–237. [Google Scholar] [CrossRef]
- Shah, N.P.; Clare, R.M.; Chiswell, K.; Navar, A.M.; Shah, B.R.; Peterson, E.D. Trends of blood pressure control in the U.S. during the COVID-19 pandemic. Am. Heart J. 2022, 247, 15–23. [Google Scholar] [CrossRef]
- Feitosa, F.; Feitosa, A.D.M.; Paiva, A.M.G.; Mota-Gomes, M.A.; Barroso, W.S.; Miranda, R.D.; Barbosa, E.C.D.; Brandao, A.A.; Lima-Filho, J.L.; Sposito, A.C.; et al. Impact of the COVID-19 pandemic on blood pressure control: A nationwide home blood pressure monitoring study. Hypertens. Res. 2022, 45, 364–368. [Google Scholar] [CrossRef]
- Gotanda, H.; Liyanage-Don, N.; Moran, A.E.; Krousel-Wood, M.; Green, J.B.; Zhang, Y.; Nuckols, T.K. Changes in Blood Pressure Outcomes Among Hypertensive Individuals During the COVID-19 Pandemic: A Time Series Analysis in Three US Healthcare Organizations. Hypertension 2022, 79, 2733–2742. [Google Scholar] [CrossRef]
- Akpek, M. Does COVID-19 Cause Hypertension? Angiology 2022, 73, 682–687. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Clinical Guidelines. In COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; National Institute for Health and Care Excellence (NICE): London, UK, 2020. [Google Scholar]
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus. 6 October 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 25 January 2023).
- Raman, B.; Bluemke, D.A.; Luscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, B.; Mohn, K.G.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Saevik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Gyongyosi, M.; Alcaide, P.; Asselbergs, F.W.; Brundel, B.; Camici, G.G.; da Costa Martins, P.; Ferdinandy, P.; Fontana, M.; Girao, H.; Gnecchi, M.; et al. Long COVID and the cardiovascular system—elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: A joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial & Pericardial Diseases. Cardiovasc. Res. 2022, cvac115. [Google Scholar] [CrossRef]
- Hastie, C.E.; Lowe, D.J.; McAuley, A.; Winter, A.J.; Mills, N.L.; Black, C.; Scott, J.T.; O’Donnell, C.A.; Blane, D.N.; Browne, S.; et al. Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat. Commun. 2022, 13, 5663. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.M.; Brunjes, D.L.; Lala, A.; Trivieri, M.G.; Contreras, J.P.; Natelson, B.H. Use of Cardiopulmonary Stress Testing for Patients with Unexplained Dyspnea Post-Coronavirus Disease. JACC Heart Fail. 2021, 9, 927–937. [Google Scholar] [CrossRef]
- Long, B.; Brady, W.J.; Bridwell, R.E.; Ramzy, M.; Montrief, T.; Singh, M.; Gottlieb, M. Electrocardiographic manifestations of COVID-19. Am. J. Emerg. Med. 2021, 41, 96–103. [Google Scholar] [CrossRef]
- Radin, J.M.; Quer, G.; Ramos, E.; Baca-Motes, K.; Gadaleta, M.; Topol, E.J.; Steinhubl, S.R. Assessment of Prolonged Physiological and Behavioral Changes Associated with COVID-19 Infection. JAMA Netw. Open 2021, 4, e2115959. [Google Scholar] [CrossRef]
- Lassen, M.C.H.; Skaarup, K.G.; Lind, J.N.; Alhakak, A.S.; Sengelov, M.; Nielsen, A.B.; Simonsen, J.O.; Johansen, N.D.; Davidovski, F.S.; Christensen, J.; et al. Recovery of cardiac function following COVID-19—ECHOVID-19: A prospective longitudinal cohort study. Eur. J. Heart Fail. 2021, 23, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Moody, W.E.; Liu, B.; Mahmoud-Elsayed, H.M.; Senior, J.; Lalla, S.S.; Khan-Kheil, A.M.; Brown, S.; Saif, A.; Moss, A.; Bradlow, W.M.; et al. Persisting Adverse Ventricular Remodeling in COVID-19 Survivors: A Longitudinal Echocardiographic Study. J. Am. Soc. Echocardiogr. 2021, 34, 562–566. [Google Scholar] [CrossRef]
- Ramadan, M.S.; Bertolino, L.; Zampino, R.; Durante-Mangoni, E.; Monaldi Hospital Cardiovascular Infection Study Group. Cardiac sequelae after coronavirus disease 2019 recovery: A systematic review. Clin. Microbiol. Infect. 2021, 27, 1250–1261. [Google Scholar] [CrossRef]
- Szekely, Y.; Lichter, Y.; Sadon, S.; Lupu, L.; Taieb, P.; Banai, A.; Sapir, O.; Granot, Y.; Hochstadt, A.; Friedman, S.; et al. Cardiorespiratory Abnormalities in Patients Recovering from Coronavirus Disease 2019. J. Am. Soc. Echocardiogr. 2021, 34, 1273–1284.e1279. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Katogiannis, K.; Thymis, J.; Korakas, E.; Pavlidis, G.; Kazakou, P.; Panagopoulos, G.; et al. Myocardial work and vascular dysfunction are partially improved at 12 months after COVID-19 infection. Eur. J. Heart Fail. 2022, 24, 727–729. [Google Scholar] [CrossRef]
- Knight, D.S.; Kotecha, T.; Razvi, Y.; Chacko, L.; Brown, J.T.; Jeetley, P.S.; Goldring, J.; Jacobs, M.; Lamb, L.E.; Negus, R.; et al. COVID-19: Myocardial Injury in Survivors. Circulation 2020, 142, 1120–1122. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Satterfield, B.A.; Bhatt, D.L.; Gersh, B.J. Cardiac involvement in the long-term implications of COVID-19. Nat. Rev. Cardiol. 2022, 19, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Yamada, A.; Fujito, H.; Hashimoto, N.; Nagao, T.; Tanaka, Y.; Fukumoto, K.; Arai, R.; Wakamatsu, Y.; Ebuchi, Y.; et al. Cardiovascular manifestations identified by multi-modality imaging in patients with long COVID. Front. Cardiovasc. Med. 2022, 9, 968584. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.O.; Banerjee, A. Long COVID and cardiovascular disease: A learning health system approach. Nat. Rev. Cardiol. 2022, 19, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Porcari, A.; Merlo, M.; Roncon, L.; Sinagra, G. One-Year Risk of myocarditis after COVID-19 infection: A systematic review and meta-analysis. Can. J. Cardiol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Joy, G.; Artico, J.; Kurdi, H.; Seraphim, A.; Lau, C.; Thornton, G.D.; Oliveira, M.F.; Adam, R.D.; Aziminia, N.; Menacho, K.; et al. Prospective Case-Control Study of Cardiovascular Abnormalities 6 Months Following Mild COVID-19 in Healthcare Workers. JACC Cardiovasc. Imaging 2021, 14, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, D.; Isaak, A.; Zimmer, S.; Mesropyan, N.; Reinert, M.; Faron, A.; Pieper, C.C.; Heine, A.; Velten, M.; Nattermann, J.; et al. Cardiac MRI in Patients with Prolonged Cardiorespiratory Symptoms after Mild to Moderate COVID-19. Radiology 2021, 301, E419–E425. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cardenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Murillo-Zamora, E.; Mendoza-Cano, O.; Delgado-Enciso, I.; Hernandez-Suarez, C.M. Predictors of severe symptomatic laboratory-confirmed SARS-CoV-2 reinfection. Public Health 2021, 193, 113–115. [Google Scholar] [CrossRef]
- Kim, Y.E.; Huh, K.; Park, Y.J.; Peck, K.R.; Jung, J. Association Between Vaccination and Acute Myocardial Infarction and Ischemic Stroke After COVID-19 Infection. JAMA 2022, 328, 887–889. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2022, 28, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Hu, M.; Zhou, C.K.; Lloyd, P.C.; Amend, K.L.; Beachler, D.C.; Secora, A.; McMahill-Walraven, C.N.; Lu, Y.; Wu, Y.; et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: A cohort study in claims databases. Lancet 2022, 399, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Barda, N.; Hoss, S.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Auster, O.; Dagan, N.; Balicer, R.D.; et al. Myocarditis after COVID-19 Vaccination in a Large Health Care Organization. N. Engl. J. Med. 2021, 385, 2132–2139. [Google Scholar] [CrossRef]
- Matta, A.; Kunadharaju, R.; Osman, M.; Jesme, C.; McMiller, Z.; Johnson, E.M.; Matta, D.; Kallamadi, R.; Bande, D. Clinical Presentation and Outcomes of Myocarditis Post mRNA Vaccination: A Meta-Analysis and Systematic Review. Cureus 2021, 13, e19240. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef]
- Heymans, S.; Cooper, L.T. Myocarditis after COVID-19 mRNA vaccination: Clinical observations and potential mechanisms. Nat. Rev. Cardiol. 2022, 19, 75–77. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Zhao, Y.; Lung, D.C.; Ye, Z.; Song, W.; Liu, F.F.; Cai, J.P.; Wong, W.M.; Yip, C.C.; et al. Intravenous Injection of Coronavirus Disease 2019 (COVID-19) mRNA Vaccine Can Induce Acute Myopericarditis in Mouse Model. Clin. Infect. Dis. 2022, 74, 1933–1950. [Google Scholar] [CrossRef]
- Le Vu, S.; Bertrand, M.; Jabagi, M.J.; Botton, J.; Drouin, J.; Baricault, B.; Weill, A.; Dray-Spira, R.; Zureik, M. Age and sex-specific risks of myocarditis and pericarditis following COVID-19 messenger RNA vaccines. Nat. Commun. 2022, 13, 3633. [Google Scholar] [CrossRef]
- Boehmer, T.K.; Kompaniyets, L.; Lavery, A.M.; Hsu, J.; Ko, J.Y.; Yusuf, H.; Romano, S.D.; Gundlapalli, A.V.; Oster, M.E.; Harris, A.M. Association Between COVID-19 and Myocarditis Using Hospital-Based Administrative Data—United States, March 2020-January 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- COVID-NET: COVID-19-Associated Hospitalization Surveillance Network, C.f.D.C.a.P. Available online: https://gis.cdc.gov/grasp/covidnet/covid19_3.html (accessed on 3 August 2022).
- Franco-Paredes, C. Transmissibility of SARS-CoV-2 among fully vaccinated individuals. Lancet Infect. Dis. 2022, 22, s1473–s3099. [Google Scholar] [CrossRef]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022, 22, 183–195. [Google Scholar] [CrossRef]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022, 327, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sorvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattor, T.H.; Tjonnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef]
- Huynh, A.; Kelton, J.G.; Arnold, D.M.; Daka, M.; Nazy, I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature 2021, 596, 565–569. [Google Scholar] [CrossRef]
- See, I.; Lale, A.; Marquez, P.; Streiff, M.B.; Wheeler, A.P.; Tepper, N.K.; Woo, E.J.; Broder, K.R.; Edwards, K.M.; Gallego, R.; et al. Case Series of Thrombosis with Thrombocytopenia Syndrome After COVID-19 Vaccination-United States, December 2020 to August 2021. Ann. Intern. Med. 2022, 175, 513–522. [Google Scholar] [CrossRef]
- Elberry, M.H.; Abdelgawad, H.A.H.; Hamdallah, A.; Abdella, W.S.; Ahmed, A.S.; Ghaith, H.S.; Negida, A. A systematic review of vaccine-induced thrombotic thrombocytopenia in individuals who received COVID-19 adenoviral-vector-based vaccines. J. Thromb. Thrombolysis 2022, 53, 798–823. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- American Society of Hematology. Vaccine-Induced Immune Thrombotic Thrombocytopenia; American Society of Hematology: Washington, DC, USA, 2022. [Google Scholar]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Ng, M.K.; Celermajer, D.S. Glucocorticoid treatment and cardiovascular disease. Heart 2004, 90, 829–830. [Google Scholar] [CrossRef]
- Christiansen, C.F.; Christensen, S.; Mehnert, F.; Cummings, S.R.; Chapurlat, R.D.; Sorensen, H.T. Glucocorticoid use and risk of atrial fibrillation or flutter: A population-based, case-control study. Arch. Intern. Med. 2009, 169, 1677–1683. [Google Scholar] [CrossRef]
- Perez-Belmonte, L.M.; Sanz-Canovas, J.; Salinas, A.; Fornie, I.S.; Mendez-Bailon, M.; Gomez-Huelgas, R.; SEMI-COVID-19 Network. Corticosteroid therapy in patients with heart failure hospitalized for COVID-19: A multicenter retrospective study. Intern. Emerg. Med. 2021, 16, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, V.; Nesukay, E.; Cherniuk, S.; Kirichenko, R.; Kozliuk, A.; Titova, N.; Titov, E.; Giresh, J. Glucocorticoids in myocarditis therapy after COVID-19. Eur. Heart J. 2021, 42, ehab724.1752. [Google Scholar] [CrossRef]
- Ferrara, F.; Vitiello, A. Efficacy of synthetic glucocorticoids in COVID-19 endothelites. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, E.; Rezig, A.O.M.; Bruzzese, D.; Ialenti, A.; Cicala, C.; Cleland, J.G.F.; Guzik, T.J.; Maffia, P.; Pellicori, P. Systemic administration of glucocorticoids, cardiovascular complications and mortality in patients hospitalised with COVID-19, SARS, MERS or influenza: A systematic review and meta-analysis of randomised trials. Pharm. Res. 2022, 176, 106053. [Google Scholar] [CrossRef]
- REMAP-CAP Investigators; Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; van Bentum-Puijk, W.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Rosas, I.O.; Brau, N.; Waters, M.; Go, R.C.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Aziz, M.S.; Cooper, N.; Douglas, I.S.; et al. Tocilizumab in Hospitalized Patients with Severe COVID-19 Pneumonia. N. Engl. J. Med. 2021, 384, 1503–1516. [Google Scholar] [CrossRef]
- Salvarani, C.; Dolci, G.; Massari, M.; Merlo, D.F.; Cavuto, S.; Savoldi, L.; Bruzzi, P.; Boni, F.; Braglia, L.; Turra, C.; et al. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized with COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 24–31. [Google Scholar] [CrossRef]
- Ghosn, L.; Chaimani, A.; Evrenoglou, T.; Davidson, M.; Grana, C.; Schmucker, C.; Bollig, C.; Henschke, N.; Sguassero, Y.; Nejstgaard, C.H.; et al. Interleukin-6 blocking agents for treating COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021, 3, CD013881. [Google Scholar] [CrossRef] [PubMed]
- Castagne, B.; Viprey, M.; Martin, J.; Schott, A.M.; Cucherat, M.; Soubrier, M. Cardiovascular safety of tocilizumab: A systematic review and network meta-analysis. PLoS ONE 2019, 14, e0220178. [Google Scholar] [CrossRef] [PubMed]
- Kleveland, O.; Kunszt, G.; Bratlie, M.; Ueland, T.; Broch, K.; Holte, E.; Michelsen, A.E.; Bendz, B.; Amundsen, B.H.; Espevik, T.; et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: A double-blind, randomized, placebo-controlled phase 2 trial. Eur. Heart J. 2016, 37, 2406–2413. [Google Scholar] [CrossRef]
- Chitturi, K.R.; Thacker, S.; Al-Saadi, M.A.; Kassi, M. Successful treatment of acute heart failure in COVID-19-induced cytokine storm with tocilizumab: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Schreiber, A.; Elango, K.; Soussu, C.; Fakhra, S.; Asad, S.; Ahsan, C. COVID-19 Induced Cardiomyopathy Successfully Treated with Tocilizumab. Case Rep. Cardiol. 2022, 2022, 9943937. [Google Scholar] [CrossRef]
- Alattar, R.; Ibrahim, T.B.H.; Shaar, S.H.; Abdalla, S.; Shukri, K.; Daghfal, J.N.; Khatib, M.Y.; Aboukamar, M.; Abukhattab, M.; Alsoub, H.A.; et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J. Med. Virol. 2020, 92, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/ (accessed on 25 January 2023).
- McLornan, D.P.; Pope, J.E.; Gotlib, J.; Harrison, C.N. Current and future status of JAK inhibitors. Lancet 2021, 398, 803–816. [Google Scholar] [CrossRef]
- Solimani, F.; Meier, K.; Ghoreschi, K. Janus kinase signaling as risk factor and therapeutic target for severe SARS-CoV-2 infection. Eur. J. Immunol. 2021, 51, 1071–1075. [Google Scholar] [CrossRef]
- Baranova, A.; Cao, H.; Zhang, F. Unraveling Risk Genes of COVID-19 by Multi-Omics Integrative Analyses. Front. Med. 2021, 8, 738687. [Google Scholar] [CrossRef] [PubMed]
- Dieter, C.; de Almeida Brondani, L.; Lemos, N.E.; Schaeffer, A.F.; Zanotto, C.; Ramos, D.T.; Girardi, E.; Pellenz, F.M.; Camargo, J.L.; Moresco, K.S.; et al. Polymorphisms in ACE1, TMPRSS2, IFIH1, IFNAR2, and TYK2 Genes Are Associated with Worse Clinical Outcomes in COVID-19. Genes 2022, 14, 29. [Google Scholar] [CrossRef]
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D.; et al. Genetic mechanisms of critical illness in COVID-19. Nature 2021, 591, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet 2022, 400, 359–368. [Google Scholar] [CrossRef]
- Ely, E.W.; Ramanan, A.V.; Kartman, C.E.; de Bono, S.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Saraiva, J.F.K.; Chakladar, S.; Marconi, V.C.; et al. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: An exploratory, randomised, placebo-controlled trial. Lancet Respir. Med. 2022, 10, 327–336. [Google Scholar] [CrossRef]
- Taylor, P.C.; Weinblatt, M.E.; Burmester, G.R.; Rooney, T.P.; Witt, S.; Walls, C.D.; Issa, M.; Salinas, C.A.; Saifan, C.; Zhang, X.; et al. Cardiovascular Safety During Treatment with Baricitinib in Rheumatoid Arthritis. Arthritis Rheumatol. 2019, 71, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, P.O.; Quirk, D.; Furtado, R.H.; Maia, L.N.; Saraiva, J.F.; Antunes, M.O.; Kalil Filho, R.; Junior, V.M.; Soeiro, A.M.; Tognon, A.P.; et al. Tofacitinib in Patients Hospitalized with COVID-19 Pneumonia. N. Engl. J. Med. 2021, 385, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; DeMasi, R.; Valdez, H.; Soma, K.; Hwang, L.J.; Boy, M.G.; Biswas, P.; McInnes, I.B. Risk Factors for Major Adverse Cardiovascular Events in Phase III and Long-Term Extension Studies of Tofacitinib in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2019, 71, 1450–1459. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Wicker, P.; Gonzalez-Gay, M.A.; Boy, M.; Zuckerman, A.; Soma, K.; Geier, J.; Kwok, K.; Riese, R. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin. Arthritis Rheum. 2016, 46, 261–271. [Google Scholar] [CrossRef]
- Kume, K.; Amano, K.; Yamada, S.; Kanazawa, T.; Ohta, H.; Hatta, K.; Amano, K.; Kuwaba, N. Tofacitinib improves atherosclerosis despite up-regulating serum cholesterol in patients with active rheumatoid arthritis: A cohort study. Rheumatol. Int. 2017, 37, 2079–2085. [Google Scholar] [CrossRef]
- Yang, L.; Han, Y.; Jaffre, F.; Nilsson-Payant, B.E.; Bram, Y.; Wang, P.; Zhu, J.; Zhang, T.; Redmond, D.; Houghton, S.; et al. An Immuno-Cardiac Model for Macrophage-Mediated Inflammation in COVID-19 Hearts. Circ. Res. 2021, 129, 33–46. [Google Scholar] [CrossRef]
- Cavalli, G.; De Luca, G.; Campochiaro, C.; Della-Torre, E.; Ripa, M.; Canetti, D.; Oltolini, C.; Castiglioni, B.; Tassan Din, C.; Boffini, N.; et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e325–e331. [Google Scholar] [CrossRef]
- Huet, T.; Beaussier, H.; Voisin, O.; Jouveshomme, S.; Dauriat, G.; Lazareth, I.; Sacco, E.; Naccache, J.-M.; Bézie, Y.; Laplanche, S.; et al. Anakinra for severe forms of COVID-19: A cohort study. Lancet Rheumatol. 2020, 2, e393–e400. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Poulakou, G.; Milionis, H.; Metallidis, S.; Adamis, G.; Tsiakos, K.; Fragkou, A.; Rapti, A.; Damoulari, C.; Fantoni, M.; et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021, 27, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- The CORIMUNO-19 Collaborative Group; Tharaux, P.-L.; Pialoux, G.; Pavot, A.; Mariette, X.; Hermine, O.; Resche-Rigon, M.; Porcher, R.; Ravaud, P.; Bureau, S.; et al. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): A randomised controlled trial. Lancet Respir. Med. 2021, 9, 295–304. [Google Scholar] [CrossRef]
- Trpkov, C.; MacMullan, P.; Feuchter, P.; Kachra, R.; Heydari, B.; Merchant, N.; Bristow, M.S.; White, J.A. Rapid Response to Cytokine Storm Inhibition Using Anakinra in a Patient with COVID-19 Myocarditis. CJC Open 2021, 3, 210–213. [Google Scholar] [CrossRef]
- Caricchio, R.; Abbate, A.; Gordeev, I.; Meng, J.; Hsue, P.Y.; Neogi, T.; Arduino, R.; Fomina, D.; Bogdanov, R.; Stepanenko, T.; et al. Effect of Canakinumab vs Placebo on Survival Without Invasive Mechanical Ventilation in Patients Hospitalized with Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 326, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Dinarello, C.A. Anakinra Therapy for Non-cancer Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1157. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Lye, D.C.B.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 Days in Patients with Severe COVID-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas Lopez, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Rafaniello, C.; Ferrajolo, C.; Sullo, M.G.; Gaio, M.; Zinzi, A.; Scavone, C.; Gargano, F.; Coscioni, E.; Rossi, F.; Capuano, A. Cardiac Events Potentially Associated to Remdesivir: An Analysis from the European Spontaneous Adverse Event Reporting System. Pharmaceuticals 2021, 14, 611. [Google Scholar] [CrossRef] [PubMed]
- Nabati, M.; Parsaee, H. Potential Cardiotoxic Effects of Remdesivir on Cardiovascular System: A Literature Review. Cardiovasc. Toxicol. 2022, 22, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Parker, B.M.; Priyadarshi, V.; Parker, J. Cardiac Adverse Events with Remdesivir in COVID-19 Infection. Cureus 2020, 12, e11132. [Google Scholar] [CrossRef]
- Brunetti, N.D.; Poliseno, M.; Bottalico, I.F.; Centola, A.; Montemurro, L.; Sica, S.; Santantonio, T.; Lo Caputo, S. Safety and heart rate changes in COVID-19 patients treated with Remdesivir. Int. J. Infect. Dis. 2021, 112, 254–257. [Google Scholar] [CrossRef]
- Choi, S.W.; Shin, J.S.; Park, S.J.; Jung, E.; Park, Y.G.; Lee, J.; Kim, S.J.; Park, H.J.; Lee, J.H.; Park, S.M.; et al. Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antivir. Res. 2020, 184, 104955. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simon-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Marzolini, C.; Kuritzkes, D.R.; Marra, F.; Boyle, A.; Gibbons, S.; Flexner, C.; Pozniak, A.; Boffito, M.; Waters, L.; Burger, D.; et al. Recommendations for the Management of Drug-Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Paxlovid) and Comedications. Clin. Pharm. Ther. 2022, 112, 1191–1200. [Google Scholar] [CrossRef]
- Nappi, F.; Iervolino, A.; Avtaar Singh, S.S. Molecular Insights of SARS-CoV-2 Antivirals Administration: A Balance between Safety Profiles and Impact on Cardiovascular Phenotypes. Biomedicines 2022, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martin-Quiros, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.H.; Au, I.C.H.; Lau, K.T.K.; Lau, E.H.Y.; Cowling, B.J.; Leung, G.M. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: A retrospective cohort study. Lancet Infect. Dis. 2022, 22, 1681–1693. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.A., 2nd; Eron, J.J., Jr.; Holman, W.; Cohen, M.S.; Fang, L.; Szewczyk, L.J.; Sheahan, T.P.; Baric, R.; Mollan, K.R.; Wolfe, C.R.; et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci. Transl. Med. 2022, 14, eabl7430. [Google Scholar] [CrossRef]
- Gentile, I.; Scotto, R.; Schiano Moriello, N.; Pinchera, B.; Villari, R.; Trucillo, E.; Ametrano, L.; Fusco, L.; Castaldo, G.; Buonomo, A.R.; et al. Nirmatrelvir/Ritonavir and Molnupiravir in the Treatment of Mild/Moderate COVID-19: Results of a Real-Life Study. Vaccines 2022, 10, 1731. [Google Scholar] [CrossRef]
- Sinha, S.; Kumarasamy, N.; Suram, V.K.; Chary, S.S.; Naik, S.; Singh, V.B.; Jain, M.K.; Suthar, C.P.; Borthakur, S.; Sawardekar, V.; et al. Efficacy and Safety of Molnupiravir in Mild COVID-19 Patients in India. Cureus 2022, 14, e31508. [Google Scholar] [CrossRef]
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhou, X.; Wu, Q.; Zhang, X.; Feng, Z.; et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis. Ann. Med. 2022, 54, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Kontos, M.C.; Grizzard, J.D.; Biondi-Zoccai, G.G.; Van Tassell, B.W.; Robati, R.; Roach, L.M.; Arena, R.A.; Roberts, C.S.; Varma, A.; et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am. J. Cardiol. 2010, 105, 1371–1377.e1371. [Google Scholar] [CrossRef]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients with ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Van Tassell, B.W.; Biondi-Zoccai, G.; Kontos, M.C.; Grizzard, J.D.; Spillman, D.W.; Oddi, C.; Roberts, C.S.; Melchior, R.D.; Mueller, G.H.; et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am. J. Cardiol. 2013, 111, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Arena, R.; Biondi-Zoccai, G.; Canada, J.M.; Oddi, C.; Abouzaki, N.A.; Jahangiri, A.; Falcao, R.A.; Kontos, M.C.; Shah, K.B.; et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 2014, 113, 321–327. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Canada, J.; Carbone, S.; Trankle, C.; Buckley, L.; Oddi Erdle, C.; Abouzaki, N.A.; Dixon, D.; Kadariya, D.; Christopher, S.; et al. Interleukin-1 Blockade in Recently Decompensated Systolic Heart Failure: Results From REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ. Heart Fail. 2017, 10, e004373. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Trankle, C.R.; Canada, J.M.; Carbone, S.; Buckley, L.; Kadariya, D.; Del Buono, M.G.; Billingsley, H.; Wohlford, G.; Viscusi, M.; et al. IL-1 Blockade in Patients with Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2018, 11, e005036. [Google Scholar] [CrossRef] [PubMed]
- Brucato, A.; Imazio, M.; Gattorno, M.; Lazaros, G.; Maestroni, S.; Carraro, M.; Finetti, M.; Cumetti, D.; Carobbio, A.; Ruperto, N.; et al. Effect of Anakinra on Recurrent Pericarditis Among Patients with Colchicine Resistance and Corticosteroid Dependence: The AIRTRIP Randomized Clinical Trial. JAMA 2016, 316, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vosko, I.; Zirlik, A.; Bugger, H. Impact of COVID-19 on Cardiovascular Disease. Viruses 2023, 15, 508. https://doi.org/10.3390/v15020508
Vosko I, Zirlik A, Bugger H. Impact of COVID-19 on Cardiovascular Disease. Viruses. 2023; 15(2):508. https://doi.org/10.3390/v15020508
Chicago/Turabian StyleVosko, Ivan, Andreas Zirlik, and Heiko Bugger. 2023. "Impact of COVID-19 on Cardiovascular Disease" Viruses 15, no. 2: 508. https://doi.org/10.3390/v15020508
APA StyleVosko, I., Zirlik, A., & Bugger, H. (2023). Impact of COVID-19 on Cardiovascular Disease. Viruses, 15(2), 508. https://doi.org/10.3390/v15020508






