EPAC1 Pharmacological Inhibition with AM-001 Prevents SARS-CoV-2 and Influenza A Virus Replication in Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of AM-001
2.2. Cell Culture and Viral Infections
2.3. Treatments with AM-001 and Remdesivir
2.4. Quantification of Viral Load in Cell Supernatants
2.5. Cellular Viability Assay
2.6. Virucidal Assay
2.7. Bioluminescence Resonance Energy Transfer (BRET) Assay
2.8. Immunoblot Assay
2.9. Statistical Analyses
3. Results
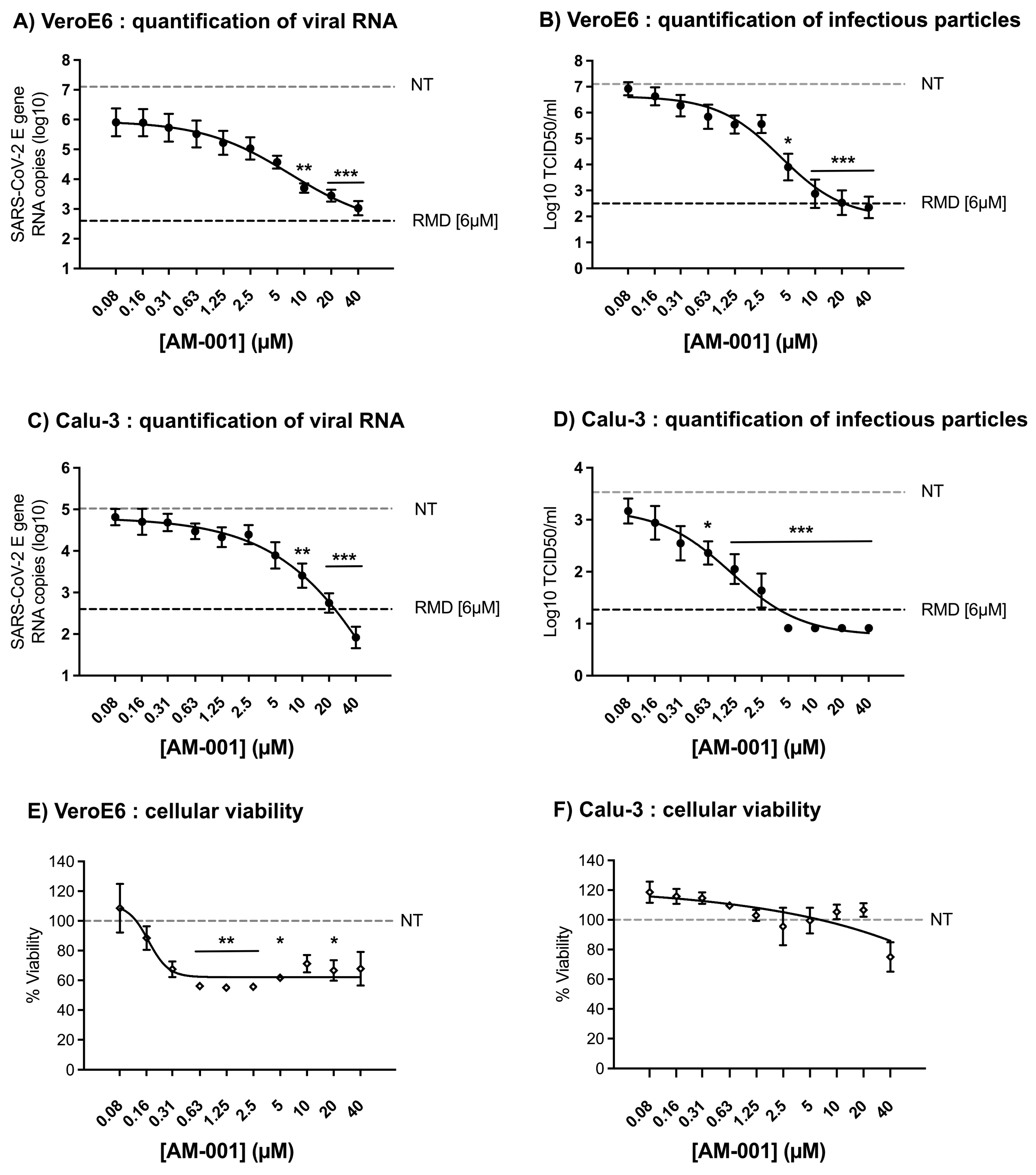
3.1. Inhibition of SARS-CoV-2 by AM-001
3.2. Time-of-Addition-Dependent Inhibition of SARS-CoV-2 by AM-001
3.3. Lack of Virucidal Activity of AM-001 against SARS-CoV-2
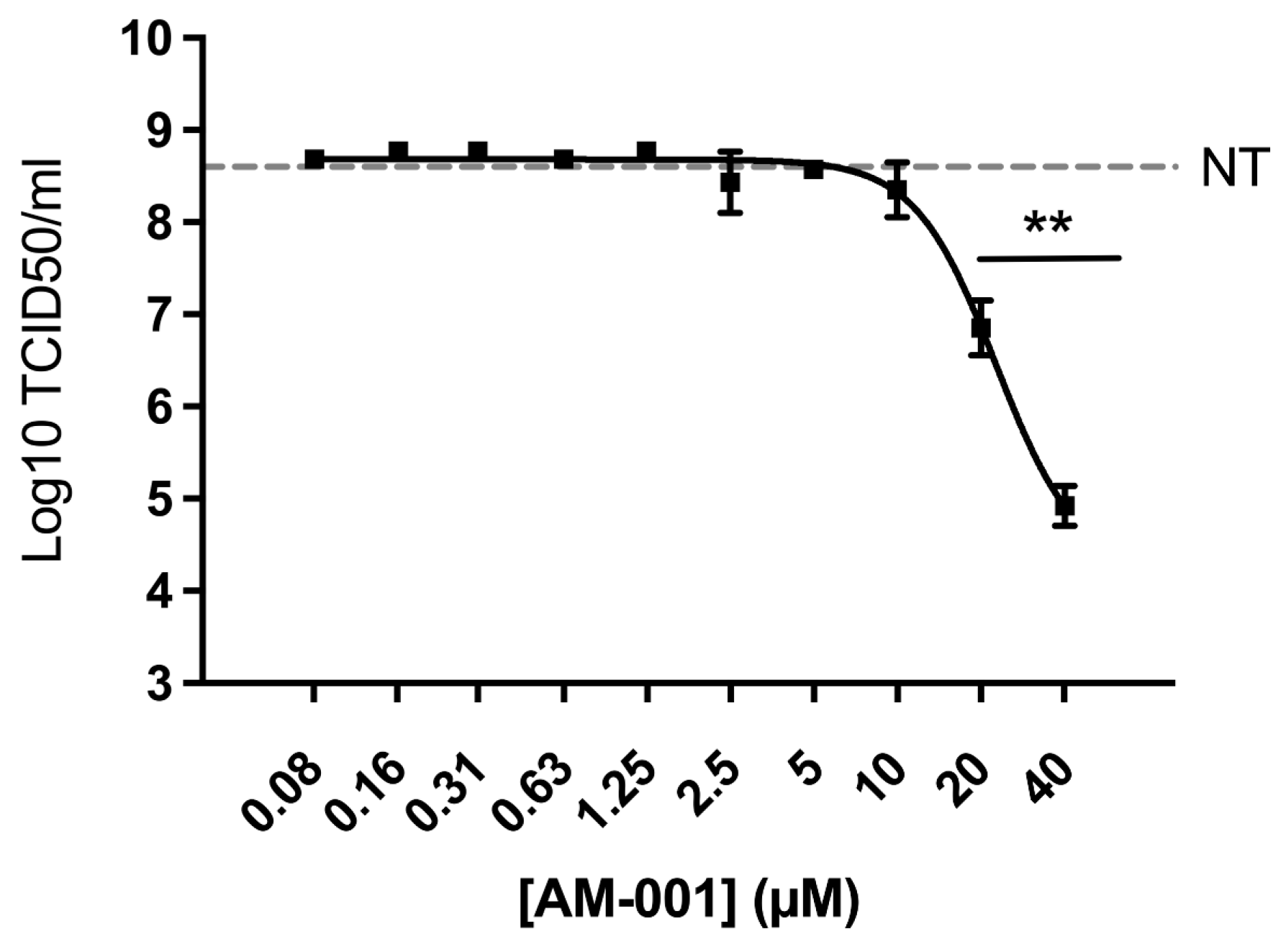
3.4. Inhibition of Influenza A Virus by AM-001
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sachs, J.D.; Karim, S.S.A.; Aknin, L.; Allen, J.; Brosbøl, K.; Colombo, F.; Barron, G.C.; Espinosa, M.F.; Gaspar, V.; Gaviria, A.; et al. The Lancet Commission on Lessons for the Future from the COVID-19 Pandemic. Lancet 2022, 400, 1224–1280. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Song, K.; Ye, J.; Li, W.; Zhong, Y.; Feng, Z.; Liang, S.; Cai, Z.; Xu, K. A Broad Antiviral Strategy: Inhibitors of Human DHODH Pave the Way for Host-Targeting Antivirals against Emerging and Re-Emerging Viruses. Viruses 2022, 14, 928. [Google Scholar] [CrossRef] [PubMed]
- Beavo, J.A.; Brunton, L.L. Cyclic Nucleotide Research—Still Expanding after Half a Century. Nat. Rev. Mol. Cell Biol. 2002, 3, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Gloerich, M.; Bos, J.L. Epac: Defining a New Mechanism for CAMP Action. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 355–375. [Google Scholar] [CrossRef]
- Robichaux, W.G.; Cheng, X. Intracellular CAMP Sensor EPAC: Physiology, Pathophysiology, and Therapeutics Development. Physiol. Rev. 2018, 98, 919–1053. [Google Scholar] [CrossRef]
- Formoso, K.; Lezoualc’h, F.; Mialet-Perez, J. Role of EPAC1 Signalosomes in Cell Fate: Friends or Foes? Cells 2020, 9, 1954. [Google Scholar] [CrossRef]
- Bouvet, M.; Blondeau, J.-P.; Lezoualc’h, F. The Epac1 Protein: Pharmacological Modulators, Cardiac Signalosome and Pathophysiology. Cells 2019, 8, 1543. [Google Scholar] [CrossRef]
- Courilleau, D.; Bisserier, M.; Jullian, J.-C.; Lucas, A.; Bouyssou, P.; Fischmeister, R.; Blondeau, J.-P.; Lezoualc’h, F. Identification of a Tetrahydroquinoline Analog as a Pharmacological Inhibitor of the CAMP-Binding Protein Epac. J. Biol. Chem. 2012, 287, 44192–44202. [Google Scholar] [CrossRef]
- Courilleau, D.; Bouyssou, P.; Fischmeister, R.; Lezoualc’h, F.; Blondeau, J.-P. The (R)-Enantiomer of CE3F4 Is a Preferential Inhibitor of Human Exchange Protein Directly Activated by Cyclic AMP Isoform 1 (Epac1). Biochem. Biophys. Res. Commun. 2013, 440, 443–448. [Google Scholar] [CrossRef]
- Laudette, M.; Coluccia, A.; Sainte-Marie, Y.; Solari, A.; Fazal, L.; Sicard, P.; Silvestri, R.; Mialet-Perez, J.; Pons, S.; Ghaleh, B.; et al. Identification of a Pharmacological Inhibitor of Epac1 That Protects the Heart against Acute and Chronic Models of Cardiac Stress. Cardiovasc. Res. 2019, 115, 1766–1777. [Google Scholar] [CrossRef]
- Prajapati, R.; Fujita, T.; Suita, K.; Nakamura, T.; Cai, W.; Hidaka, Y.; Umemura, M.; Yokoyama, U.; Knollmann, B.C.; Okumura, S.; et al. Usefulness of Exchanged Protein Directly Activated by CAMP (Epac)1-Inhibiting Therapy for Prevention of Atrial and Ventricular Arrhythmias in Mice. Circ. J. Off. J. Jpn. Circ. Soc. 2019, 83, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Mei, F.; Agrawal, A.; Peters, C.J.; Ksiazek, T.G.; Cheng, X.; Tseng, C.-T.K. Blocking of Exchange Proteins Directly Activated by CAMP Leads to Reduced Replication of Middle East Respiratory Syndrome Coronavirus. J. Virol. 2014, 88, 3902–3910. [Google Scholar] [CrossRef] [PubMed]
- Drelich, A.; Judy, B.; He, X.; Chang, Q.; Yu, S.; Li, X.; Lu, F.; Wakamiya, M.; Popov, V.; Zhou, J.; et al. Exchange Protein Directly Activated by CAMP Modulates Ebola Virus Uptake into Vascular Endothelial Cells. Viruses 2018, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-J.; Wu, W.; Cong, X.; Zhang, K.; Luo, J.; Ye, S.; Wang, P.; Suresh, A.; Ullah, U.M.; Zhou, J.; et al. Broad Impact of Exchange Protein Directly Activated by CAMP 2 (EPAC2) on Respiratory Viral Infections. Viruses 2021, 13, 1179. [Google Scholar] [CrossRef]
- Ren, J.; Wu, W.; Zhang, K.; Choi, E.-J.; Wang, P.; Ivanciuc, T.; Peniche, A.; Qian, Y.; Garofalo, R.P.; Zhou, J.; et al. Exchange Protein Directly Activated by CAMP 2 Enhances Respiratory Syncytial Virus-Induced Pulmonary Disease in Mice. Front. Immunol. 2021, 12, 757758. [Google Scholar] [CrossRef]
- Kamal El-Dean, A.M.; Gaber, A.E.-A.M.; El-Gaby, M.S.A.; Eyada, H.A.; Al-Kamali, A.S.N. Recent Trends in the Chemistry of Thienopyridazines. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 321–344. [Google Scholar] [CrossRef]
- Bakhite, E.A.; Abdel Rahman, A.E.; Mohamed, O.S.; Thabet, E.A. Synthesis and Reactions of New Thienopyridines, Pyridothieniopyrimidines and Pyridothienotriazines. Bull. Korean Chem. Soc. 2002, 23, 1709–1714. [Google Scholar]
- de Wit, E.; Spronken, M.I.J.; Bestebroer, T.M.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Efficient Generation and Growth of Influenza Virus A/PR/8/34 from Eight CDNA Fragments. Virus Res. 2004, 103, 155–161. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-NCoV) by Real-Time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- Li, X.; Lidsky, P.V.; Xiao, Y.; Wu, C.-T.; Garcia-Knight, M.; Yang, J.; Nakayama, T.; Nayak, J.V.; Jackson, P.K.; Andino, R.; et al. Ethacridine Inhibits SARS-CoV-2 by Inactivating Viral Particles. PLoS Pathog. 2021, 17, e1009898. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-NCoV) in Vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Fazal, L.; Laudette, M.; Paula-Gomes, S.; Pons, S.; Conte, C.; Tortosa, F.; Sicard, P.; Sainte-Marie, Y.; Bisserier, M.; Lairez, O.; et al. Multifunctional Mitochondrial Epac1 Controls Myocardial Cell Death. Circ. Res. 2017, 120, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Ladilov, Y. Targeting the SAC-Dependent CAMP Pool to Prevent SARS-CoV-2 Infection. Cells 2020, 9, 1962. [Google Scholar] [CrossRef] [PubMed]
- Laudette, M.; Formoso, K.; Lezoualc’h, F. GRKs and Epac1 Interaction in Cardiac Remodeling and Heart Failure. Cells 2021, 10, 154. [Google Scholar] [CrossRef]
- Parnell, E.; Yarwood, S.J. Interactions between Epac1 and Ezrin in the Control of Endothelial Barrier Function. Biochem. Soc. Trans. 2014, 42, 274–278. [Google Scholar] [CrossRef]
- Lezoualc’h, F.; Fazal, L.; Laudette, M.; Conte, C. Cyclic AMP Sensor EPAC Proteins and Their Role in Cardiovascular Function and Disease. Circ. Res. 2016, 118, 881–897. [Google Scholar] [CrossRef]
- Bedi, S.; Ono, A. Friend or Foe: The Role of the Cytoskeleton in Influenza A Virus Assembly. Viruses 2019, 11, 46. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foret-Lucas, C.; Figueroa, T.; Bertin, A.; Bessière, P.; Lucas, A.; Bergonnier, D.; Wasniewski, M.; Servat, A.; Tessier, A.; Lezoualc’h, F.; et al. EPAC1 Pharmacological Inhibition with AM-001 Prevents SARS-CoV-2 and Influenza A Virus Replication in Cells. Viruses 2023, 15, 319. https://doi.org/10.3390/v15020319
Foret-Lucas C, Figueroa T, Bertin A, Bessière P, Lucas A, Bergonnier D, Wasniewski M, Servat A, Tessier A, Lezoualc’h F, et al. EPAC1 Pharmacological Inhibition with AM-001 Prevents SARS-CoV-2 and Influenza A Virus Replication in Cells. Viruses. 2023; 15(2):319. https://doi.org/10.3390/v15020319
Chicago/Turabian StyleForet-Lucas, Charlotte, Thomas Figueroa, Alexandre Bertin, Pierre Bessière, Alexandre Lucas, Dorian Bergonnier, Marine Wasniewski, Alexandre Servat, Arnaud Tessier, Frank Lezoualc’h, and et al. 2023. "EPAC1 Pharmacological Inhibition with AM-001 Prevents SARS-CoV-2 and Influenza A Virus Replication in Cells" Viruses 15, no. 2: 319. https://doi.org/10.3390/v15020319
APA StyleForet-Lucas, C., Figueroa, T., Bertin, A., Bessière, P., Lucas, A., Bergonnier, D., Wasniewski, M., Servat, A., Tessier, A., Lezoualc’h, F., & Volmer, R. (2023). EPAC1 Pharmacological Inhibition with AM-001 Prevents SARS-CoV-2 and Influenza A Virus Replication in Cells. Viruses, 15(2), 319. https://doi.org/10.3390/v15020319






