Pro-Viral and Anti-Viral Roles of the RNA-Binding Protein G3BP1
Abstract
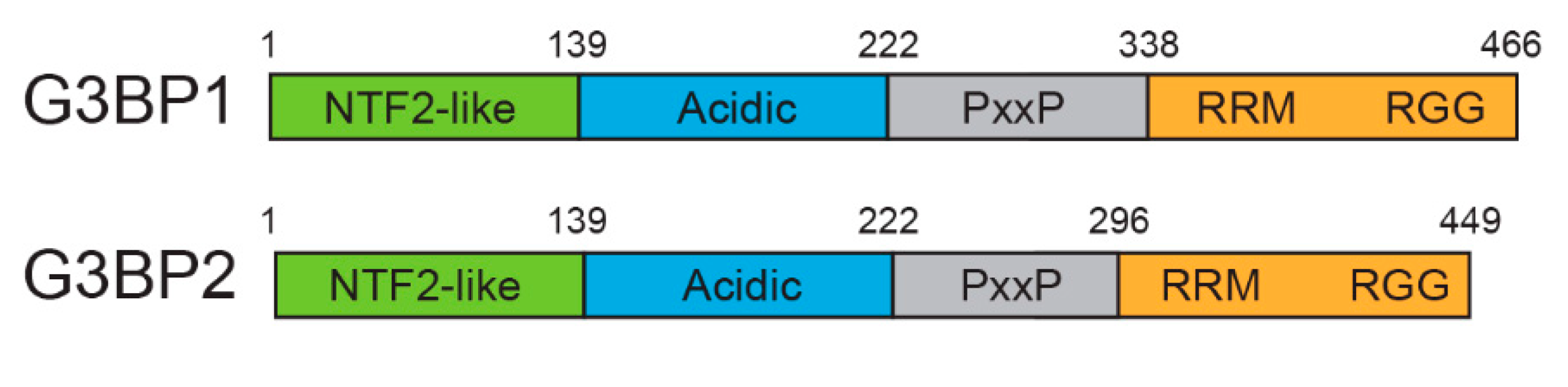
1. Introduction
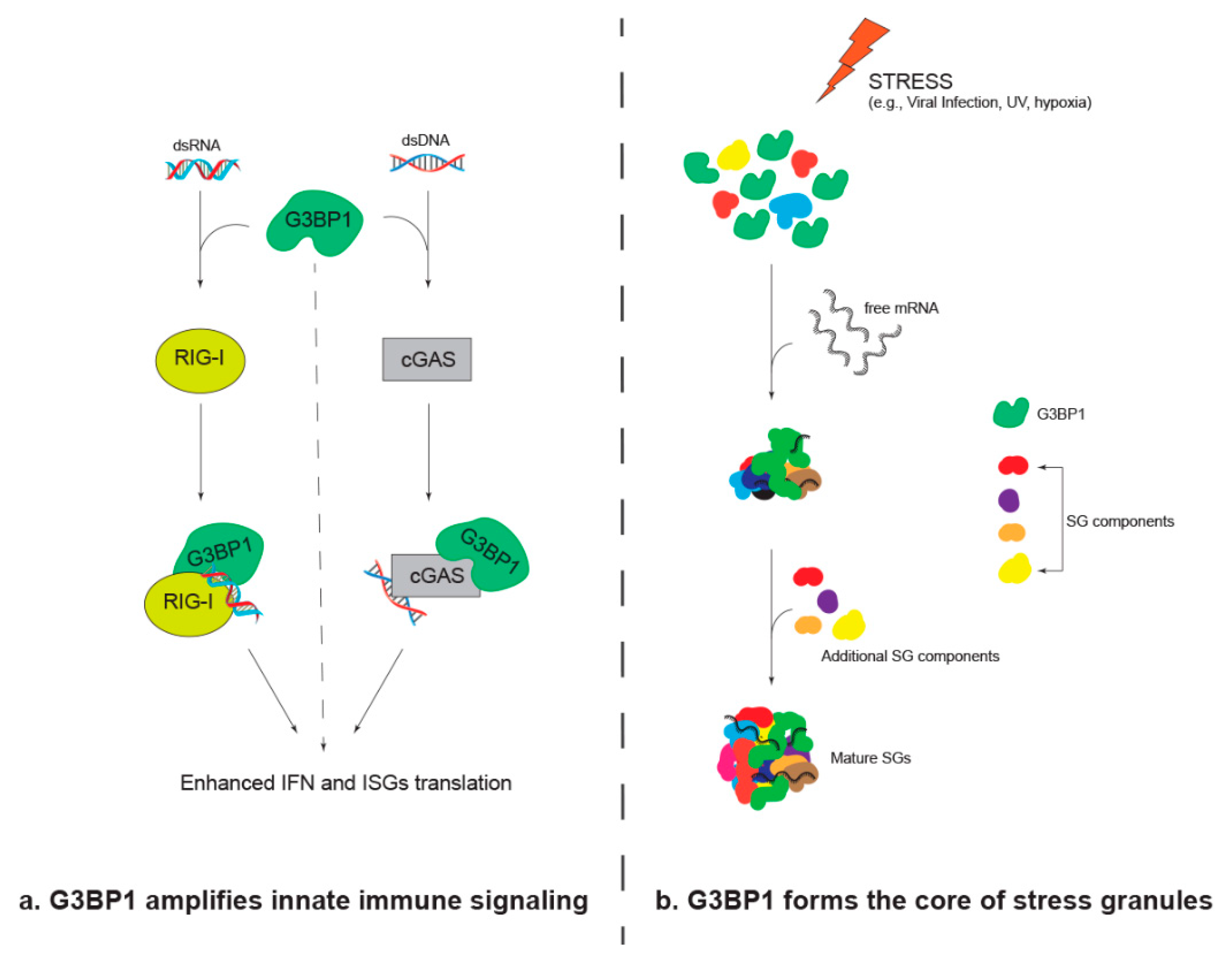
2. G3BP1 Amplifies Innate Immune Signaling
3. G3BP1 Forms the Core of Stress Granules
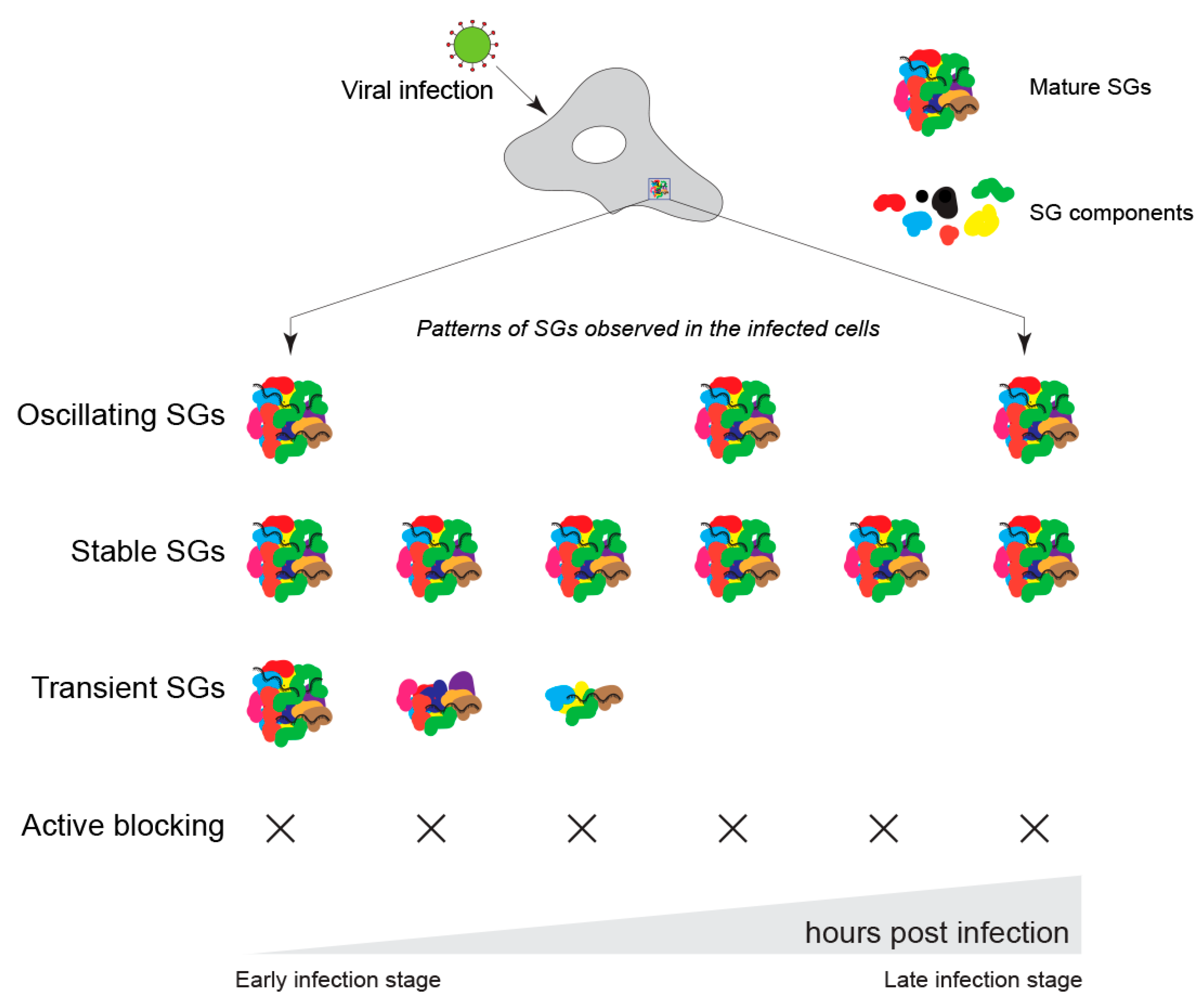
4. Role of G3BP1 during Viral Infection
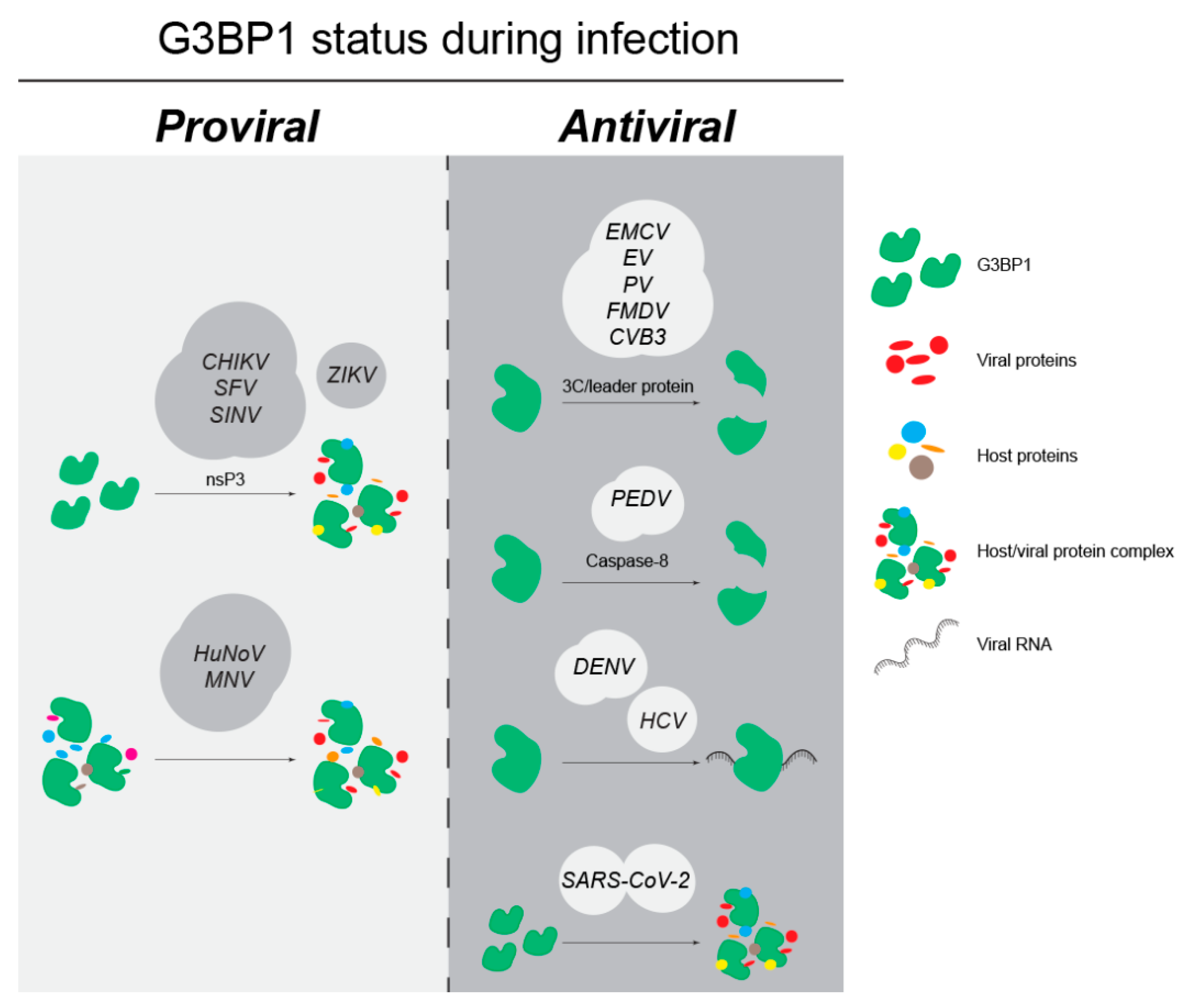
4.1. G3BP1 as an Anti-Viral Factor
4.1.1. Picornaviruses Inhibit G3BP1 Function through Protease-Mediated Cleavage
4.1.2. Coronaviruses Modulate G3BP1 Function Either by Cleavage or Sequestration
4.2. G3BP1 as a Pro-Viral Factor
4.2.1. Togaviruses Sequester G3BP1 to Facilitate Viral Replication and Translation
4.2.2. Caliciviruses Remodel the G3BP1 Interactome during Infection
4.3. G3BP1 Is Differentially Utilized within the Flaviviridae Family
4.3.1. Dengue Virus (DENV) Modulates G3BP1 Function by Interacting with 3′UTR vRNA
4.3.2. ZIKV and HCV Require G3BP1 for Proper Viral Replication
4.4. Viruses without a Defined Role for G3BP1 in Infection
4.4.1. Depletion or Overexpression of G3BP1 Does Not Affect PRRSV Infection
4.4.2. G3BP1 Is Sequestered within Ebola Virus Inclusions
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Zid, B.M.; O’Shea, E.K. Promoter Sequences Direct Cytoplasmic Localization and Translation of MRNAs during Starvation in Yeast. Nature 2014, 514, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Yamazaki, T.; Kroemer, G. Linking Cellular Stress Responses to Systemic Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 731–745. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-Damage Response in Human Biology and Disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The Unfolded Protein Response: Controlling Cell Fate Decisions under ER Stress and Beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Rouse, B.T.; Sehrawat, S. Immunity and Immunopathology to Viruses: What Decides the Outcome? Nat. Rev. Immunol. 2010, 10, 514–526. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Ma, Z.; Damania, B. CGAS and STING: At the Intersection of DNA and RNA Virus-Sensing Networks. PLoS Pathog. 2018, 14, e1007148. [Google Scholar] [CrossRef]
- Lester, S.N.; Li, K. Toll-like Receptors in Antiviral Innate Immunity. J. Mol. Biol. 2014, 426, 1246–1264. [Google Scholar] [CrossRef] [PubMed]
- Katze, M.G.; He, Y.; Gale, M. Viruses and Interferon: A Fight for Supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; Rice, C.M. Interferon-Stimulated Genes and Their Antiviral Effector Functions. Curr. Opin. Virol. 2011, 1, 519–525. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Gack, M.U. RIG-I-like Receptors: Their Regulation and Roles in RNA Sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Ellegast, J.; Kim, S.; Brzózka, K.; Jung, A.; Kato, H.; Poeck, H.; Akira, S.; Conzelmann, K.-K.; Schlee, M.; et al. 5′-Triphosphate RNA Is the Ligand for RIG-I. Science 2006, 314, 994–997. [Google Scholar] [CrossRef]
- Payne, S. Virus Interactions With the Cell. Viruses 2017, 23, 23–35. [Google Scholar] [CrossRef]
- Raoult, D.; Audic, S.; Robert, C.; Abergel, C.; Renesto, P.; Ogata, H.; La Scola, B.; Suzan, M.; Claverie, J.-M. The 1.2-Megabase Genome Sequence of Mimivirus. Science 2004, 306, 1344–1350. [Google Scholar] [CrossRef]
- Moore, C.H.; Farron, F.; Bohnert, D.; Weissmann, C. Possible Origin of a Minor Virus Specific Protein (A1) in Q-Beta Particles. Nat. New Biol. 1971, 234, 204–206. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Lloyd, R.E. Cytoplasmic RNA Granules and Viral Infection. Annu. Rev. Virol. 2014, 1, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Laver, J.D.; Ly, J.; Winn, A.K.; Karaiskakis, A.; Lin, S.; Nie, K.; Benic, G.; Jaberi-Lashkari, N.; Cao, W.X.; Khademi, A.; et al. The RNA-Binding Protein Rasputin/G3BP Enhances the Stability and Translation of Its Target MRNAs. Cell Rep. 2020, 30, 3353–3367.e7. [Google Scholar] [CrossRef]
- Somasekharan, S.P.; Zhang, F.; Saxena, N.; Huang, J.N.; Kuo, I.C.; Low, C.; Bell, R.; Adomat, H.; Stoynov, N.; Foster, L.; et al. G3BP1-Linked MRNA Partitioning Supports Selective Protein Synthesis in Response to Oxidative Stress. Nucleic Acids Res. 2020, 48, 6855–6873. [Google Scholar] [CrossRef]
- Fischer, J.W.; Busa, V.F.; Shao, Y.; Leung, A.K.L. Structure-Mediated RNA Decay by UPF1 and G3BP1. Mol. Cell 2020, 78, 70–84.e6. [Google Scholar] [CrossRef]
- Bidet, K.; Dadlani, D.; Garcia-Blanco, M.A. G3BP1, G3BP2 and CAPRIN1 Are Required for Translation of Interferon Stimulated MRNAs and Are Targeted by a Dengue Virus Non-Coding RNA. PLoS Pathog. 2014, 10, e1004242. [Google Scholar] [CrossRef]
- Sanchez, I.I.; Nguyen, T.B.; England, W.E.; Lim, R.G.; Vu, A.Q.; Miramontes, R.; Byrne, L.M.; Markmiller, S.; Lau, A.L.; Orellana, I.; et al. Huntington’s Disease Mice and Human Brain Tissue Exhibit Increased G3BP1 Granules and TDP43 Mislocalization. J. Clin. Investig. 2021, 131, e140723. [Google Scholar] [CrossRef]
- Scholte, F.E.M.; Tas, A.; Albulescu, I.C.; Žusinaite, E.; Merits, A.; Snijder, E.J.; van Hemert, M.J. Stress Granule Components G3BP1 and G3BP2 Play a Proviral Role Early in Chikungunya Virus Replication. J. Virol. 2015, 89, 4457–4469. [Google Scholar] [CrossRef]
- Cho, E.; Than, T.T.; Kim, S.-H.; Park, E.-R.; Kim, M.-Y.; Lee, K.H.O.; Shin, H.J. G3BP1 Depletion Increases Radiosensitisation by Inducing Oxidative Stress in Response to DNA Damage. Anticancer Res. 2019, 39, 6087–6095. [Google Scholar] [CrossRef] [PubMed]
- Dou, N.; Chen, J.; Yu, S.; Gao, Y.; Li, Y. G3BP1 Contributes to Tumor Metastasis via Upregulation of Slug Expression in Hepatocellular Carcinoma. Am. J. Cancer Res. 2016, 6, 2641–2650. [Google Scholar] [PubMed]
- Ge, Y.; Jin, J.; Li, J.; Ye, M.; Jin, X. The Roles of G3BP1 in Human Diseases (Review). Gene 2022, 821, 146294. [Google Scholar] [CrossRef]
- Irvine, K.; Stirling, R.; Hume, D.; Kennedy, D. Rasputin, More Promiscuous than Ever: A Review of G3BP. Int. J. Dev. Biol. 2004, 48, 1065–1077. [Google Scholar] [CrossRef]
- Matsuki, H.; Takahashi, M.; Higuchi, M.; Makokha, G.N.; Oie, M.; Fujii, M. Both G3BP1 and G3BP2 Contribute to Stress Granule Formation. Genes Cells 2013, 18, 135–146. [Google Scholar] [CrossRef]
- Kedersha, N.; Panas, M.D.; Achorn, C.A.; Lyons, S.; Tisdale, S.; Hickman, T.; Thomas, M.; Lieberman, J.; McInerney, G.M.; Ivanov, P.; et al. G3BP-Caprin1-USP10 Complexes Mediate Stress Granule Condensation and Associate with 40S Subunits. J. Cell Biol. 2016, 212, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.Y.; Sze, L.; Lam, K.P. The Stress Granule Protein G3BP1 Binds Viral DsRNA and RIG-I to Enhance Interferon-β Response. J. Biol. Chem. 2019, 294, 6430–6438. [Google Scholar] [CrossRef]
- Hosmillo, M.; Lu, J.; McAllaster, M.R.; Eaglesham, J.B.; Wang, X.; Emmott, E.; Domingues, P.; Chaudhry, Y.; Fitzmaurice, T.J.; Tung, M.K.; et al. Noroviruses Subvert the Core Stress Granule Component G3BP1 to Promote Viral VPg-Dependent Translation. Elife 2019, 8, e46681. [Google Scholar] [CrossRef]
- Riggs, C.L.; Kedersha, N.; Ivanov, P.; Anderson, P. Mammalian Stress Granules and P Bodies at a Glance. J. Cell Sci. 2020, 133, jcs242487. [Google Scholar] [CrossRef] [PubMed]
- Tourrière, H.; Chebli, K.; Zekri, L.; Courselaud, B.; Blanchard, J.M.; Bertrand, E.; Tazi, J. The RasGAP-Associated Endoribonuclease G3BP Assembles Stress Granules. J. Cell Biol. 2003, 160, 823–831. [Google Scholar] [CrossRef]
- Liu, Z.S.; Cai, H.; Xue, W.; Wang, M.; Xia, T.; Li, W.J.; Xing, J.Q.; Zhao, M.; Huang, Y.J.; Chen, S.; et al. G3BP1 Promotes DNA Binding and Activation of CGAS. Nat. Immunol. 2019, 20, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Sun, H.; Yin, L.; Li, J.; Mei, S.; Xu, F.; Wu, C.; Liu, X.; Zhao, F.; Zhang, D.; et al. PKR-Dependent Cytosolic CGAS Foci Are Necessary for Intracellular DNA Sensing. Sci. Signal. 2019, 12, eaav7934. [Google Scholar] [CrossRef]
- Yang, W.; Ru, Y.; Ren, J.; Bai, J.; Wei, J.; Fu, S.; Liu, X.; Li, D.; Zheng, H. G3BP1 Inhibits RNA Virus Replication by Positively Regulating RIG-I-Mediated Cellular Antiviral Response. Cell Death Dis. 2019, 10, 946. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, P.; Kedersha, N.; Anderson, P. Stress Granules and Processing Bodies in Translational Control. Cold Spring Harb. Perspect. Biol. 2019, 11, a032813. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-N.; Kavianpour, S.; Zhang, T.; Zhang, X.; Nguyen, D.; Thombre, R.; He, L.; Wang, J. MARK2 Phosphorylates EIF2α in Response to Proteotoxic Stress. PLoS Biol. 2021, 19, e3001096. [Google Scholar] [CrossRef]
- Taniuchi, S.; Miyake, M.; Tsugawa, K.; Oyadomari, M.; Oyadomari, S. Integrated Stress Response of Vertebrates Is Regulated by Four EIF2α Kinases. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Gebauer, F.; Hentze, M.W. Molecular Mechanisms of Translational Control. Nat. Rev. Mol. Cell Biol. 2004, 5, 827–835. [Google Scholar] [CrossRef]
- Marmor-Kollet, H.; Siany, A.; Kedersha, N.; Knafo, N.; Rivkin, N.; Danino, Y.M.; Moens, T.G.; Olender, T.; Sheban, D.; Cohen, N.; et al. Spatiotemporal Proteomic Analysis of Stress Granule Disassembly Using APEX Reveals Regulation by SUMOylation and Links to ALS Pathogenesis. Mol. Cell 2020, 80, 876–891.e6. [Google Scholar] [CrossRef]
- Gwon, Y.; Maxwell, B.A.; Kolaitis, R.-M.; Zhang, P.; Kim, H.J.; Paul Taylor, J. Ubiquitination of G3BP1 Mediates Stress Granule Disassembly in a Context-Specific Manner. Science 2021, 372, eabf6548. [Google Scholar] [CrossRef] [PubMed]
- Ohn, T.; Kedersha, N.; Hickman, T.; Tisdale, S.; Anderson, P. A Functional RNAi Screen Links O-GlcNAc Modification of Ribosomal Proteins to Stress Granule and Processing Body Assembly. Nat. Cell Biol. 2008, 10, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Gayatri, S.; Reineke, L.C.; Sbardella, G.; Bedford, M.T.; Lloyd, R.E. Arginine Demethylation of G3BP1 Promotes Stress Granule Assembly. J. Biol. Chem. 2016, 291, 22671–22685. [Google Scholar] [CrossRef]
- Leung, A.K.L.; Vyas, S.; Rood, J.E.; Bhutkar, A.; Sharp, P.A.; Chang, P. Poly(ADP-Ribose) Regulates Stress Responses and MicroRNA Activity in the Cytoplasm. Mol. Cell 2011, 42, 489–499. [Google Scholar] [CrossRef]
- Jayabalan, A.K.; Sanchez, A.; Park, R.Y.; Yoon, S.P.; Kang, G.Y.; Baek, J.H.; Anderson, P.; Kee, Y.; Ohn, T. NEDDylation Promotes Stress Granule Assembly. Nat. Commun. 2016, 7, 12125. [Google Scholar] [CrossRef]
- Jayabalan, A.K.; Adivarahan, S.; Koppula, A.; Abraham, R.; Batish, M.; Zenklusen, D.; Griffin, D.E.; Leung, A.K.L. Stress Granule Formation, Disassembly, and Composition Are Regulated by Alphavirus ADP-Ribosylhydrolase Activity. Proc. Natl. Acad. Sci. USA 2021, 118, e2021719118. [Google Scholar] [CrossRef] [PubMed]
- Matheny, T.; Van Treeck, B.; Huynh, T.N.; Parker, R. RNA Partitioning into Stress Granules Is Based on the Summation of Multiple Interactions. Rna 2021, 27, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Markmiller, S.; Soltanieh, S.; Server, K.L.; Mak, R.; Jin, W.; Fang, M.Y.; Luo, E.-C.; Krach, F.; Yang, D.; Sen, A.; et al. Context-Dependent and Disease-Specific Diversity in Protein Interactions within Stress Granules. Cell 2018, 172, 590–604.e13. [Google Scholar] [CrossRef]
- Yang, P.; Mathieu, C.; Kolaitis, R.M.; Zhang, P.; Messing, J.; Yurtsever, U.; Yang, Z.; Wu, J.; Li, Y.; Pan, Q.; et al. G3BP1 Is a Tunable Switch That Triggers Phase Separation to Assemble Stress Granules. Cell 2020, 181, 325–345.e28. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.W.; Kedersha, N.; Lee, D.S.W.; Strom, A.R.; Drake, V.; Riback, J.A.; Bracha, D.; Eeftens, J.M.; Iwanicki, A.; Wang, A.; et al. Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization. Cell 2020, 181, 306–324.e28. [Google Scholar] [CrossRef]
- Guillén-Boixet, J.; Kopach, A.; Holehouse, A.S.; Wittmann, S.; Jahnel, M.; Schlüßler, R.; Kim, K.; Trussina, I.R.E.A.; Wang, J.; Mateju, D.; et al. RNA-Induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation. Cell 2020, 181, 346–361.e17. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, A.; Dazert, E.; Metz, P.; Hofmann, S.; Bergeest, J.-P.; Mazur, J.; Bankhead, P.; Hiet, M.-S.; Kallis, S.; Alvisi, G.; et al. Dynamic Oscillation of Translation and Stress Granule Formation Mark the Cellular Response to Virus Infection. Cell Host Microbe 2012, 12, 71–85. [Google Scholar] [CrossRef] [PubMed]
- McInerney, G.M.; Kedersha, N.L.; Kaufman, R.J.; Anderson, P.; Liljeström, P. Importance of EIF2α Phosphorylation and Stress Granule Assembly in Alphavirus Translation Regulation. Mol. Biol. Cell 2005, 16, 3753–3763. [Google Scholar] [CrossRef]
- Le Sage, V.; Cinti, A.; McCarthy, S.; Amorim, R.; Rao, S.; Daino, G.L.; Tramontano, E.; Branch, D.R.; Mouland, A.J. Ebola Virus VP35 Blocks Stress Granule Assembly. Virology 2017, 502, 73–83. [Google Scholar] [CrossRef]
- Weissbach, R.; Scadden, A.D.J. Tudor-SN and ADAR1 Are Components of Cytoplasmic Stress Granules. RNA 2012, 18, 462–471. [Google Scholar] [CrossRef]
- Law, L.M.J.; Razooky, B.S.; Li, M.M.H.; You, S.; Jurado, A.; Rice, C.M.; Macdonald, M.R. ZAP’s Stress Granule Localization Is Correlated with Its Antiviral Activity and Induced by Virus Replication. PLoS Pathog. 2019, 15, e1007798. [Google Scholar] [CrossRef] [PubMed]
- Onomoto, K.; Jogi, M.; Yoo, J.-S.; Narita, R.; Morimoto, S.; Takemura, A.; Sambhara, S.; Kawaguchi, A.; Osari, S.; Nagata, K.; et al. Critical Role of an Antiviral Stress Granule Containing RIG-I and PKR in Viral Detection and Innate Immunity. PLoS ONE 2012, 7, e43031. [Google Scholar] [CrossRef]
- Reineke, L.C.; Lloyd, R.E. Diversion of Stress Granules and P-Bodies during Viral Infection. Virology 2013, 436, 255–267. [Google Scholar] [CrossRef]
- Deater, M.; Tamhankar, M.; Lloyd, R.E. TDRD3 Is an Antiviral Restriction Factor That Promotes IFN Signaling with G3BP1. PLoS Pathog. 2022, 18, e1010249. [Google Scholar] [CrossRef]
- Ng, C.S.; Jogi, M.; Yoo, J.-S.; Onomoto, K.; Koike, S.; Iwasaki, T.; Yoneyama, M.; Kato, H.; Fujita, T. Encephalomyocarditis Virus Disrupts Stress Granules, the Critical Platform for Triggering Antiviral Innate Immune Responses. J. Virol. 2013, 87, 9511–9522. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, D.; Ru, Y.; Bai, J.; Ren, J.; Zhang, J.; Li, L.; Liu, X.; Zheng, H. Foot-and-Mouth Disease Virus 3A Protein Causes Upregulation of Autophagy-Related Protein LRRC25 To Inhibit the G3BP1-Mediated RIG-Like Helicase-Signaling Pathway. J. Virol. 2020, 94, e02086-19. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.J.; Medina, G.N.; Rabouw, H.H.; de Groot, R.J.; Langereis, M.A.; de Los Santos, T.; van Kuppeveld, F.J.M. Foot-and-Mouth Disease Virus Leader Protease Cleaves G3BP1 and G3BP2 and Inhibits Stress Granule Formation. J. Virol. 2019, 93, e00922-18. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.J.; Langereis, M.A.; Rabouw, H.H.; Wahedi, M.; Muntjewerff, E.M.; de Groot, R.J.; van Kuppeveld, F.J.M. Essential Role of Enterovirus 2A Protease in Counteracting Stress Granule Formation and the Induction of Type I Interferon. J. Virol. 2019, 93, e00222-19. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, L.; Xu, X.; Han, H.; Li, P.; Zou, D.; Li, X.; Zheng, L.; Cheng, L.; Shen, Y.; et al. Enterovirus 71 Inhibits Cytoplasmic Stress Granule Formation during the Late Stage of Infection. Virus Res. 2018, 255, 55–67. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, S.; Zhu, C.; Liu, S.; Li, J.; Kang, J.; Wang, Z.; Wang, T. Typical Stress Granule Proteins Interact with the 3′ Untranslated Region of Enterovirus D68 To Inhibit Viral Replication. J. Virol. 2020, 94, e02041-19. [Google Scholar] [CrossRef]
- White, J.P.; Cardenas, A.M.; Marissen, W.E.; Lloyd, R.E. Inhibition of Cytoplasmic MRNA Stress Granule Formation by a Viral Proteinase. Cell Host Microbe 2007, 2, 295–305. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Lin, L.; Si, X.; Wang, T.; Zhong, X.; Tong, L.; Luan, Y.; Chen, Y.; Li, X.; et al. Protease 2A Induces Stress Granule Formation during Coxsackievirus B3 and Enterovirus 71 Infections. Virol. J. 2014, 11, 192. [Google Scholar] [CrossRef]
- Fung, G.; Ng, C.S.; Zhang, J.; Shi, J.; Wong, J.; Piesik, P.; Han, L.; Chu, F.; Jagdeo, J.; Jan, E.; et al. Production of a Dominant-Negative Fragment Due to G3BP1 Cleavage Contributes to the Disruption of Mitochondria-Associated Protective Stress Granules during CVB3 Infection. PLoS ONE 2013, 8, e79546. [Google Scholar] [CrossRef]
- Bonenfant, G.; Williams, N.; Netzband, R.; Schwarz, M.C.; Evans, M.J.; Pager, C.T. Zika Virus Subverts Stress Granules To Promote and Restrict Viral Gene Expression. J. Virol. 2019, 93, e00520-19. [Google Scholar] [CrossRef]
- Amorim, R.; Temzi, A.; Griffin, B.D.; Mouland, A.J. Zika Virus Inhibits EIF2α-Dependent Stress Granule Assembly. PLoS Negl. Trop. Dis. 2017, 11, e0005775. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Kumar, A.; Xu, Z.; Airo, A.M.; Stryapunina, I.; Wong, C.P.; Branton, W.; Tchesnokov, E.; Götte, M.; Power, C.; et al. Zika Virus Hijacks Stress Granule Proteins and Modulates the Host Stress Response. J. Virol. 2017, 91, e00474-17. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Chen, X.; Xu, F.; Wang, Y.; Shi, Y.; Li, Y.; He, J.; Zhang, P. Dengue Virus Infection Induces Formation of G3BP1 Granules in Human Lung Epithelial Cells. Arch. Virol. 2015, 160, 2991–2999. [Google Scholar] [CrossRef]
- Yi, Z.; Pan, T.; Wu, X.; Song, W.; Wang, S.; Xu, Y.; Rice, C.M.; Macdonald, M.R.; Yuan, Z. Hepatitis C Virus Co-Opts Ras-GTPase-Activating Protein-Binding Protein 1 for Its Genome Replication. J. Virol. 2011, 85, 6996–7004. [Google Scholar] [CrossRef]
- Garaigorta, U.; Heim, M.H.; Boyd, B.; Wieland, S.; Chisari, F.V. Hepatitis C Virus (HCV) Induces Formation of Stress Granules Whose Proteins Regulate HCV RNA Replication and Virus Assembly and Egress. J. Virol. 2012, 86, 11043–11056. [Google Scholar] [CrossRef] [PubMed]
- Panas, M.D.; Ahola, T.; McInerney, G.M. The C-Terminal Repeat Domains of NsP3 from the Old World Alphaviruses Bind Directly to G3BP. J. Virol. 2014, 88, 5888–5893. [Google Scholar] [CrossRef] [PubMed]
- Panas, M.D.; Varjak, M.; Lulla, A.; Eng, K.E.; Merits, A.; Hedestam, G.B.K.; McInerney, G.M. Sequestration of G3BP Coupled with Efficient Translation Inhibits Stress Granules in Semliki Forest Virus Infection. Mol. Biol. Cell 2012, 23, 4701–4712. [Google Scholar] [CrossRef]
- Foy, N.J.; Akhrymuk, M.; Akhrymuk, I.; Atasheva, S.; Bopda-Waffo, A.; Frolov, I.; Frolova, E.I. Hypervariable Domains of NsP3 Proteins of New World and Old World Alphaviruses Mediate Formation of Distinct, Virus-Specific Protein Complexes. J. Virol. 2013, 87, 1997–2010. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, H.; Ming, X.; Bo, Z.; Shin, H.-J.; Jung, Y.-S.; Qian, Y. Porcine Epidemic Diarrhea Virus Infection Induces Caspase-8-Mediated G3BP1 Cleavage and Subverts Stress Granules To Promote Viral Replication. J. Virol. 2021, 95, e02344-20. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Zhong, S.; Diel, D.G.; Hou, Y.; Wang, Q.; Nelson, E.; Wang, X. GTPase-Activating Protein-Binding Protein 1 (G3BP1) Plays an Antiviral Role against Porcine Epidemic Diarrhea Virus. Vet. Microbiol. 2019, 236, 108392. [Google Scholar] [CrossRef]
- Zheng, Z.-Q.; Wang, S.-Y.; Xu, Z.-S.; Fu, Y.-Z.; Wang, Y.-Y. SARS-CoV-2 Nucleocapsid Protein Impairs Stress Granule Formation to Promote Viral Replication. Cell Discovery 2021, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, Z.; Zhao, T.; Ju, X.; Ma, P.; Jin, B.; Zhou, Y.; He, S.; Huang, J.; Xu, X.; et al. SARS-CoV-2 Nucleocapsid Protein Phase Separates with G3BPs to Disassemble Stress Granules and Facilitate Viral Production. Sci. Bull. 2021, 66, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Ye, Q.; Singh, D.; Cao, Y.; Diedrich, J.K.; Yates, J.R.; Villa, E.; Cleveland, D.W.; Corbett, K.D. The SARS-CoV-2 Nucleocapsid Phosphoprotein Forms Mutually Exclusive Condensates with RNA and the Membrane-Associated M Protein. Nat. Commun. 2021, 12, 502. [Google Scholar] [CrossRef]
- Brocard, M.; Iadevaia, V.; Klein, P.; Hall, B.; Lewis, G.; Lu, J.; Burke, J.; Willcocks, M.M.; Parker, R.; Goodfellow, I.G.; et al. Norovirus Infection Results in EIF2α Independent Host Translation Shut-off and Remodels the G3BP1 Interactome Evading Stress Granule Formation. PLoS Pathog. 2020, 16, e1008250. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, N.; Meng, X.-J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV)-Induced Stress Granules Are Associated with Viral Replication Complexes and Suppression of Host Translation. Virus Res. 2019, 265, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fang, L.; Wang, D.; Cai, K.; Chen, H.; Xiao, S. Porcine Reproductive and Respiratory Syndrome Virus Infection Induces Stress Granule Formation Depending on Protein Kinase R-like Endoplasmic Reticulum Kinase (PERK) in MARC-145 Cells. Front. Cell. Infect. Microbiol. 2017, 7, 111. [Google Scholar] [CrossRef]
- Nelson, E.V.; Schmidt, K.M.; Deflubé, L.R.; Doğanay, S.; Banadyga, L.; Olejnik, J.; Hume, A.J.; Ryabchikova, E.; Ebihara, H.; Kedersha, N.; et al. Ebola Virus Does Not Induce Stress Granule Formation during Infection and Sequesters Stress Granule Proteins within Viral Inclusions. J. Virol. 2016, 90, 7268–7284. [Google Scholar] [CrossRef]
- Kim, S.S.-Y.; Sim, D.C.N.; Carissimo, G.; Lim, H.-H.; Lam, K.-P. Bruton’s Tyrosine Kinase Phosphorylates Scaffolding and RNA-Binding Protein G3BP1 to Induce Stress Granule Aggregation during Host Sensing of Foreign Ribonucleic Acids. J. Biol. Chem. 2022, 298, 102231. [Google Scholar] [CrossRef] [PubMed]
- Galan, A.; Lozano, G.; Piñeiro, D.; Martinez-Salas, E. G3BP1 Interacts Directly with the FMDV IRES and Negatively Regulates Translation. FEBS J. 2017, 284, 3202–3217. [Google Scholar] [CrossRef]
- Dougherty, J.D.; White, J.P.; Lloyd, R.E. Poliovirus-Mediated Disruption of Cytoplasmic Processing Bodies. J. Virol. 2011, 85, 64–75. [Google Scholar] [CrossRef]
- Zhai, X.; Wu, S.; Lin, L.; Wang, T.; Zhong, X.; Chen, Y.; Xu, W.; Tong, L.; Wang, Y.; Zhao, W.; et al. Stress Granule Formation Is One of the Early Antiviral Mechanisms for Host Cells Against Coxsackievirus B Infection. Virol. Sin. 2018, 33, 314–322. [Google Scholar] [CrossRef]
- Eckei, L.; Krieg, S.; Bütepage, M.; Lehmann, A.; Gross, A.; Lippok, B.; Grimm, A.R.; Kümmerer, B.M.; Rossetti, G.; Lüscher, B.; et al. The Conserved Macrodomains of the Non-Structural Proteins of Chikungunya Virus and Other Pathogenic Positive Strand RNA Viruses Function as Mono-ADP-Ribosylhydrolases. Sci. Rep. 2017, 7, 41746. [Google Scholar] [CrossRef] [PubMed]
- Cristea, I.M.; Carroll, J.-W.N.; Rout, M.P.; Rice, C.M.; Chait, B.T.; MacDonald, M.R. Tracking and Elucidating Alphavirus-Host Protein Interactions. J. Biol. Chem. 2006, 281, 30269–30278. [Google Scholar] [CrossRef]
- Humoud, M.N.; Doyle, N.; Royall, E.; Willcocks, M.M.; Sorgeloos, F.; van Kuppeveld, F.; Roberts, L.O.; Goodfellow, I.G.; Langereis, M.A.; Locker, N. Feline Calicivirus Infection Disrupts Assembly of Cytoplasmic Stress Granules and Induces G3BP1 Cleavage. J. Virol. 2016, 90, 6489–6501. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, Z.A.; Kieft, J.S. Viral RNA Structure-Based Strategies to Manipulate Translation. Nat. Rev. Microbiol. 2019, 17, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Ciccosanti, F.; Di Rienzo, M.; Romagnoli, A.; Colavita, F.; Refolo, G.; Castilletti, C.; Agrati, C.; Brai, A.; Manetti, F.; Botta, L.; et al. Proteomic Analysis Identifies the RNA Helicase DDX3X as a Host Target against SARS-CoV-2 Infection. Antiviral Res. 2021, 190, 105064. [Google Scholar] [CrossRef]
- Zheng, Y.; Deng, J.; Han, L.; Zhuang, M.-W.; Xu, Y.; Zhang, J.; Nan, M.-L.; Xiao, Y.; Zhan, P.; Liu, X.; et al. SARS-CoV-2 NSP5 and N Protein Counteract the RIG-I Signaling Pathway by Suppressing the Formation of Stress Granules. Signal Transduct Target Ther 2022, 7, 22. [Google Scholar] [CrossRef]
- Liu, H.; Bai, Y.; Zhang, X.; Gao, T.; Liu, Y.; Li, E.; Wang, X.; Cao, Z.; Zhu, L.; Dong, Q.; et al. SARS-CoV-2 N Protein Antagonizes Stress Granule Assembly and IFN Production by Interacting with G3BPs to Facilitate Viral Replication. J. Virol. 2022, 96, e0041222. [Google Scholar] [CrossRef]
- Lindquist, M.E.; Lifland, A.W.; Utley, T.J.; Santangelo, P.J.; Crowe, J.E. Respiratory Syncytial Virus Induces Host RNA Stress Granules To Facilitate Viral Replication. J. Virol. 2010, 84, 12274–12284. [Google Scholar] [CrossRef]
- Götte, B.; Panas, M.D.; Hellström, K.; Liu, L.; Samreen, B.; Larsson, O.; Ahola, T.; McInerney, G.M. Separate Domains of G3BP Promote Efficient Clustering of Alphavirus Replication Complexes and Recruitment of the Translation Initiation Machinery. PLoS Pathog. 2019, 15, e1007842. [Google Scholar] [CrossRef]
- McPherson, R.L.; Abraham, R.; Sreekumar, E.; Ong, S.E.; Cheng, S.J.; Baxter, V.K.; Kistemaker, H.A.V.; Filippov, D.V.; Griffin, D.E.; Leung, A.K.L. ADP-Ribosylhydrolase Activity of Chikungunya Virus Macrodomain Is Critical for Virus Replication and Virulence. Proc. Natl. Acad. Sci. USA 2017, 114, 1666–1671. [Google Scholar] [CrossRef]
- Alhammad, Y.M.O.; Kashipathy, M.M.; Roy, A.; Gagné, J.-P.; McDonald, P.; Gao, P.; Nonfoux, L.; Battaile, K.P.; Johnson, D.K.; Holmstrom, E.D.; et al. The SARS-CoV-2 Conserved Macrodomain Is a Mono-ADP-Ribosylhydrolase. bioRxiv 2020, 95, e01969-20. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.L.; McPherson, R.L.; Griffin, D.E. Macrodomain ADP-Ribosylhydrolase and the Pathogenesis of Infectious Diseases. PLoS Pathog. 2018, 14, e1006864. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.L.; Griffin, D.E.; Bosch, J.; Fehr, A.R. The Conserved Macrodomain Is a Potential Therapeutic Target for Coronaviruses and Alphaviruses. Pathogens 2022, 11, 94. [Google Scholar] [CrossRef]
- Abraham, R.; Hauer, D.; McPherson, R.L.; Utt, A.; Kirby, I.T.; Cohen, M.S.; Merits, A.; Leung, A.K.L.; Griffin, D.E. ADP-Ribosyl-Binding and Hydrolase Activities of the Alphavirus NsP3 Macrodomain Are Critical for Initiation of Virus Replication. Proc. Natl. Acad. Sci. USA 2018, 115, E10457–E10466. [Google Scholar] [CrossRef]
- Kim, D.Y.; Reynaud, J.M.; Rasalouskaya, A.; Akhrymuk, I.; Mobley, J.A.; Frolov, I.; Frolova, E.I. New World and Old World Alphaviruses Have Evolved to Exploit Different Components of Stress Granules, FXR and G3BP Proteins, for Assembly of Viral Replication Complexes. PLoS Pathog. 2016, 12, e1005810. [Google Scholar] [CrossRef]
- Fritzlar, S.; Aktepe, T.E.; Chao, Y.-W.; Kenney, N.D.; McAllaster, M.R.; Wilen, C.B.; White, P.A.; Mackenzie, J.M. Mouse Norovirus Infection Arrests Host Cell Translation Uncoupled from the Stress Granule-PKR-EIF2α Axis. MBio 2019, 10, e00960-19. [Google Scholar] [CrossRef]
- Gao, P.; Liu, Y.; Wang, H.; Chai, Y.; Weng, W.; Zhang, Y.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; et al. Viral Evasion of PKR Restriction by Reprogramming Cellular Stress Granules. Proc. Natl. Acad. Sci. USA 2022, 119, e2201169119. [Google Scholar] [CrossRef] [PubMed]
- Panas, M.D.; Schulte, T.; Thaa, B.; Sandalova, T.; Kedersha, N.; Achour, A.; McInerney, G.M. Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation. PLoS Pathog. 2015, 11, e1004659. [Google Scholar] [CrossRef]
- Katsafanas, G.C.; Moss, B. Colocalization of Transcription and Translation within Cytoplasmic Poxvirus Factories Coordinates Viral Expression and Subjugates Host Functions. Cell Host Microbe 2007, 2, 221–228. [Google Scholar] [CrossRef]
- Katsafanas, G.C.; Moss, B. Vaccinia Virus Intermediate Stage Transcription Is Complemented by Ras-GTPase-Activating Protein SH3 Domain-Binding Protein (G3BP) and Cytoplasmic Activation/Proliferation-Associated Protein (P137) Individually or as a Heterodimer. J. Biol. Chem. 2004, 279, 52210–52217. [Google Scholar] [CrossRef] [PubMed]
| Family | Species | SG Dynamics | Duration Monitored for SG Presence | eIF2alphα Phosphorylation Status | Responsible Kinase | Cell Line Tested | References |
|---|---|---|---|---|---|---|---|
| Picornaviridae | EMCV | Transient | 4, 12 hpi | *ND* | PKR | HeLa | [61] |
| FMDV | Block | 4–6 hpi | *ND* | *ND* | PK-15 | [62,63] | |
| EV | Transient | 0–24 hpi | 8 h | PKR | HeLa, RD | [64,65,66] | |
| PV | 0–6 hpi | *ND* | *ND* | HeLa, 293T, MCF7, Vero | [67] | ||
| CVB3 | 1–7 hpi | 6 h | *ND* | HeLa | [68,69] | ||
| Flaviviridae | Zika | Block | 24 hpi | 24 h | PKR | A549, Huh7, Vero | [70,71,72] |
| DENV | Unknown | 6–24 hpi | 12 h | PKR | A549, Huh7 | [21,73] | |
| HCV | Oscillating | 0–96 hpi | 24 h | PKR | Huh7, HEK293T | [53,74,75] | |
| Togaviridae | CHIKV | Transient | 0–12 hpi | 6 h | *ND* | U2OS, HEK293, Vero | [23,47] |
| SFV | Transient | 2–8 hpi | 5 h | *ND* | MEF | [54,76,77] | |
| SINV | Transient | 6 h | PKR | MEF | [78] | ||
| Coronaviridae | PEDV | Transient | 0–36 hpi | *ND* | *ND* | Vero E6, Vero-76 | [79,80] |
| SARS-CoV-2 | Block | 0, 10, 24 hpi | *ND* | PKR | HeLa | [81,82,83] | |
| Caliciviridae | MNV | Unknown | 9, 12 hpi | 9 h | PKR, GCN2 | BMDM, BV2, RAW264.7 | [31,84] |
| Arteriviridae | PRRSV | Stable | 12–48 hpi | 12 h | PERK | MARK-145 | [85,86] |
| Filoviridae | EVD | Block | 0.5–24 hpi | *ND* | *ND* | U2OS, Vero, Huh7 | [55,87] |
| Family | Species | G3BP1 Status | Role of G3BP1 | Effect of Viral Protein/RNA or Titer Value | Interaction with Viral Protein | Interaction with Viral RNA | Proposed Mechanism of Action | References | |
|---|---|---|---|---|---|---|---|---|---|
| G3BP1 KD | G3BP1 OE | ||||||||
| Picornaviridae | EMCV | Cleavage | Anti-viral | ↑ | *ND* | 3C protease cleaves G3BP1 at Q325 | [61] | ||
| FMDV | ↑ | ↓ | 3A | Interacts with IRES | Leader protein cleaves G3BP1, G3BP1 dephosphorylated, G3BP1 binds to FMDV IRES region, 3A protein degrades G3BP1 through autophagy | [62,63,89] | |||
| EV | ↑ | ↓ | Interacts with 3′UTR | 3C proteinase cleaves G3BP1 at Q326 | [64,65,66] | ||||
| PV | *ND* | ↓ | 3C protease cleaves G3BP1, but not G3BP2, at Q326 | [67,90] | |||||
| CVB3 | ↑ | ↓ | 3C protease cleaves G3BP1 at Q325 | [69,91] | |||||
| Flaviviridae | Zika | Sequestration | Pro-viral | ↓ | ↑ | Interacts with Capsid, colocalizes with envelope protein | Interacts with genomic RNA, localize with replication complexes | Sequester G3BP1 and facilitates viral replication | [70,71,72] |
| DENV | Anti-viral | ↑ | *ND* | Subgenomic viral RNA binds to G3BP1 and antagonizes its function | [21,73] | ||||
| HCV | Pro-viral | ↓ | *ND* | NS5B | Localizes to vRC | G3BP1 requires at early and late stages of infection | [53,74,75] | ||
| Togaviridae | CHIKV | Sequestration | Pro-viral | ↓ | *ND* | nsP3 | yes | Binding through FGDF motif, Reduction G3BP1 ADP-ribosylation | [23,47,92] |
| SFV | ↓ | *ND* | nsP3 | yes | Binds through FGDF motif; recruits ribosomal proteins to nsP3 | [54,76,77] | |||
| SINV | ↓ | *ND* | nsP3, nsP4 | Colocalizes with vRNA | Binding through FGDF motif block SG assembly | [78,93] | |||
| Coronaviridae | PEDV | Cleavage | Anti-viral | ↑ | ↓ | Caspase-8-mediated G3BP1 cleavage at Asp168 and Asp169 at late infection stages | [79,80] | ||
| SARS-CoV-2 | Sequestration | Both proviral & antiviral roles have been reported | ↑ | *ND* | N protein, nsP1 | N protein interacts/phase separates with G3BP1 | [81,82,83] | ||
| Caliciviridae | MNV | Sequestration | Pro-viral | ↓ | *ND* | NS3, VPg | Colocalizes with vRCs | Remodels G3BP1 interactome, doesn’t affect SGs | [31,84,94] |
| FCV | Cleavage | *ND* | NS6-mediated G3BP1 cleavage at E405 | ||||||
| Arteriviridae | PRRSV | *ND* | Not involved | No changes | G3BP1 closely associated with vRCs | [85,86] | |||
| Filoviridae | EVD | Sequestration | *ND* | *ND* | VP5 | Sequestered within viral inclusions | [55,87] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayabalan, A.K.; Griffin, D.E.; Leung, A.K.L. Pro-Viral and Anti-Viral Roles of the RNA-Binding Protein G3BP1. Viruses 2023, 15, 449. https://doi.org/10.3390/v15020449
Jayabalan AK, Griffin DE, Leung AKL. Pro-Viral and Anti-Viral Roles of the RNA-Binding Protein G3BP1. Viruses. 2023; 15(2):449. https://doi.org/10.3390/v15020449
Chicago/Turabian StyleJayabalan, Aravinth Kumar, Diane E. Griffin, and Anthony K. L. Leung. 2023. "Pro-Viral and Anti-Viral Roles of the RNA-Binding Protein G3BP1" Viruses 15, no. 2: 449. https://doi.org/10.3390/v15020449
APA StyleJayabalan, A. K., Griffin, D. E., & Leung, A. K. L. (2023). Pro-Viral and Anti-Viral Roles of the RNA-Binding Protein G3BP1. Viruses, 15(2), 449. https://doi.org/10.3390/v15020449





