Caffeic Acid Has Antiviral Activity against Ilhéus Virus In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Virus, and Chemicals
2.2. Cytotoxicity Analysis
2.3. Viral Infection Assays by Viral Titration
2.4. Virus Plaque-Forming Unit Assay
2.5. Plaque Reduction Assay
2.6. Immunofluorescence Assay
2.7. RNA Extraction and RT-qPCR
2.8. Data Analysis
2.9. E Protein Dimer Molecular Modeling and Molecular Dynamics
2.10. Molecular Docking and Interaction Analysis
3. Results
3.1. Caffeic Acid Is Not Toxic in Cell Culture
3.2. Inhibition of ILHV Replication by Caffeic Acid Is Concentration-Dependent
3.3. Caffeic Acid Inhibited ILHV Lysis Plaque Formation in Cell Culture
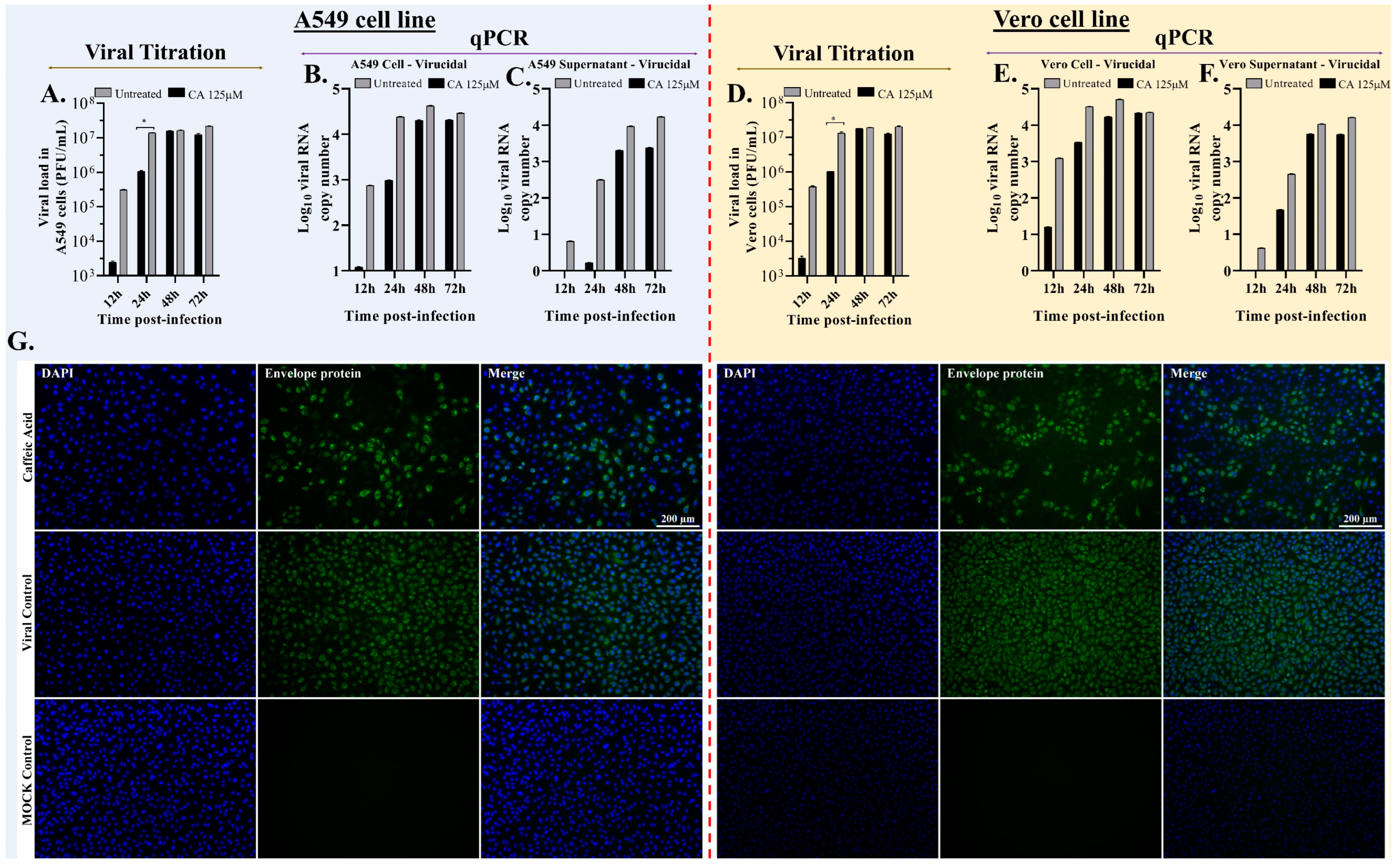
3.4. Caffeic Acid Is Virucidal against Ilhéus Virus
3.5. Caffeic Acid Reduces Ilhéus Virus Progeny Yield
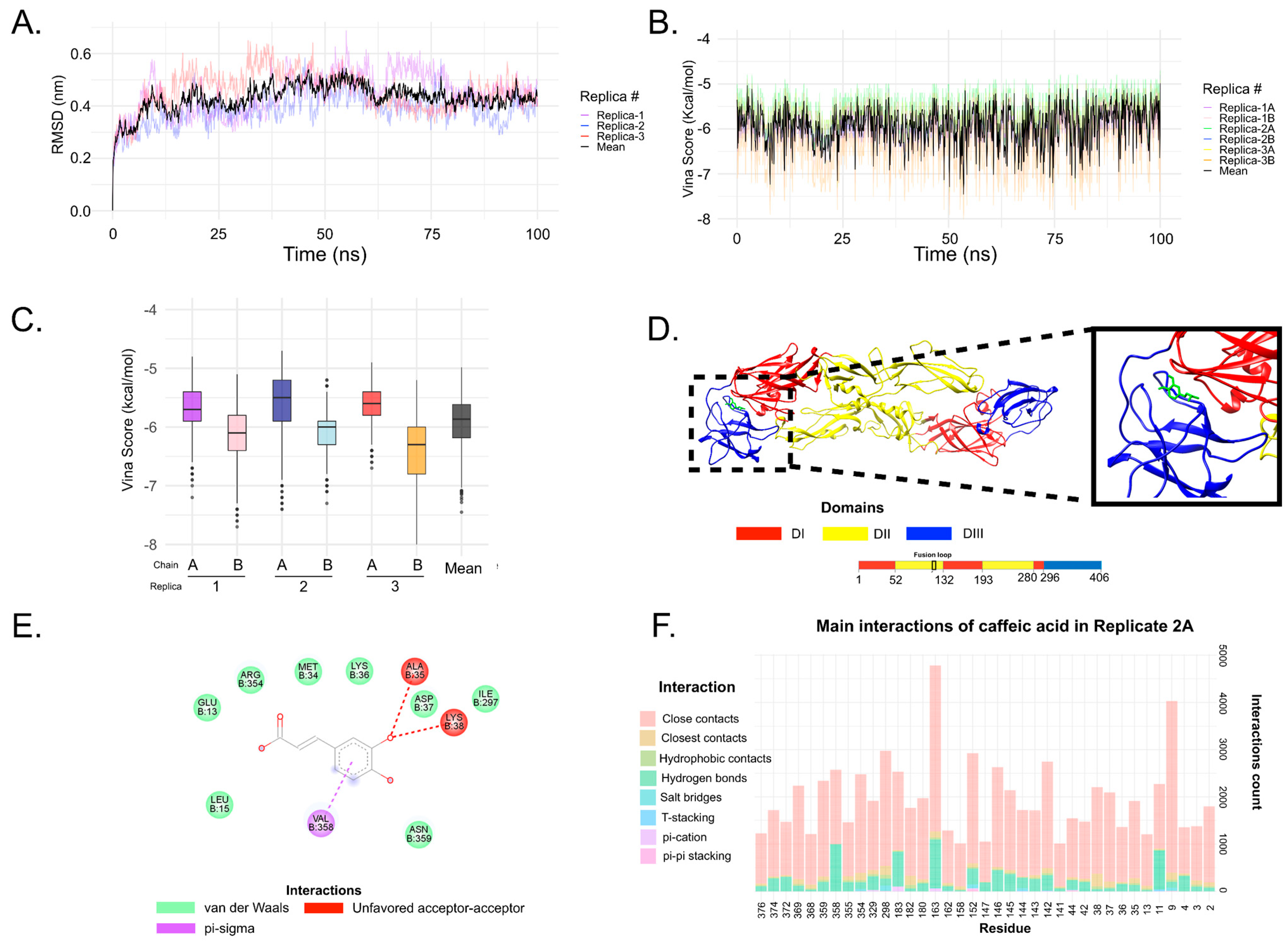
3.6. In Silico Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sips, G.J.; Wilschut, J.; Smit, J.M. Neuroinvasive Flavivirus Infections. Rev. Med. Virol. 2012, 22, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R. Waiting in the Wings: The Potential of Mosquito Transmitted Flaviviruses to Emerge. Crit. Rev. Microbiol. 2017, 43, 405–422. [Google Scholar] [CrossRef] [PubMed]
- Immunologists, A.A. of The Virus of Ilhéus Encephalitis: Isolation, Serological Specificity and Transmission. J. Immunol. 1947, 55, 61–67. [Google Scholar]
- Johnson, B.W.; Cruz, C.; Felices, V.; Espinoza, W.R.; Manock, S.R.; Guevara, C.; Olson, J.G.; Kochel, T.J. Ilheus Virus Isolate from a Human, Ecuador. Emerg. Infect. Dis. 2007, 13, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Venegas, E.A.; Aguilar, P.V.; Cruz, C.; Guevara, C.; Kochel, T.J.; Vargas, J.; Halsey, E.S. Ilheus Virus Infection in Human, Bolivia. Emerg. Infect. Dis. 2012, 18, 516–518. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The Continued Threat of Emerging Flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- Southam, C.M.; Moore, A.E. West Nile, Ilheus, and Bunyamwera Virus Infections in Man. Am. J. Trop. Med. Hyg. 1951, 31, 724–741. [Google Scholar] [CrossRef]
- Causey, O.R.; Causey, C.E.; Maroja, O.M.; Macedo, D.G. The Isolation of Arthropod-Borne Viruses, Including Members of Two Hitherto Undescribed Serological Groups, in the Amazon Region of Brazil. Am. J. Trop. Med. Hyg. 1961, 10, 227–249. [Google Scholar] [CrossRef]
- Spence, L.; Anderson, C.R.; Downs, W.G. Isolation of Ilhéus Virus from Human Beings in Trinidad, West Indies☆. Trans. R. Soc. Trop. Med. Hyg. 1962, 56, 504–509. [Google Scholar] [CrossRef]
- Srihongse, S.; Johnson, C.M. The Isolation of Ilhéus Virus from Man in Panamá. Am. J. Trop. Med. Hyg. 1967, 16, 516–518. [Google Scholar] [CrossRef]
- Prías-Landínez, E.; Bernal-Cubides, C.; Morales-Alarcón, A. Isolation of Ilhéus Virus from Man in Colombia. Am. J. Trop. Med. Hyg. 1968, 17, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Nassar, E.S.; Coimbra, T.L.M.; Rocco, I.M.; Pereira, L.E.; Ferreira, I.B.; de Souza, L.T.M.; de Souza, D.M.; Ueda-Ito, M.; Moura, J.P.; Bergo, R.C.F. Human Disease Caused by an Arbovirus Closely Related to Ilheus Virus: Report of Five Cases. Intervirology 1997, 40, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Milhim, B.H.G.A.; Estofolete, C.F.; da Rocha, L.C.; Liso, E.; Brienze, V.M.S.; Vasilakis, N.; Terzian, A.C.B.; Nogueira, M.L. Fatal Outcome of Ilheus Virus in the Cerebrospinal Fluid of a Patient Diagnosed with Encephalitis. Viruses 2020, 12, 957. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Desprès, P. Serological Diagnosis of Flavivirus-Associated Human Infections. Diagnostics 2020, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- da Costa, V.G.; Saivish, M.V.; Lino, N.A.B.; Bittar, C.; de Freitas Calmon, M.; Nogueira, M.L.; Rahal, P. Clinical Landscape and Rate of Exposure to Ilheus Virus: Insights from Systematic Review and Meta-Analysis. Viruses 2023, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Bollati, M.; Alvarez, K.; Assenberg, R.; Baronti, C.; Canard, B.; Cook, S.; Coutard, B.; Decroly, E.; de Lamballerie, X.; Gould, E.A.; et al. Structure and Functionality in Flavivirus NS-Proteins: Perspectives for Drug Design. Antivir. Res. 2010, 87, 125–148. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, K.; Quek, J.P.; Luo, D. Molecular Insights into the Flavivirus Replication Complex. Viruses 2021, 13, 956. [Google Scholar] [CrossRef]
- Fishburn, A.T.; Pham, O.H.; Kenaston, M.W.; Beesabathuni, N.S.; Shah, P.S. Let’s Get Physical: Flavivirus-Host Protein–Protein Interactions in Replication and Pathogenesis. Front. Microbiol. 2022, 13, 847588. [Google Scholar] [CrossRef]
- Newton, N.D.; Hardy, J.M.; Modhiran, N.; Hugo, L.E.; Amarilla, A.A.; Bibby, S.; Venugopal, H.; Harrison, J.J.; Traves, R.J.; Hall, R.A.; et al. The Structure of an Infectious Immature Flavivirus Redefines Viral Architecture and Maturation. Sci. Adv. 2021, 7, eabe4507. [Google Scholar] [CrossRef]
- Sevvana, M.; Long, F.; Miller, A.S.; Klose, T.; Buda, G.; Sun, L.; Kuhn, R.J.; Rossmann, M.G. Refinement and Analysis of the Mature Zika Virus Cryo-EM Structure at 3.1 Å Resolution. Structure 2018, 26, 1169–1177. [Google Scholar] [CrossRef]
- Dey, D.; Poudyal, S.; Rehman, A.; Hasan, S.S. Structural and Biochemical Insights into Flavivirus Proteins. Virus Res. 2021, 296, 198343. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Khaliq, M.; Zhou, Z.; Post, C.B.; Kuhn, R.J.; Cushman, M. Design, Synthesis, and Biological Evaluation of Antiviral Agents Targeting Flavivirus Envelope Proteins. J. Med. Chem. 2008, 51, 4660–4671. [Google Scholar] [CrossRef] [PubMed]
- de Wispelaere, M.; Lian, W.; Potisopon, S.; Li, P.-C.; Jang, J.; Ficarro, S.B.; Clark, M.J.; Zhu, X.; Kaplan, J.B.; Pitts, J.D.; et al. Inhibition of Flaviviruses by Targeting a Conserved Pocket on the Viral Envelope Protein. Cell Chem. Biol. 2018, 25, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Anasir, M.I.; Ramanathan, B.; Poh, C.L. Structure-Based Design of Antivirals against Envelope Glycoprotein of Dengue Virus. Viruses 2020, 12, 367. [Google Scholar] [CrossRef]
- Li, P.-C.; Jang, J.; Hsia, C.-Y.; Groomes, P.V.; Lian, W.; de Wispelaere, M.; Pitts, J.D.; Wang, J.; Kwiatkowski, N.; Gray, N.S.; et al. Small Molecules Targeting the Flavivirus E Protein with Broad-Spectrum Activity and Antiviral Efficacy in Vivo. ACS Infect. Dis. 2019, 5, 460–472. [Google Scholar] [CrossRef]
- Perera, R.; Khaliq, M.; Kuhn, R.J. Closing the Door on Flaviviruses: Entry as a Target for Antiviral Drug Design. Antivir. Res. 2008, 80, 11–22. [Google Scholar] [CrossRef]
- Kampmann, T.; Yennamalli, R.; Campbell, P.; Stoermer, M.J.; Fairlie, D.P.; Kobe, B.; Young, P.R. In Silico Screening of Small Molecule Libraries Using the Dengue Virus Envelope E Protein Has Identified Compounds with Antiviral Activity against Multiple Flaviviruses. Antivir. Res. 2009, 84, 234–241. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinski, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A New Class of Highly Potent, Broadly Neutralizing Antibodies Isolated from Viremic Patients Infected with Dengue Virus. Nat. Immunol. 2015, 16, 170–177. [Google Scholar] [CrossRef]
- Ogawa, M.; Shirasago, Y.; Tanida, I.; Kakuta, S.; Uchiyama, Y.; Shimojima, M.; Hanada, K.; Saijo, M.; Fukasawa, M. Structural Basis of Antiviral Activity of Caffeic Acid against Severe Fever with Thrombocytopenia Syndrome Virus. J. Infect. Chemother. 2021, 27, 397–400. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant Activity of Caffeic Acid (3,4-Dihydroxycinnamic Acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus Aureus Clinical Strains. BioMed Res. Int. 2018, 2018, e7413504. [Google Scholar] [CrossRef] [PubMed]
- Bailly, F.; Cotelle, P. Anti-HIV Activities of Natural Antioxidant Caffeic Acid Derivatives: Toward an Antiviral Supplementation Diet. Curr. Med. Chem. 2005, 12, 1811–1818. [Google Scholar] [CrossRef]
- Wu, Z.-M.; Yu, Z.-J.; Cui, Z.-Q.; Peng, L.-Y.; Li, H.-R.; Zhang, C.-L.; Shen, H.-Q.; Yi, P.-F.; Fu, B.-D. In Vitro Antiviral Efficacy of Caffeic Acid against Canine Distemper Virus. Microb. Pathog. 2017, 110, 240–244. [Google Scholar] [CrossRef]
- Langland, J.; Jacobs, B.; Wagner, C.E.; Ruiz, G.; Cahill, T.M. Antiviral Activity of Metal Chelates of Caffeic Acid and Similar Compounds towards Herpes Simplex, VSV-Ebola Pseudotyped and Vaccinia Viruses. Antivir. Res. 2018, 160, 143–150. [Google Scholar] [CrossRef]
- Shen, J.; Wang, G.; Zuo, J. Caffeic Acid Inhibits HCV Replication via Induction of IFNα Antiviral Response through P62-Mediated Keap1/Nrf2 Signaling Pathway. Antivir. Res. 2018, 154, 166–173. [Google Scholar] [CrossRef]
- Wang, G.-F.; Shi, L.-P.; Ren, Y.-D.; Liu, Q.-F.; Liu, H.-F.; Zhang, R.-J.; Li, Z.; Zhu, F.-H.; He, P.-L.; Tang, W.; et al. Anti-Hepatitis B Virus Activity of Chlorogenic Acid, Quinic Acid and Caffeic Acid in Vivo and in Vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Ichinose, M.; Ikeda, K.; Uozaki, M.; Morishita, J.; Kuwahara, T.; Koyama, A.H.; Yamasaki, H. Inhibition by Caffeic Acid of the Influenza A Virus Multiplication in Vitro. Int. J. Mol. Med. 2014, 34, 1020–1024. [Google Scholar] [CrossRef]
- Ogawa, M.; Shirasago, Y.; Ando, S.; Shimojima, M.; Saijo, M.; Fukasawa, M. Caffeic Acid, a Coffee-Related Organic Acid, Inhibits Infection by Severe Fever with Thrombocytopenia Syndrome Virus in Vitro. J. Infect. Chemother. 2018, 24, 597–601. [Google Scholar] [CrossRef]
- Adem, Ş.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Zahoor, A.F.; Ali, M.; Abdalla, M.; Ibrahim, I.M.; Elfiky, A.A. Caffeic Acid Derivatives (CAFDs) as Inhibitors of SARS-CoV-2: CAFDs-Based Functional Foods as a Potential Alternative Approach to Combat COVID-19. Phytomedicine 2021, 85, 153310. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Mong, M.; Chan, K.; Yin, M. Anti-Glycative and Anti-Inflammatory Effects of Caffeic Acid and Ellagic Acid in Kidney of Diabetic Mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Burleson, F.G.; Chambers, T.M.; Wiedbrauk, D.L. Virology: A Laboratory Manual; Academic Press: San Diego, CA, USA, 1992; ISBN 978-1-4832-9676-0. [Google Scholar]
- Patel, P.; Landt, O.; Kaiser, M.; Faye, O.; Koppe, T.; Lass, U.; Sall, A.A.; Niedrig, M. Development of One-Step Quantitative Reverse Transcription PCR for the Rapid Detection of Flaviviruses. Virol. J. 2013, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, I.R.; Pei, J.; Baek, M.; Krishnakumar, A.; Anishchenko, I.; Ovchinnikov, S.; Zhang, J.; Ness, T.J.; Banjade, S.; Bagde, S.R.; et al. Computed Structures of Core Eukaryotic Protein Complexes. Science 2021, 374, eabm4805. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate Prediction of Protein Structures and Interactions Using a Three-Track Neural Network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and Better Reference Data for Improved All-Atom Structure Validation: PROTEIN SCIENCE.ORG. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical p K a Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved Side-Chain Torsion Potentials for the Amber Ff99SB Protein Force Field: Improved Protein Side-Chain Potentials. Proteins Struct. Funct. Bioinforma. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Menezes, G.D.L.; Saivish, M.V.; Nogueira, M.L.; da Silva, R.A. Virtual Screening of Small Natural Compounds against NS1 Protein of DENV, YFV and ZIKV. J. Biomol. Struct. Dyn. 2022, 41, 1–11. [Google Scholar] [CrossRef]
- de Lima Menezes, G.; da Silva, R.A. Identification of Potential Drugs against SARS-CoV-2 Non-Structural Protein 1 (Nsp1). J. Biomol. Struct. Dyn. 2021, 39, 5657–5667. [Google Scholar] [CrossRef]
- de Oliveira, F.M.; Procopio, V.O.; Menezes, G.D.L.; da Silva, R.A.; Kipnis, A.; Junqueira-Kipnis, A.P. Mycobacterium Bovis PknG R242P Mutation Results in Structural Changes with Enhanced Virulence in the Mouse Model of Infection. Microorganisms 2022, 10, 673. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kollman, P.A. Settle: An Analytical Version of the SHAKE and RATTLE Algorithm for Rigid Water Models. J. Comput. Chem. 1992, 13, 952–962. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for Molecular Simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Hutter, J. Car-Parrinello Molecular Dynamics: Car-Parrinello Molecular Dynamics. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 604–612. [Google Scholar] [CrossRef]
- Sharma, N.; Kumar, P.; Giri, R. Polysaccharides like Pentagalloylglucose, Parishin a and Stevioside Inhibits the Viral Entry by Binding the Zika Virus Envelope Protein. J. Biomol. Struct. Dyn. 2021, 39, 6008–6020. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.; Xiao, D.; Yin, J.; Song, M.; Xu, Y.; Zhao, L.; Dai, Q.; Li, Y.; Wang, C.; et al. Nafamostat Mesylate as a Broad-Spectrum Candidate for the Treatment of Flavivirus Infections by Targeting Envelope Proteins. Antivir. Res. 2022, 202, 105325. [Google Scholar] [CrossRef]
- Sharma, N.; Murali, A.; Singh, S.K.; Giri, R. Epigallocatechin Gallate, an Active Green Tea Compound Inhibits the Zika Virus Entry into Host Cells via Binding the Envelope Protein. Int. J. Biol. Macromol. 2017, 104, 1046–1054. [Google Scholar] [CrossRef]
- Durrant, J.D.; McCammon, J.A. BINANA: A Novel Algorithm for Ligand-Binding Characterization. J. Mol. Graph. Model. 2011, 29, 888–893. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, prot095505. [Google Scholar] [CrossRef] [PubMed]
- Goo, L.; VanBlargan, L.A.; Dowd, K.A.; Diamond, M.S.; Pierson, T.C. A Single Mutation in the Envelope Protein Modulates Flavivirus Antigenicity, Stability, and Pathogenesis. PLoS Pathog. 2017, 13, e1006178. [Google Scholar] [CrossRef] [PubMed]
- Mazeaud, C.; Freppel, W.; Chatel-Chaix, L. The Multiples Fates of the Flavivirus RNA Genome During Pathogenesis. Front. Genet. 2018, 9, 595. [Google Scholar] [CrossRef]
- Fulton, B.O.; Sachs, D.; Schwarz, M.C.; Palese, P.; Evans, M.J. Transposon Mutagenesis of the Zika Virus Genome Highlights Regions Essential for RNA Replication and Restricted for Immune Evasion. J. Virol. 2017, 91, e00698-17. [Google Scholar] [CrossRef]
- Hung, S.-J.; Huang, S.-W. Contributions of Genetic Evolution to Zika Virus Emergence. Front. Microbiol. 2021, 12, 655065. [Google Scholar] [CrossRef]
- Kaptein, S.J.; Neyts, J. Towards Antiviral Therapies for Treating Dengue Virus Infections. Curr. Opin. Pharmacol. 2016, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.B.; Chunsuttiwat, S.; Nisalak, A.; Mammen, M.P.; Libraty, D.H.; Rothman, A.L.; Green, S.; Vaughn, D.W.; Ennis, F.A.; Endy, T.P. Burden of Symptomatic Dengue Infection in Children at Primary School in Thailand: A Prospective Study. Lancet 2007, 369, 1452–1459. [Google Scholar] [CrossRef]
- Alson, S.G.; Jansen, O.; Cieckiewicz, E.; Rakotoarimanana, H.; Rafatro, H.; Degotte, G.; Francotte, P.; Frederich, M. In-Vitro and in-Vivo Antimalarial Activity of Caffeic Acid and Some of Its Derivatives. J. Pharm. Pharmacol. 2018, 70, 1349–1356. [Google Scholar] [CrossRef]
- Chao, P.; Hsu, C.; Yin, M. Anti-Inflammatory and Anti-Coagulatory Activities of Caffeic Acid and Ellagic Acid in Cardiac Tissue of Diabetic Mice. Nutr. Metab. 2009, 6, 33. [Google Scholar] [CrossRef]
- da Cunha, F.M.; Duma, D.; Assreuy, J.; Buzzi, F.C.; Niero, R.; Campos, M.M.; Calixto, J.B. Caffeic Acid Derivatives: In Vitro and In Vivo Anti-Inflammatory Properties. Free. Radic. Res. 2004, 38, 1241–1253. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic Acids and Other Cinnamates—Nature, Occurrence, Dietary Burden, Absorption and Metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; José Núñez, M.; Parajó, J.C. Natural Antioxidants from Residual Sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Fan, W.; Qian, S.; Qian, P.; Li, X. Antiviral Activity of Luteolin against Japanese Encephalitis Virus. Virus Res. 2016, 220, 112–116. [Google Scholar] [CrossRef]
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin Inhibits Zika and Chikungunya Virus Infection by Inhibiting Cell Binding. Antivir. Res. 2017, 142, 148–157. [Google Scholar] [CrossRef]
- Frumence, E.; Roche, M.; Krejbich-Trotot, P.; El-Kalamouni, C.; Nativel, B.; Rondeau, P.; Missé, D.; Gadea, G.; Viranaicken, W.; Desprès, P. The South Pacific Epidemic Strain of Zika Virus Replicates Efficiently in Human Epithelial A549 Cells Leading to IFN-β Production and Apoptosis Induction. Virology 2016, 493, 217–226. [Google Scholar] [CrossRef]
- El Kalamouni, C.; Frumence, E.; Bos, S.; Turpin, J.; Nativel, B.; Harrabi, W.; Wilkinson, D.A.; Meilhac, O.; Gadea, G.; Desprès, P.; et al. Subversion of the Heme Oxygenase-1 Antiviral Activity by Zika Virus. Viruses 2018, 11, 2. [Google Scholar] [CrossRef]
- Viranaicken, W.; Nativel, B.; Krejbich-Trotot, P.; Harrabi, W.; Bos, S.; El Kalamouni, C.; Roche, M.; Gadea, G.; Desprès, P. ClearColi BL21(DE3)-Based Expression of Zika Virus Antigens Illustrates a Rapid Method of Antibody Production against Emerging Pathogens. Biochimie 2017, 142, 179–182. [Google Scholar] [CrossRef]
- Turpin, J.; Frumence, E.; Harrabi, W.; Haddad, J.G.; El Kalamouni, C.; Desprès, P.; Krejbich-Trotot, P.; Viranaïcken, W. Zika Virus Subversion of Chaperone GRP78/BiP Expression in A549 Cells during UPR Activation. Biochimie 2020, 175, 99–105. [Google Scholar] [CrossRef]
- Simonetti, P.; Gardana, C.; Pietta, P. Plasma Levels of Caffeic Acid and Antioxidant Status after Red Wine Intake. J. Agric. Food Chem. 2001, 49, 5964–5968. [Google Scholar] [CrossRef]
- Renouf, M.; Guy, P.A.; Marmet, C.; Fraering, A.-L.; Longet, K.; Moulin, J.; Enslen, M.; Barron, D.; Dionisi, F.; Cavin, C.; et al. Measurement of Caffeic and Ferulic Acid Equivalents in Plasma after Coffee Consumption: Small Intestine and Colon Are Key Sites for Coffee Metabolism. Mol. Nutr. Food Res. 2010, 54, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.K.; Khan, J. Chapter 7—Formulation Strategies to Improve the Bioavailability of Poorly Absorbed Drugs. In Advances and Challenges in Pharmaceutical Technology; Nayak, A.K., Pal, K., Banerjee, I., Maji, S., Nanda, U., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 229–242. ISBN 978-0-12-820043-8. [Google Scholar]
- Chellasamy, S.K.; Devarajan, S. Identification of Potential Lead Molecules for Zika Envelope Protein from In Silico Perspective. Avicenna J. Med. Biotechnol. 2019, 11, 10. [Google Scholar]
- Ikeda, K.; Tsujimoto, K.; Uozaki, M.; Nishide, M.; Suzuki, Y.; Koyama, A.H.; Yamasaki, H. Inhibition of Multiplication of Herpes Simplex Virus by Caffeic Acid. Int. J. Mol. Med. 2011, 28, 595–598. [Google Scholar] [CrossRef] [PubMed]





| Inhibition Efficacy (µM) * | Cell Viability (%) * | ||||||
|---|---|---|---|---|---|---|---|
| EC50 | A549 | HepG2 | Vero | ||||
| A549 | HepG2 | Vero | 1000µM | 72.4 ± 2.4 | 91.4 ± 5.2 | 100.5 ± 14.6 | |
| Virucidal | 390.3 ± 32.4 | 140.6 ± 23.4 | 234.0 ± 39.2 | 500 µM | 77 ± 6.6 | 96.2 ± 5.1 | 101.4 ± 9.7 |
| Pre-treatment | Not achieved | Not achieved | Not achieved | 250 µM | 83.3 ± 6.8 | 94.7± 3.2 | 96 ± 9.2 |
| Co-treatment | 370.4 ± 19.9 | 167.9 ± 26.3 | 228.9 ± 58.4 | 125 µM | 86.4 ± 4.3 | 96.5 ± 2.1 | 95.5 ± 7.6 |
| Post-treatment | 340.1 ± 34.4 | 187.4 ± 42.1 | 168.7 ± 33.3 | 62.5 µM | 87.4 ± 11,2 | 93.8 ± 4.7 | 94.5 ± 7.2 |
| Vina Score from Ensemble Docking | |||
|---|---|---|---|
| Structure | Vina Score (Kcal/mol) | ||
| Replicate (Chain) | Min. | Max. | 3rd Quartile |
| 1 (A) | −7.20 | −4.80 | −5.40 |
| 1 (B) | −8.10 | −5.10 | −5.80 |
| 2 (A) | −7.40 | −4.70 | −5.20 |
| 2 (B) | −7.30 | −5.20 | −5.90 |
| 3 (A) | −6.70 | −4.90 | −5.40 |
| 3 (B) | −8.00 | −5.20 | −6.00 |
| Average | −7.45 | −4.98 | −5.62 |
| Estimated Binding Free Energy by Vina | |||
| PDB ID | Vina Score (Kcal/mol) | ||
| 4N0S | −5.102 | ||
| 6YRI | −4.273 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saivish, M.V.; Pacca, C.C.; da Costa, V.G.; de Lima Menezes, G.; da Silva, R.A.; Nebo, L.; da Silva, G.C.D.; de Aguiar Milhim, B.H.G.; da Silva Teixeira, I.; Henrique, T.; et al. Caffeic Acid Has Antiviral Activity against Ilhéus Virus In Vitro. Viruses 2023, 15, 494. https://doi.org/10.3390/v15020494
Saivish MV, Pacca CC, da Costa VG, de Lima Menezes G, da Silva RA, Nebo L, da Silva GCD, de Aguiar Milhim BHG, da Silva Teixeira I, Henrique T, et al. Caffeic Acid Has Antiviral Activity against Ilhéus Virus In Vitro. Viruses. 2023; 15(2):494. https://doi.org/10.3390/v15020494
Chicago/Turabian StyleSaivish, Marielena Vogel, Carolina Colombelli Pacca, Vivaldo Gomes da Costa, Gabriela de Lima Menezes, Roosevelt Alves da Silva, Liliane Nebo, Gislaine Celestino Dutra da Silva, Bruno Henrique Gonçalves de Aguiar Milhim, Igor da Silva Teixeira, Tiago Henrique, and et al. 2023. "Caffeic Acid Has Antiviral Activity against Ilhéus Virus In Vitro" Viruses 15, no. 2: 494. https://doi.org/10.3390/v15020494
APA StyleSaivish, M. V., Pacca, C. C., da Costa, V. G., de Lima Menezes, G., da Silva, R. A., Nebo, L., da Silva, G. C. D., de Aguiar Milhim, B. H. G., da Silva Teixeira, I., Henrique, T., Mistrão, N. F. B., Hernandes, V. M., Zini, N., de Carvalho, A. C., Fontoura, M. A., Rahal, P., Sacchetto, L., Marques, R. E., & Nogueira, M. L. (2023). Caffeic Acid Has Antiviral Activity against Ilhéus Virus In Vitro. Viruses, 15(2), 494. https://doi.org/10.3390/v15020494









