Abstract
Uveitis is one of the most common ocular complications in people living with the Human immunodeficiency virus (HIV) and can be classified into HIV-induced uveitis, co-infection related uveitis, immune recovery uveitis, and drug-induced uveitis. The introduction of antiretroviral therapy has considerably changed the incidence, diagnosis, and treatment of different types of HIV-related uveitis. Furthermore, the specific immune condition of patients infected with HIV makes diagnosing HIV-related uveitis difficult. Recent studies have focused on the growing prevalence of syphilis/tuberculosis co-infection in uveitis. Simultaneously, more studies have demonstrated that HIV can directly contribute to the incidence of uveitis. However, the detailed mechanism has not been studied. Immune recovery uveitis is diagnosed by exclusion, and recent studies have addressed the role of biomarkers in its diagnosis. This review highlights recent updates on HIV-related uveitis. Furthermore, it aims to draw the attention of infectious disease physicians and ophthalmologists to the ocular health of patients infected with HIV.
1. Introduction
Human immunodeficiency virus (HIV) infection is a retrovirus-induced multisystemic disease. In 1981, the first HIV case was identified in Los Angeles, and HIV had infected more than 38 million people by 2019 [1]. At the peak of the pandemic, HIV resulted in the death of >1.7 million people in 2005 [2]. Over the past two decades, the incidence of HIV infection worldwide has stabilized due to the introduction of antiretroviral therapy (ART). However, in developing countries, especially those in sub-Saharan Africa, HIV infection is still a great burden. HIV prevalence in sub-Saharan Africa is approximately 5%, which is much greater than that in other regions [3]. Moreover, in 2019, less than half of the people living with HIV (PLWH) had suppressed viral loads in >28% of countries, indicating that they were at risk of transmitting the virus [3].
The survival time of patients with HIV is increasing with the application of ART. However, their vision quality has become a matter of concern. Almost all patients with HIV complain of ocular manifestations that cause trouble in their daily life [4,5]. Uveitis is the main cause of blindness and is a common ocular complication of HIV [4,6]. The outcomes of HIV-associated uveitis are not ideal. Hence, ophthalmologists should be aware of this disease, improve patients’ awareness, and achieve the best management to prevent vision loss. This paper focuses on the latest developments in the different causes of uveitis in patients with HIV.
2. Prevalence of HIV and Its Related Ocular Compilations
More than half of HIV-infected patients present with ocular manifestations. Although this number has rapidly decreased since the introduction of ART, it remains a serious problem for patients with HIV. Ocular complications of patients with HIV can occur at any stage and segment in the eye [7], involving the eyelids, eyelashes, and anterior and posterior eye segments. The prevalence of different ocular manifestations varies in different regions, with the most common presentations including dry eyes, cytomegalovirus (CMV) retinitis, uveitis, and keratitis [4,7,8]. CMV retinitis is an acquired immunodeficiency syndrome (AIDS)-defining opportunistic infection. In the pre-ART era, CMV retinitis was the most common ocular manifestation of HIV infection, with a prevalence of 30% [9]. However, with the emergence of ART, the burden of CMV retinitis has decreased by >95% [10], although it is still one of the most common ocular manifestations in PLWH [4,11,12,13]. Actually, the involvement of the anterior segment is more common than that of the posterior segment in the post-ART era. According to a study from Tunisia, dry eye (8%) is the most common manifestation in the anterior segment [14]. The prevalence of uveitis in patients with HIV is 1.1–24% [4,7,14]. However, considering that two-thirds of PLWH live in HIV high-burden countries [3], where the awareness of uveitis still needs to be improved, it is still an emergency for ophthalmologists to have more access to patients with HIV-related uveitis.
Compared with HIV-negative patients, HIV-positive patients have a five-to-six-fold higher chance of being diagnosed with uveitis [15]. It has been well demonstrated that HIV could enter the central nervous system (CNS) and replicate within ocular tissue [16,17]. Meanwhile, the depletion of CD4+ T cells induced by HIV and consequent opportunistic infections and co-infections have increased the prevalence of uveitis as an HIV-related ocular complication [18]. However, patients may only complain of decreased vision at the onset of uveitis; thus, it is easily neglected by the clinician, leading to blindness. The causes of uveitis in HIV infection can be divided into four main categories: HIV-induced uveitis, co-infections-related uveitis, immune recovery uveitis (IRU), and drug-induced uveitis.
3. Causes of Uveitis and Its Clinical Manifestation
3.1. HIV-Induced Uveitis
It has been demonstrated that HIV can break the blood-brain barrier (BBB) and consequently cause nerve degeneration. As early as 1988, detectable HIV viral loads were found in ocular tissues [19]. HIV was found in the vitreous, retina, iris, conjunctiva, and even tears [20,21,22], indicating that the virus could cross the blood-retina barrier and enter the intraocular space. Several recent studies have focused on the intraocular replication of HIV. Rothova et al. found that the intraocular HIV-1 RNA viral load largely exceeds the plasma HIV-1 RNA load in HIV patients with uveitis and no evidence of other antigens causing uveitis [17]. A research from 2012 has demonstrated the clinical manifestations of HIV-induced uveitis. All six patients had mild anterior uveitis, and none received ART. Inflammation is sensitive to ART rather than traditional steroid treatment [23]. According to research from Thailand, intraocular HIV viral load was detectable in 32% of uveitis patients with HIV [22]. It is worth noting that four of them had higher intraocular HIV viral loads than plasma, and intraocular HIV viral loads rapidly decreased after ART, suggesting that uveitis was caused directly by HIV. Plasma viral load and breakdown of the BBB are suggested to be positive factors of intraocular viral load. However, they did not find a correlation between CD4+ T cells and intraocular viral loads. Furthermore, this implies that HIV can replicate within the ocular space and cause uveitis. Overall, when patients with HIV who have not received ART show uveitis manifestation, especially anterior uveitis, or uveitis without retinal manifestation, HIV-induced uveitis should be suspected.
The HIV Tat protein, a transactivator protein, is important for HIV replication and infection. Research has confirmed that the HIV Tat protein participates in the breakdown of the blood-retina barrier [24]. In addition, within the ocular environment, HIV Tat induces the death of retinal microvascular endothelial cells via N-methyl-D-aspartate receptors, activates Muller cells, and increases pro-inflammatory cytokines [25]. However, further investigations of the detailed mechanism are required.
3.2. Co-Infection-Related Uveitis
3.2.1. Syphilitic Uveitis
Syphilis incidence has been reported to increase since the beginning of the 20th century in both developed and developing countries [26], especially among males who have sex with males [27]. Over the last two decades, this increased incidence was primarily due to high-risk sexual behavior [28]. PLWH have a 77 times higher chance of being co-infected with syphilis than HIV-negative individuals, according to a report from the United States [29]. Syphilitic uveitis is one of the most common ocular complications of syphilis; as many as 9% of patients with HIV/syphilis co-infection have ocular manifestation [30,31], while according to a study from The Netherlands, 58.8% of patients with syphilis complain of blurred vision that affects driving [32]. Considering the high prevalence of HIV in areas with a high prevalence of syphilis infection, the number of HIV/syphilis co-infected patients is sizable. Hence, syphilitic uveitis in patients with HIV deserves more attention.
Syphilis serologic testing involves treponemal and nontreponemal tests. Treponemal tests detect infection by testing immunoglobulin (Ig)M and IgG directly against T. pallidum, while nontreponemal tests are indirect tests that measure biomarkers released from cells damaged by T. pallidum. Although treponemal tests are more sensitive than nontreponemal tests during early infection, they are more complicated [33]. Serologic diagnosis of syphilis requires two positive tests, at least one of which must be a treponemal test. Typically, serological testing for syphilis is based on initial screening with a non-treponemal test and confirmation with a treponemal test if the screening result is positive. In recent two decades, reserve sequence screening, a newer treponemal test, has been used in some laboratories. Reserve sequence screening is based on the application of automatable treponemal enzyme and chemiluminescence immunoassays (EIA/CIA). Considering its advantages of simple operation and lower price, reserve sequence screening is typically performed as the first test. Reserve sequence screening is more sensitive in the early or latent stage [34]. However, compared with non-treponemal tests, the reserve sequence screening algorithm has a greater potential for false positive results [35]. Therefore, the Centers for Disease Control and Prevention recommends that a specimen with a positive result in reverse sequence screening should be tested again with a quantitative nontreponemal test [34,36,37].
As the name of “the great imitator” indicates, the clinical findings of syphilitic uveitis are highly diverse and nonspecific. Any segment or disease stage may be associated with syphilitic uveitis. Thus, diagnosing syphilitic uveitis is difficult, and it is easily overlooked and misdiagnosed. The involvement of panuveitis and posterior uveitis is the most common manifestation of syphilitic uveitis (Figure 1). In contrast, anterior uveitis is more common among HIV-negative patients with syphilis [27]. Chorioretinitis and bilateral involvement are also more common in syphilitic uveitis than in general uveitis [27,32,38,39,40]. Serological testing is useful for diagnosing syphilitic uveitis, while patients with HIV might need more time to respond to the test [41]. Co-infection with HIV and syphilis is common. Hence, routine testing for either virus is highly recommended for patients diagnosed with HIV or syphilis.
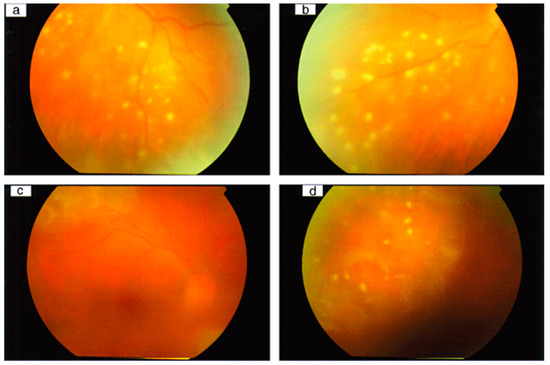
Figure 1.
Syphilitic panuveitis patients with punctuate inner retinitis. Color fundus photographs of the left eye of a patient with syphilis, showing (a) punctuate inner retinitis overlying deeper retinal inflammation inferotemporally and (b) inferonasally without deeper involvement. Color fundus photographs of the right eye of another patient with syphilis, showing (c) posterior pole and (d) superior views with inner punctuate retinitis and deep diffuse retinitis superior to the superotemporal arcade, with ARTerial sheathing. (Adapted with permission from Ref. [27]. 2010, Edward H Hughes et al.).
The majority of uveitis clinicians choose antibiotic treatments for their patients. The most commonly used antibiotic is intravenous penicillin, continued for 10–14 days or longer depending on the patient’s status [27,42]. Administering ART does not seem to prevent syphilis infection; however, when combined with intravenous penicillin, ART could help relieve early manifestations [43,44]. Steroids were administered to selected patients. However, a study showed that administering steroids as local injections may even worsen the final outcomes, although this may be related to the time frame between antibiotic treatment and steroids [45]. Syphilitic uveitis is often accompanied by syphilitic retinitis and ocular neurosyphilis, which are risk factors for adverse outcomes [25]. Better baseline best-corrected distance visual acuity (BCVA) and visual improvement in the early stages were significantly correlated with better outcomes. Therefore, early diagnosis and treatment are vital for the outcomes [46].
3.2.2. Tubercular Uveitis (TBU)
HIV spread in Africa began in the 1970s. Currently, about two-thirds of patients with HIV live in Sub-Saharan Africa [47], which is also an area with a high prevalence of tuberculosis (TB). HIV infection is an independent risk factor for active TB [48], and from the World Health Organization (WHO) report in 2013, M. tuberculosis co-infection was the leading cause of death in patients with HIV. Meanwhile, compared with immunocompetent people, patients with HIV, especially those whose CD4+ T cell count is <200 cells/µL, have a two to three times higher chance of getting TBU with more rapid progress [49,50]. By 2010, approximately 14 million individuals had HIV and TB co-infection [50]. In fact, due to the absence of authoritative uniform guidelines for diagnosing TBU and false-negative results caused by the low CD4+ T cell count, the number of HIV and TB co-infection cases might be underestimated [51].
The detection of M. tuberculosis from aqueous/vitreous sampling or detection of the M. tuberculosis gene (MPB64 or IS6110) with PCR testing is the most affirmative evidence for diagnosing TBU. However, it is difficult to confirm microbiologic evidence from such limited materials. Furthermore, most studies’ diagnostic criteria for TBU included ocular manifestations suggestive of uveitis, tuberculosis-related systematic manifestation (caught, fever, night sweats, and weight loss), and laboratory investigations suggesting TB infection [52,53,54]. The prevalence of posterior segment involvement and panuveitis in TBU was higher than that in general uveitis (Figure 2). Compared with non-TBU uveitis, chronic uveitis is also more common in patients with TBU.

Figure 2.
Fundus photograph of the left eye at presentation showing a chorioretinal granuloma, papilledema, retinal vasculitis, and minimal vitritis. (Adapted with permission from Ref [64]. 2014, Erik Schaftenaar et al.).
Commonly used laboratory investigation methods include the tuberculin skin test (TST) and interferon-gamma release assay (IGRA). However, the sensitivity and specificity of the TST are not satisfactory for diagnosing TBU. According to previous reports, the general sensitivity of the TST is between 7.59–87.0% [52,54]. However, this number drops to 17.2% in PLWH. Hence, a positive TST result in a PLWH could only suggest that this patient has a higher risk of developing TB and does not necessarily indicate active TB disease, especially for those with CD4+ T cell counts <200 cells/mm3 [55,56]. IGRA may be the best available laboratory investigation for TB since its sensitivity ranges from 65% to 93%, and its specificity is up to 99% [57,58]. However, IGRA is still unideal for patients with HIV. The presence of HIV reduces the sensitivity of IGRA to 63% [59]. Mainly, this is because IGRA depends on the immune response to the M. tuberculosis antigen, and the specificity of this test is decreased due to cross-reactivity with other co-infection viruses. According to La Distia Nora et al., compared with the idiopathic uveitis group, the TBU group had less positive QFT-G results, and the positive result was more related to previous or latent infection of M. tuberculosis instead of active TB lesions [53]. Other systemic investigations, such as chest computed tomography scans, have also provided important tips for diagnosing TBU.
Antituberculosis Treatment (ATT) with or without steroids is highly recommended for the treatment of TBU. A study in 2018 showed that ATT treatment was beneficial, even for HIV-infected patients and latent TB [60,61]. Experts recommend that the decision to start ATT should depend on the phenotypes of TBU and the incidence of TB in the patients’ geographical region [62]. Steroids could be administered as an adjunct therapy, especially for patients with signs of an autoimmune reaction to the molecular mimicry of latent TB [52]. Prophylactic treatment with isoniazid is recommended for patients with HIV regardless of whether the TST result is positive for potential lifesaving benefits [56]. Considering the interaction between ART and Rifampicin, clinicians should assess whether ART is needed before ATT [63].
3.3. Immune Recovery Uveitis
Although it has reduced the prevalence of opportunistic infections, ART has also increased the incidence of immune reconstitution inflammatory syndrome (IRIS). The prevalence of IRIS from global research is unavailable, and the number based on a single study is significantly different. Approximately 10–32% of patients with AIDS who received ART experienced IRIS [65,66]. IRU is one of the most common ocular manifestations of IRIS. It is defined as any new inflammation in an eye with controlled CMV retinitis or other ocular infections, not attributable to an alternative cause, following substantial recovery of immunity [12]. Currently, IRU has become an important cause of vision loss in patients with HIV who received ART [67].
The pathology of IRU is not fully understood, but studies have suggested that it is associated with recovering the immune response to CMV infection. Destruction of existing CD4+ T cells and the inability to activate new CD4+ T cells caused by HIV infection reduced the absolute CD4+ T cell count and increased the risk of opportunistic infection [18]. CMV, the most common opportunistic infection, causes a break in the blood-retina barrier and intraocular inflammation [68]. Treatment with ART effectively inhibited HIV infection and increased CD4+ T cell counts. With the recovery of immune function, the immune system can respond to existing antigens such as CMV and cause uveitis, which is also why CMV retinitis and IRU commonly co-exist.
Since IRU is closely related to CMV retinitis, the diagnosis of CMV retinitis should be mentioned. The diagnosis of CMV retinitis is simple, as the clinical features of CMV retinitis are easily recognized. An experienced ophthalmologist diagnoses CMV retinitis through indirect ophthalmoscopy by observing the characteristic patterns of CMV retinitis: (1) the “pizza” or “cottage cheese with ketchup retinopathy” presentation, which is characterized by centripetal necrotic retinal areas with associated hemorrhage. This is the most common presentation of CMV retinitis; (2) variable small dot-like lesions; and (3) retinal vasculitis with perivascular sheathing [69,70]. CMV retinitis causes full-thickness retinal necrosis, which leaves an atrophic and gliotic scar [71]. Compared with other opportunistic pathogens that involve the retina and choroid, CMV retinitis is more likely to be accompanied by minimal vitritis and iritis, following massive retinal necrosis, and fine, non-granulomatous, karetic precipitates on the corneal epithelium [72]. Early diagnosis is an effective way to reduce the incidence of blindness caused by CMV retinitis and the risk of IRU. However, in many HIV high-burden countries, a shortage of ophthalmologists is a prevailing problem. A study from Thailand showed that remote CMV retinitis diagnosis by trained nonexpert clinicians is satisfactory, and this might solve the ophthalmologist shortage [73].
Recently, few studies have focused on biomarkers for the diagnosis of IRU. Currently, the risk factors most associated with IRU are HIV viral load and CD4+ T cell count [74]. Duraikkannu et al. found that the expression of miRNA-192 in patients with IRU was significantly decreased, which might help us identify the status of PLWH [75]. Additionally, three metabolites were identified as the most specific indices to distinguish IRIS before ART: (i) oxidized cysteinyl-glycine (Cys-Gly Oxidized), (ii) 1-myristoyl-2-palmitoyl GPC (14:0/16:0), and (iii) the sulfate of piperine metabolite (C18H21NO3). However, after 1 month of ART, quinolinate, gluconate, and serine were the indices driving the distinction [76]. In addition, plasma tumor necrosis factor-α and mucin domain 3 levels were significantly increased after ART in HIV-infected patients with IRIS [77].
Typically, ART is initiated as soon as HIV is diagnosed. However, a study in 2005 demonstrated that delayed ART reduced the incidence and severity of CMV-associated IRU [78]. Controlling CMV retinitis before starting ART can significantly reduce the occurrence and severity of IRU [79]. Therefore, continuing anti-CMV treatment to minimize lesions until the immune system is strong enough to control retinitis is necessary [80]. Most experts recommend starting ART within one to two weeks after anti-CMV treatment [71]; however, further research is required to support this recommendation. Once an IRU is present, most clinicians recommend local or systemic steroid treatment based on the position and degree of ocular inflammation. However, with the application of steroids, TBU should be monitored because steroid use is an important risk factor for the occurrence of TBU [81,82].
3.4. Drug-Related Uveitis
Only 0.5% of uveitis cases are caused by drugs, [83] and related reports are limited. HIV-related uveitis generally presents as posterior segment disease. However, drug-induced uveitis in patients with HIV is mostly anterior uveitis.
Most drug-induced uveitis cases are caused by cidofovir and rifabutin, although the use of these two drugs has greatly reduced in the ART era. Cidofovir is effective for treating CMV retinitis and is widely used in patients with HIV. However, there are reports on the occurrence of uveitis after using cidofovir. Anterior uveitis occurred in 26% of patients who used cidofovir [84]. Evidence supporting cidofovir-induced uveitis includes dose-related uveitis manifestation and probenecid, a drug against cidofovir, which is effective for cidofovir-induced uveitis [85,86]. Reduced intraocular pressure (IOP) can be observed in the development of cidofovir-induced uveitis. Thus, the administration of cidofovir should be assessed in patients with HIV with a low IOP. Once cidofovir-induced uveitis is diagnosed, steroids and cycloplegic/mydriatic agents can be administered as treatment [87]. Cidofovir-induced uveitis is more likely to occur in the eye with previous CMV disease [88], indicating the development of cidofovir-induced uveitis is related to immune recovery. The use of cidofovir could stimulate the immune system and thus increase the number of CD4+ T cells, causing ocular inflammation and leading to uveitis [89]. The stage of cidofovir-induced uveitis emergence is close to that of IRU; thus, cidofovir-induced uveitis is likely to be overlooked or misdiagnosed. However, the clinical manifestation of cidofovir-induced uveitis is quite different from that of IRU. Compared with cidofovir-induced uveitis, anterior or intermediate uveitis is more likely to be present in IRU, while posterior synechiae or granulomatous keratic precipitates are more common in cidofovir-induced uveitis.
Rifabutin is commonly administered to treat Mycobacterium avium intracellular complex infection in patients with HIV whose CD4+ T cell count is <100 cells/µL. Recently, Nair et al. reported the occurrence of uveitis following rifabutin treatment as a complication of IRIS in patients with HIV [90]. The underlying mechanism of rifabutin-induced uveitis is related to drug toxicity and immune responses. Drug toxicity was directly proven by dose-dependent manifestation and recovery after adjusting the rifabutin dosage. The occurrence of uveitis after rifabutin administration is highly associated with combination treatment with clarithromycin [91,92], which is a CYP3A inhibitor that increases serum levels of rifabutin [93]. Therefore, a daily dose of <150 mg is administered when a protease inhibitor is combined with rifabutin. Rifabutin-induced uveitis is typically treated with corticosteroids and mydriatics with satisfactory outcomes.
4. Occupational Exposure to HIV in Ophthalmology
As the number of PLWH increases, healthcare workers, especially those in developing countries, are frequently at risk of exposure to the patients’ blood and body fluids. In general, the risk of HIV transmission from patients to healthcare workers is 0.3% [94]. The risk of HIV transmission through needlestick injury is approximately 0.3% [95], and the risk through mucous membrane exposure is 0.1%. Exposure to a large amount of blood from patients with late AIDS increases the risk of transmission, as exposure to blood is more dangerous than exposure to other body fluids, and serum viral load is an important risk factor [96,97]. More than 20% of patients living in parts of Southern Africa who needed surgical procedures were HIV positive, and surgeons have an average of three HIV exposures per person, per year [98]. In 2005, the WHO reported that approximately 3 million percutaneous exposures to healthcare workers occur annually, and the most common exposure is through accidental puncture by a needle or other sharp devices [99]. Furthermore, according to research from India, only half of the occupational exposure was reported [100]. Therefore, the risk of HIV exposure to healthcare workers might be largely underestimated, and healthcare workers, especially surgeons, are considered a high-risk population of HIV infection.
Although ophthalmologists are not generally considered to be at high risk of HIV infection, they should be aware of HIV protection. Ophthalmologists can be exposed to HIV through mucous membrane contact during medical treatment, drainage of aqueous/vitreous humor, injection procedure, and surgery. Therefore, it is necessary for ophthalmologists to improve their awareness of occupational exposure to HIV. Further research is needed on the extent of ophthalmologists’ exposure to HIV and the prevention measures.
Most clinicians from developing countries are reported to lack knowledge about occupational exposure and protection measures against HIV [101]. All healthcare workers, including ophthalmologists, should be trained on standard prevention measures for occupational exposure. Increased awareness will reduce HIV-related stigma and discrimination among healthcare workers. Furthermore, health and safety guidelines should be placed in the working station or easily visible places for quick reference. Healthcare workers should be provided with personal protective equipment (PPE), such as boots, aprons, masks, and glasses, to prevent exposure to body fluids during an operation. It is appalling that healthcare workers in public hospitals in developing countries reuse PPE due to resource constraints, and this problem was exacerbated by the coronavirus disease pandemic [102]. For healthcare workers, proper handling of infectious materials and adhering to the correct waste disposal protocol is a common but easily overlooked problem [102]. Almost half of the healthcare workers will not report an incident of exposure to HIV [103], which negates the use of post-exposure prophylaxis (PEP). Nevertheless, ART remains a priority for protecting healthcare workers from HIV infection, and one should take PEP within 72 h after HIV exposure. Overall, a standard occupation protocol should be established, and healthcare workers should be encouraged to adhere to it.
5. Conclusions
As ART becomes widespread and the survival of patients with HIV increases, quality of life has become a new issue of concern. The causes of uveitis in patients with HIV could be complex. Hence, raising awareness of uveitis and its clinical manifestation is important for both patients with HIV and infectious disease physicians. When patients with HIV present with uveitis-related changes, a complete systematic assessment should be started, including the duration and treatment of HIV, CD4+ T cell count, HIV viral loads, and serological tests. Early diagnosis and treatment can help achieve optimal outcomes and a higher quality of life for patients with HIV. Although occupational exposure to HIV is very rare, ophthalmologists should still be familiar with the precautions and management of occupational exposure to HIV.
Author Contributions
Conceptualization, K.K.; Writing—Original Draft Preparation, M.Y.; Writing—Review and Editing, K.K., Y.Z., J.Z. and K.O.-M.; Funding acquisition, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI (grant no. JP 20K09824), a Grant on Rare and Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan (grant no. 22FC0201), and a Research Program on Emerging and Re-emerging Infectious Disease grant form Japan Agency for Medical and Development, AMED (grant no. JP21fk0108124j).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data related to this study are presented and published here.
Conflicts of Interest
The authors declare no conflict of interest.
References
- HIV/AIDS JUNPo. 2020 Global AIDS Update Seizing the Moment-Tackling Entrenched Inequalities to End Epidemics. Available online: https://www.unaids.org/en/resources/documents/2020/global-aids-report (accessed on 20 January 2023).
- Murray, C.J.; Ortblad, K.F.; Guinovart, C.; Lim, S.S.; Wolock, T.M.; Roberts, D.A.; Dansereau, E.A.; Graetz, N.; Barber, R.M.; Brown, J.C.; et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 1005–1070. [Google Scholar] [CrossRef] [PubMed]
- Delpech, V. The HIV epidemic: Global and United Kingdom trends. Medicine 2022, 50, 202–204. [Google Scholar] [CrossRef]
- Arora, R.; Sandhu, N.; Dokania, P.; Subramanian, A. Ocular Manifestations in Patients of HIV(Human Immunodeficiency Virus) Infection on Combined Anti-Retroviral Therapy (CART). Ocul. Immunol. Inflamm. 2021, 30, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Hodge, W.G.; Seiff, S.R.; Margolis, T.P. Ocular opportunistic infection incidences among patients who are HIV positive compared to patients who are HIV negative. Ophthalmology 1998, 105, 895–900. [Google Scholar] [CrossRef] [PubMed]
- de-la-Torre, A. Virus-Induced Anterior Uveitis (VIAU) in Immunocompromised Patients. Ocul. Immunol. Inflamm. 2018, 26, 807–817. [Google Scholar] [CrossRef]
- Becker, K.N.; Becker, N.M. Ocular manifestations seen in HIV. Dis. Mon. 2014, 60, 268–275. [Google Scholar] [CrossRef]
- Saini, N.; Hasija, S.; Kaur, P.; Kaur, M.; Pathania, V.; Singh, A. Study of prevalence of ocular manifestations in HIV positive patients. Nepal. J. Ophthalmol. 2019, 11, 11–18. [Google Scholar] [CrossRef]
- Hoover, D.R.; Peng, Y.; Saah, A.; Semba, R.; Detels, R.R.; Rinaldo, C.R., Jr.; Phair, J.P. Occurrence of cytomegalovirus retinitis after human immunodeficiency virus immunosuppression. Arch. Ophthalmol. 1996, 114, 821–827. [Google Scholar] [CrossRef]
- Sugar, E.A.; Jabs, D.A.; Ahuja, A.; Thorne, J.E.; Danis, R.P.; Meinert, C.L. Incidence of cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am. J. Ophthalmol. 2012, 153, 1016–1024.e1015. [Google Scholar] [CrossRef]
- Holland, G.N. AIDS and ophthalmology: The first quarter century. Am. J. Ophthalmol. 2008, 145, 397–408. [Google Scholar] [CrossRef]
- Sudharshan, S.; Nair, N.; Curi, A.; Banker, A.; Kempen, J.H. Human immunodeficiency virus and intraocular inflammation in the era of highly active anti retroviral therapy—An update. Indian J. Ophthalmol. 2020, 68, 1787–1798. [Google Scholar] [CrossRef]
- Sadik, M.T.; Aksu Ceylan, N.; Cebeci, Z.; Kir, N.; Oray, M.; Tugal-Tutkun, I. Patterns of cytomegalovirus retinitis at a tertiary referral center in Turkey. Int. Ophthalmol. 2021, 41, 2981–2992. [Google Scholar] [CrossRef] [PubMed]
- Saadouli, D.; Ammari, L.; Ben Mansour, K.; Yahyaoui, Y.; Aissa, S.; Mohamed Ali, E.A.; Yahyaoui, S.; Tiouri, H. Ocular manifestations of people living with HIV in Tunisia. South. Afr. J. HIV Med. 2021, 22, 1193. [Google Scholar] [CrossRef]
- Rothova, A.; Hajjaj, A.; de Hoog, J.; Thiadens, A.; Dalm, V. Uveitis causes according to immune status of patients. Acta Ophthalmol. 2019, 97, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Persidsky, Y.; Poluektova, L. Immune privilege and HIV-1 persistence in the CNS. Immunol. Rev. 2006, 213, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Rothova, A.; Schneider, M.; de Groot-Mijnes, J.D. Human immunodeficiency virus-induced uveitis: Intraocular and plasma human immunodeficiency virus-1 RNA loads. Ophthalmology 2008, 115, 2062–2064. [Google Scholar] [CrossRef]
- Nussenblatt, R.B.; Lane, H.C. Human immunodeficiency virus disease: Changing patterns of intraocular inflammation. Am. J. Ophthalmol. 1998, 125, 374–382. [Google Scholar] [CrossRef]
- Cantrill, H.L.; Henry, K.; Jackson, B.; Erice, A.; Ussery, F.M.; Balfour, H.H., Jr. Recovery of human immunodeficiency virus from ocular tissues in patients with acquired immune deficiency syndrome. Ophthalmology 1988, 95, 1458–1462. [Google Scholar] [CrossRef]
- Pham, V.T.; Wen, L.; McCluskey, P.; Madigan, M.C.; Penfold, P.L. Human retinal microglia express candidate receptors for HIV-1 infection. Br. J. Ophthalmol. 2005, 89, 753–757. [Google Scholar] [CrossRef]
- Rummelt, V.; Rummelt, C.; Jahn, G.; Wenkel, H.; Sinzger, C.; Mayer, U.M.; Naumann, G.O. Triple retinal infection with human immunodeficiency virus type 1, cytomegalovirus, and herpes simplex virus type 1. Light and electron microscopy, immunohistochemistry, and in situ hybridization. Ophthalmology 1994, 101, 270–279. [Google Scholar] [CrossRef]
- Pathanapitoon, K.; Riemens, A.; Kongyai, N.; Sirirungsi, W.; Leechanachai, P.; Ausayakhun, S.; Kalinina Ayuso, V.; Kunavisarut, P.; de Groot-Mijnes, J.D.; Rothova, A. Intraocular and plasma HIV-1 RNA loads and HIV uveitis. AIDS 2011, 25, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kunavisarut, P.; Sirirungsi, W.; Pathanapitoon, K.; Rothova, A. Clinical manifestations of human immunodeficiency virus-induced uveitis. Ophthalmology 2012, 119, 1455–1459. [Google Scholar] [CrossRef]
- Che, X.; He, F.; Deng, Y.; Xu, S.; Fan, X.; Gu, P.; Wang, Z. HIV-1 Tat-mediated apoptosis in human blood-retinal barrier-associated cells. PLoS ONE 2014, 9, e95420. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, G.; Chatterjee, N. Differential immune mechanism to HIV-1 Tat variants and its regulation by AEA [corrected]. Sci. Rep. 2015, 5, 9887. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.E.; Aubin, M.; Atwell, L.; Matthias, J.; Cope, A.; Mobley, V.; Goode, A.; Minnerly, S.; Stoltey, J.; Bauer, H.M.; et al. Ocular Syphilis—Eight Jurisdictions, United States, 2014–2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1185–1188. [Google Scholar] [CrossRef]
- Hughes, E.H.; Guzowski, M.; Simunovic, M.P.; Hunyor, A.P.; McCluskey, P. Syphilitic retinitis and uveitis in HIV-positive adults. Clin. Exp. Ophthalmol. 2010, 38, 851–856. [Google Scholar] [CrossRef]
- Traeger, M.W.; Cornelisse, V.J.; Asselin, J.; Price, B.; Roth, N.J.; Willcox, J.; Tee, B.K.; Fairley, C.K.; Chang, C.C.; Armishaw, J.; et al. Association of HIV Preexposure Prophylaxis With Incidence of Sexually Transmitted Infections Among Individuals at High Risk of HIV Infection. JAMA 2019, 321, 1380–1390. [Google Scholar] [CrossRef]
- Chesson, H.W.; Heffelfinger, J.D.; Voigt, R.F.; Collins, D. Estimates of primary and secondary syphilis rates in persons with HIV in the United States, 2002. Sex. Transm. Dis. 2005, 32, 265–269. [Google Scholar] [CrossRef]
- Rose-Nussbaumer, J.; Goldstein, D.A.; Thorne, J.E.; Arantes, T.E.; Acharya, N.R.; Shakoor, A.; Jeng, B.H.; Yeh, S.; Rahman, H.; Vemulakonda, G.A.; et al. Uveitis in human immunodeficiency virus-infected persons with CD4+ T-lymphocyte count over 200 cells/mL. Clin. Exp. Ophthalmol. 2014, 42, 118–125. [Google Scholar] [CrossRef]
- Mathew, D.; Smit, D. Clinical and laboratory characteristics of ocular syphilis and neurosyphilis among individuals with and without HIV infection. Br. J. Ophthalmol. 2021, 105, 70–74. [Google Scholar] [CrossRef]
- Bollemeijer, J.G.; Wieringa, W.G.; Missotten, T.O.; Meenken, I.; ten Dam-van Loon, N.H.; Rothova, A.; Los, L.I. Clinical Manifestations and Outcome of Syphilitic Uveitis. Invest. Ophthalmol. Vis. Sci. 2016, 57, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Seña, A.C.; White, B.L.; Sparling, P.F. Novel Treponema pallidum serologic tests: A paradigm shift in syphilis screening for the 21st century. Clin. Infect. Dis. 2010, 51, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Forrestel, A.K.; Kovarik, C.L.; Katz, K.A. Sexually acquired syphilis: Laboratory diagnosis, management, and prevention. J. Am. Acad. Dermatol. 2020, 82, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal tests for initial screening--four laboratories, New York City, 2005-2006. MMWR Morb Mortal Wkly. Rep. 2008, 57, 872–875. [Google Scholar]
- Centers for Disease Control and Prevention. Discordant results from reverse sequence syphilis screening--five laboratories, United States, 2006-2010. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 133–137. [Google Scholar]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef]
- Tyagi, M.; Kaza, H.; Pathengay, A.; Agrawal, H.; Behera, S.; Lodha, D.; Pappuru, R.R.; Basu, S.; Murthy, S. Clinical manifestations and outcomes of ocular syphilis in Asian Indian population: Analysis of cases presenting to a tertiary referral center. Indian J. Ophthalmol. 2020, 68, 1881–1886. [Google Scholar] [CrossRef]
- Oliver, G.F.; Stathis, R.M.; Furtado, J.M.; Arantes, T.E.; McCluskey, P.J.; Matthews, J.M.; International Ocular Syphilis Study, G.; Smith, J.R. Current ophthalmology practice patterns for syphilitic uveitis. Br. J. Ophthalmol. 2019, 103, 1645–1649. [Google Scholar] [CrossRef]
- Rasoldier, V.; Gueudry, J.; Chapuzet, C.; Bodaghi, B.; Muraine, M.; Tubiana, R.; Paris, L.; Pestel-Caron, M.; Caron, F.; Caumes, E. Early symptomatic neurosyphilis and ocular syphilis: A comparative study between HIV-positive and HIV-negative patients. Infect. Dis. Now. 2021, 51, 351–356. [Google Scholar] [CrossRef]
- Janier, M.; Unemo, M.; Dupin, N.; Tiplica, G.S.; Potočnik, M.; Patel, R. 2020 European guideline on the management of syphilis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 574–588. [Google Scholar] [CrossRef]
- Testi, I.; Ahmed, S.; Shah, C.; Agrawal, R. Challenges in Treating Intraocular Inflammation in HIV Patients. Ocul. Immunol. Inflamm. 2020, 28, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Testi, I.; Mahajan, S.; Agrawal, R.; Agarwal, A.; Marchese, A.; Curi, A.; Khairallah, M.; Leo, Y.S.; Nguyen, Q.D.; Gupta, V. Management of Intraocular Infections in HIV. Ocul. Immunol. Inflamm. 2020, 28, 1099–1108. [Google Scholar] [CrossRef]
- Sudharshan, S.; Menia, N.K.; Selvamuthu, P.; Tyagi, M.; Kumarasamy, N.; Biswas, J. Ocular syphilis in patients with human immunodeficiency virus/acquired immunodeficiency syndrome in the era of highly active antiretroviral therapy. Indian J. Ophthalmol. 2020, 68, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Hoogewoud, F.; Frumholtz, L.; Loubet, P.; Charlier, C.; Blanche, P.; Lebeaux, D.; Benhaddou, N.; Sedira, N.; Coutte, L.; Vanhaecke, C.; et al. Prognostic Factors in Syphilitic Uveitis. Ophthalmology 2017, 124, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Gong, H.Z.; Hu, K.R.; Zheng, H.Y.; Wan, X.; Li, J. Effect of syphilis infection on HIV acquisition: A systematic review and meta-analysis. Sex. Transm. Infect. 2021, 97, 525–533. [Google Scholar] [CrossRef] [PubMed]
- De Cock, K.M.; Jaffe, H.W.; Curran, J.W. The evolving epidemiology of HIV/AIDS. Aids 2012, 26, 1205–1213. [Google Scholar] [CrossRef]
- Mesfin, Y.M.; Hailemariam, D.; Biadgilign, S.; Kibret, K.T. Association between HIV/AIDS and multi-drug resistance tuberculosis: A systematic review and meta-analysis. PLoS ONE 2014, 9, e82235. [Google Scholar] [CrossRef]
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat. Rev. Microbiol. 2018, 16, 80–90. [Google Scholar] [CrossRef]
- Getahun, H.; Gunneberg, C.; Granich, R.; Nunn, P. HIV infection-associated tuberculosis: The epidemiology and the response. Clin. Infect. Dis. 2010, 50 (Suppl. 3), S201–S207. [Google Scholar] [CrossRef]
- Gupta, R.K.; Lucas, S.B.; Fielding, K.L.; Lawn, S.D. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: A systematic review and meta-analysis. Aids 2015, 29, 1987–2002. [Google Scholar] [CrossRef]
- Agrawal, R.; Gunasekeran, D.V.; Grant, R.; Agarwal, A.; Kon, O.M.; Nguyen, Q.D.; Pavesio, C.; Gupta, V. Clinical Features and Outcomes of Patients With Tubercular Uveitis Treated With Antitubercular Therapy in the Collaborative Ocular Tuberculosis Study (COTS)-1. JAMA Ophthalmol. 2017, 135, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- La Distia Nora, R.; Sitompul, R.; Bakker, M.; Susiyanti, M.; Edwar, L.; Sjamsoe, S.; Singh, G.; van Hagen, M.P.; Rothova, A. Tuberculosis and other causes of uveitis in Indonesia. Eye 2018, 32, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Hsia, N.Y.; Hwang, D.K.; Hwang, Y.S.; Chang, Y.C.; Lee, Y.C.; Hsu, Y.R.; Yeh, P.T.; Lin, C.P.; Chen, H.F.; et al. Clinical Manifestations and Outcomes of Tubercular Uveitis in Taiwan-A Ten-Year Multicenter Retrospective Study. Medicina 2022, 58, 376. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, N.; Hansen, N.I.; Hopewell, P.C.; Glassroth, J.; Kvale, P.A.; Mangura, B.T.; Wilcosky, T.C.; Wallace, J.M.; Rosen, M.J.; Reichman, L.B. Incidence of tuberculosis in the United States among HIV-infected persons. The Pulmonary Complications of HIV Infection Study Group. Ann. Intern. Med. 1997, 126, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, R.; Piyaworawong, S.; Prachamat, P.; Wongyai, J.; Bupachat, S.; Yamada, N.; Summanapan, S.; Yanai, H.; Mahasirimongkol, S. High tuberculosis burden among HIV-infected populations in Thailand due to a low-sensitivity tuberculin skin test. J. Infect. Public Health 2020, 13, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.W.; Hur, M. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection: An updated review. Ann. Clin. Lab. Sci 2013, 43, 221–229. [Google Scholar] [PubMed]
- Mehta, S.; Peters, R.P.; Smit, D.P.; Gupta, V. Ocular Tuberculosis in HIV-infected Individuals. Ocul. Immunol. Inflamm. 2020, 28, 1251–1258. [Google Scholar] [CrossRef]
- Smit, D.P.; Esterhuizen, T.M.; Meyer, D. The Role of QuantiFERON(®)-TB Gold and Tuberculin Skin Test as Diagnostic Tests for Intraocular Tuberculosis in HIV-Positive and HIV-Negative Patients in South Africa. Ocul. Immunol. Inflamm. 2018, 26, 853–858. [Google Scholar] [CrossRef]
- Tomkins-Netzer, O.; Leong, B.C.S.; Zhang, X.; Lightman, S.; McCluskey, P.J. Effect of Antituberculous Therapy on Uveitis Associated With Latent Tuberculosis. Am. J. Ophthalmol. 2018, 190, 164–170. [Google Scholar] [CrossRef]
- Agrawal, R.; Gunasekeran, D.V.; Raje, D.; Agarwal, A.; Nguyen, Q.D.; Kon, O.M.; Pavesio, C.; Gupta, V. Global Variations and Challenges With Tubercular Uveitis in the Collaborative Ocular Tuberculosis Study. Invest. Ophthalmol. Vis. Sci. 2018, 59, 4162–4171. [Google Scholar] [CrossRef]
- Agrawal, R.; Testi, I.; Bodaghi, B.; Barisani-Asenbauer, T.; McCluskey, P.; Agarwal, A.; Kempen, J.H.; Gupta, A.; Smith, J.R.; de Smet, M.D.; et al. Collaborative Ocular Tuberculosis Study Consensus Guidelines on the Management of Tubercular Uveitis-Report 2: Guidelines for Initiating Antitubercular Therapy in Anterior Uveitis, Intermediate Uveitis, Panuveitis, and Retinal Vasculitis. Ophthalmology 2021, 128, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Laovirojjanakul, W.; Thanathanee, O. Opportunistic ocular infections in the setting of HIV. Curr. Opin. Ophthalmol. 2018, 29, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Schaftenaar, E.; Meenken, C.; Baarsma, G.S.; Verjans, G.M.; Peters, R.P. Good visual outcome of tuberculous chorioretinitis after ART initiation in a HIV-infected patient. Int. Ophthalmol. 2014, 34, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Sudharshan, S.; Kaleemunnisha, S.; Banu, A.A.; Shrikrishna, S.; George, A.E.; Babu, B.R.; Devaleenal, B.; Kumarasamy, N.; Biswas, J. Ocular lesions in 1,000 consecutive HIV-positive patients in India: A long-term study. J. Ophthalmic. Inflamm. Infect. 2013, 3, 2. [Google Scholar] [CrossRef]
- Ratnam, I.; Chiu, C.; Kandala, N.B.; Easterbrook, P.J. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin. Infect. Dis. 2006, 42, 418–427. [Google Scholar] [CrossRef]
- Yeo, T.H.; Yeo, T.K.; Wong, E.P.; Agrawal, R.; Teoh, S.C. Immune recovery uveitis in HIV patients with cytomegalovirus retinitis in the era of HAART therapy-a 5-year study from Singapore. J. Ophthalmic. Inflamm. Infect. 2016, 6, 41. [Google Scholar] [CrossRef]
- Schrier, R.D.; Song, M.K.; Smith, I.L.; Karavellas, M.P.; Bartsch, D.U.; Torriani, F.J.; Garcia, C.R.; Freeman, W.R. Intraocular viral and immune pathogenesis of immune recovery uveitis in patients with healed cytomegalovirus retinitis. Retina 2006, 26, 165–169. [Google Scholar] [CrossRef]
- Port, A.D.; Orlin, A.; Kiss, S.; Patel, S.; D’Amico, D.J.; Gupta, M.P. Cytomegalovirus Retinitis: A Review. J. Ocul. Pharmacol. Ther. 2017, 33, 224–234. [Google Scholar] [CrossRef]
- Wons, J.; Kempen, J.; Garweg, J.G. HIV-induced Retinitis. Ocul. Immunol. Inflamm. 2020, 28, 1259–1268. [Google Scholar] [CrossRef]
- Jabs, D.A. Cytomegalovirus retinitis and the acquired immunodeficiency syndrome--bench to bedside: LXVII Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2011, 151, 198–216.e191. [Google Scholar] [CrossRef]
- Zegans, M.E.; Walton, R.C.; Holland, G.N.; O’Donnell, J.J.; Jacobson, M.A.; Margolis, T.P. Transient vitreous inflammatory reactions associated with combination antiretroviral therapy in patients with AIDS and cytomegalovirus retinitis. Am. J. Ophthalmol. 1998, 125, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.; Ausayakhun, S.; Chen, J.; Ausayakhun, S.; Jirawison, C.; Heiden, D.; Holland, G.N.; Margolis, T.P.; Keenan, J.D. Telemedicine diagnosis of cytomegalovirus retinitis by nonophthalmologists. JAMA Ophthalmol. 2014, 132, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Wandel, S.; Colebunders, R.; Attia, S.; Furrer, H.; Egger, M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Duraikkannu, D.; Akbar, A.B.; Sudharshan, S.; Poongulali, S.; Kumarasamy, N.; Jayavelu, T.; Chatterjee, N. Differential Expression of miRNA-192 is a Potential Biomarker for HIV Associated Immune Recovery Uveitis. Ocul. Immunol. Inflamm. 2022, 1–10. [Google Scholar] [CrossRef]
- Pei, L.; Fukutani, K.F.; Tibúrcio, R.; Rupert, A.; Dahlstrom, E.W.; Galindo, F.; Laidlaw, E.; Lisco, A.; Manion, M.; Andrade, B.B.; et al. Plasma Metabolomics Reveals Dysregulated Metabolic Signatures in HIV-Associated Immune Reconstitution Inflammatory Syndrome. Front. Immunol. 2021, 12, 693074. [Google Scholar] [CrossRef] [PubMed]
- Ramon-Luing, L.A.; Ocaña-Guzman, R.; Téllez-Navarrete, N.A.; Preciado-García, M.; Romero-Rodríguez, D.P.; Espinosa, E.; Reyes-Terán, G.; Chavez-Galan, L. High Levels of TNF-α and TIM-3 as a Biomarker of Immune Reconstitution Inflammatory Syndrome in People with HIV Infection. Life 2021, 11, 527. [Google Scholar] [CrossRef]
- Ortega-Larrocea, G.; Espinosa, E.; Reyes-Terán, G. Lower incidence and severity of cytomegalovirus-associated immune recovery uveitis in HIV-infected patients with delayed highly active antiretroviral therapy. Aids 2005, 19, 735–738. [Google Scholar] [CrossRef]
- Kuppermann, B.D.; Holland, G.N. Immune recovery uveitis. Am. J. Ophthalmol. 2000, 130, 103–106. [Google Scholar] [CrossRef]
- National Institutes of Health; Centers for Disease Control and Prevention; HIV Medicine Association; Infectious Diseases Society of America. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/whats-new (accessed on 20 January 2023).
- Agarwal, A.; Handa, S.; Aggarwal, K.; Sharma, M.; Singh, R.; Sharma, A.; Agrawal, R.; Sharma, K.; Gupta, V. The Role of Dexamethasone Implant in the Management of Tubercular Uveitis. Ocul. Immunol. Inflamm. 2018, 26, 884–892. [Google Scholar] [CrossRef]
- Jain, L.; Panda, K.G.; Basu, S. Clinical Outcomes of Adjunctive Sustained-Release Intravitreal Dexamethasone Implants in Tuberculosis-Associated Multifocal Serpigenoid Choroiditis. Ocul. Immunol. Inflamm. 2018, 26, 877–883. [Google Scholar] [CrossRef]
- Fraunfelder, F.W.; Rosenbaum, J.T. Drug-induced uveitis. Incidence, prevention and treatment. Drug Saf. 1997, 17, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.L.; Taskintuna, I.; Freeman, W.R.; Weinberg, D.V.; Feuer, W.J.; Leonard, R.E. Iritis and hypotony after treatment with intravenous cidofovir for cytomegalovirus retinitis. Arch. Ophthalmol. 1997, 115, 733–737. [Google Scholar] [CrossRef]
- Kirsch, L.S.; Arevalo, J.F.; Chavez de la Paz, E.; Munguia, D.; de Clercq, E.; Freeman, W.R. Intravitreal cidofovir (HPMPC) treatment of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology 1995, 102, 533–542; discussion 533–542. [Google Scholar] [CrossRef]
- Chavez-de la Paz, E.; Arevalo, J.F.; Kirsch, L.S.; Munguia, D.; Rahhal, F.M.; De Clercq, E.; Freeman, W.R. Anterior nongranulomatous uveitis after intravitreal HPMPC (cidofovir) for the treatment of cytomegalovirus retinitis. Analysis and prevention. Ophthalmology 1997, 104, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Testi, I.; Agarwal, A.; Agrawal, R.; Mahajan, S.; Marchese, A.; Miserocchi, E.; Gupta, V. Drug-induced Uveitis in HIV Patients with Ocular Opportunistic Infections. Ocul. Immunol. Inflamm. 2020, 28, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, J.W.; Raina, J.; Shah, S.M.; Ainsworth, J.; Pinching, A.J. Ocular complications of intravenous cidofovir for cytomegalovirus retinitis in patients with AIDS. Eye 1999, 13 Pt 3a, 353–356. [Google Scholar] [CrossRef]
- Ambati, J.; Wynne, K.B.; Angerame, M.C.; Robinson, M.R. Anterior uveitis associated with intravenous cidofovir use in patients with cytomegalovirus retinitis. Br. J. Ophthalmol. 1999, 83, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Sudharshan, S.; Koladiya, N.A.; Biswas, J. Rifabutin induced hypopyon uveitis mimicking endophthalmitis as a manifestation of IRU in patients with HIV. Indian J. Pharmacol. 2022, 54, 67–68. [Google Scholar] [CrossRef]
- Shafran, S.D.; Deschênes, J.; Miller, M.; Phillips, P.; Toma, E. Uveitis and pseudojaundice during a regimen of clarithromycin, rifabutin, and ethambutol. MAC Study Group of the Canadian HIV Trials Network. N. Engl. J. Med. 1994, 330, 438–439. [Google Scholar] [CrossRef]
- Kelleher, P.; Helbert, M.; Sweeney, J.; Anderson, J.; Parkin, J.; Pinching, A. Uveitis associated with rifabutin and macrolide therapy for Mycobacterium avium intracellulare infection in AIDS patients. Genitourin. Med. 1996, 72, 419–421. [Google Scholar] [CrossRef]
- Agarwal, M.; Dutta Majumder, P.; Babu, K.; Konana, V.K.; Goyal, M.; Touhami, S.; Stanescu-Segall, D.; Bodaghi, B. Drug-induced uveitis: A review. Indian J. Ophthalmol. 2020, 68, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, D.J.; Williams, R.A.; Gelfand, D.V.; Wilson, S.E. The surgeon and AIDS: Twenty years later. Arch. Surg. 2005, 140, 961–967. [Google Scholar] [CrossRef]
- Asanati, K.; Majeed, A.; Shemtob, L.; Cresswell, F. Healthcare workers potentially exposed to HIV: An update. J. R. Soc. Med. 2022, 115, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Cardo, D.M.; Culver, D.H.; Ciesielski, C.A.; Srivastava, P.U.; Marcus, R.; Abiteboul, D.; Heptonstall, J.; Ippolito, G.; Lot, F.; McKibben, P.S.; et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N. Engl. J. Med. 1997, 337, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Kuhar, D.T.H.; David, K.; Struble, K.A.; Heneine, W.; Thomas, V.; Cheever, L.W.; Gomaa, A.; Panlilio, A.L.; USPHS Working Group on Occupational Postexposure Prophylaxis; National Center for Emerging and Zoonotic Infectious Diseases (U.S.); et al. Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HIV and Recommendations for Postexposure Prophylaxis. Available online: https://stacks.cdc.gov/view/cdc/20711 (accessed on 20 January 2023).
- Kingham, T.P.; Kamara, T.B.; Daoh, K.S.; Kabbia, S.; Kushner, A.L. Universal precautions and surgery in Sierra Leone: The unprotected workforce. World J. Surg. 2009, 33, 1194–1196. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Rapiti, E.; Hutin, Y. Estimation of the global burden of disease attributable to contaminated sharps injuries among health-care workers. Am. J. Ind. Med. 2005, 48, 482–490. [Google Scholar] [CrossRef]
- Doebbeling, B.N.; Vaughn, T.E.; McCoy, K.D.; Beekmann, S.E.; Woolson, R.F.; Ferguson, K.J.; Torner, J.C. Percutaneous injury, blood exposure, and adherence to standard precautions: Are hospital-based health care providers still at risk? Clin. Infect. Dis. 2003, 37, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xue, X.F.; Shah, D.; Zhao, J.; Hwang, L.Y.; Zhuang, G. Knowledge, Attitude, and Practices Regarding Occupational HIV Exposure and Protection among Health Care Workers in China: Census Survey in a Rural Area. J. Int. Assoc. Provid. AIDS Care 2016, 15, 363–369. [Google Scholar] [CrossRef]
- Mashoto, K.O.; Mubyazi, G.M.; Makundi, E.; Mohamed, H.; Malebo, H.M. Estimated risk of HIV acquisition and practice for preventing occupational exposure: A study of healthcare workers at Tumbi and Dodoma Hospitals, Tanzania. BMC Health Serv. Res. 2013, 13, 369. [Google Scholar] [CrossRef]
- Kumakech, E.; Achora, S.; Berggren, V.; Bajunirwe, F. Occupational exposure to HIV: A conflict situation for health workers. Int. Nurs. Rev. 2011, 58, 454–462. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).