Recombinant Forms of HIV-1 in the Last Decade of the Epidemic in the Russian Federation
Abstract
:1. Introduction
- CRF03_AB—in the late 1990s in Kaliningrad—is the result of recombination between viruses of genetic variants A6 and B;
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. RNA and DNA Extraction, Amplification, and HIV-1 Sequencing
- for A protocol: PRsec1 (CAAAAATTGGGCCTGAAAATCCATA), PPOS2 (GCTAATTTTTTAGGGAAGATCTGGCCTT), RTsec1 (CAAAAATTGGGCCTGAAAATCCATA), RTOA (TGCCTCTGTTAATTGTTTTACATCATTAGTGTG);
- for B protocol: F2111 (CAAAGGGAGGCCAGGAAATTT), polR1 (TCTCTTCTGTTAATGGCCATTGTTTAA), RT1A (GTTGACTCAGCTTGGTTGTAC), R3271 (ACTGTCCATTTGTCAGGATG).
2.3. Preliminary HIV-1 Subtyping
2.4. Recombination Analysis
2.5. Phylogenic Analysis
2.6. Drug Resistance Mutation Analysis
2.7. Statistical Analysis
- HIV-1 nucleotide sequences were sourced from a minimum of 4 (50%) federal districts annually;
- In the event that Condition No. 1 was not met, the option to aggregate data from multiple years or incorporate supplementary sequences from the international database of the Los Alamos Laboratory (https://www.hiv.lanl.gov/content/sequence/HIV/mainpage.html accessed on 1 March 2023) was permitted, ensuring characteristics aligned with the examined sequences (pol gene; coordinates: 2253–3554, HXB2-numbering; with a minimum sequence length of 919 bp).
3. Results
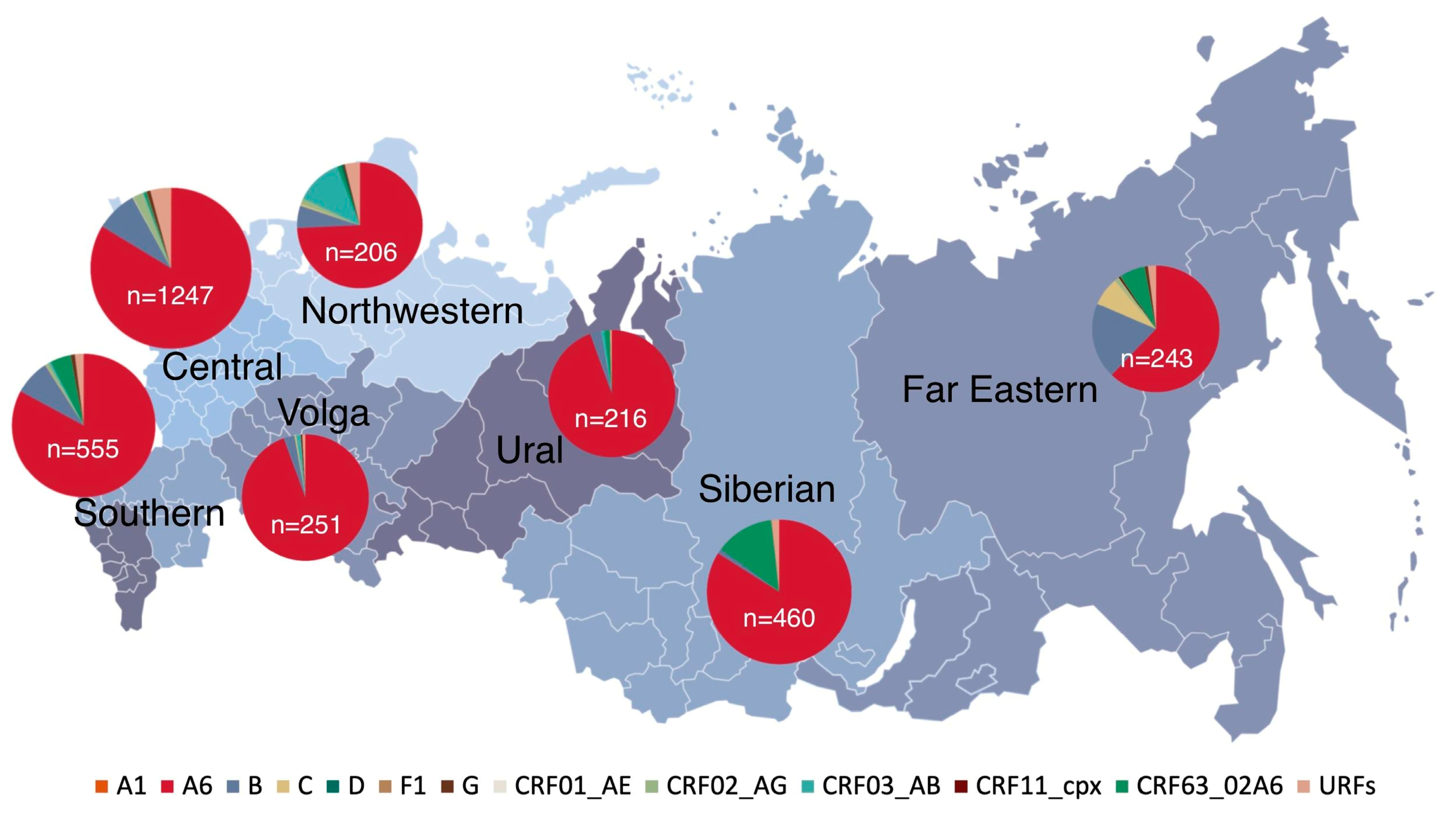
3.1. Characteristics of the Study Population
3.2. Primary HIV-1 Subtyping
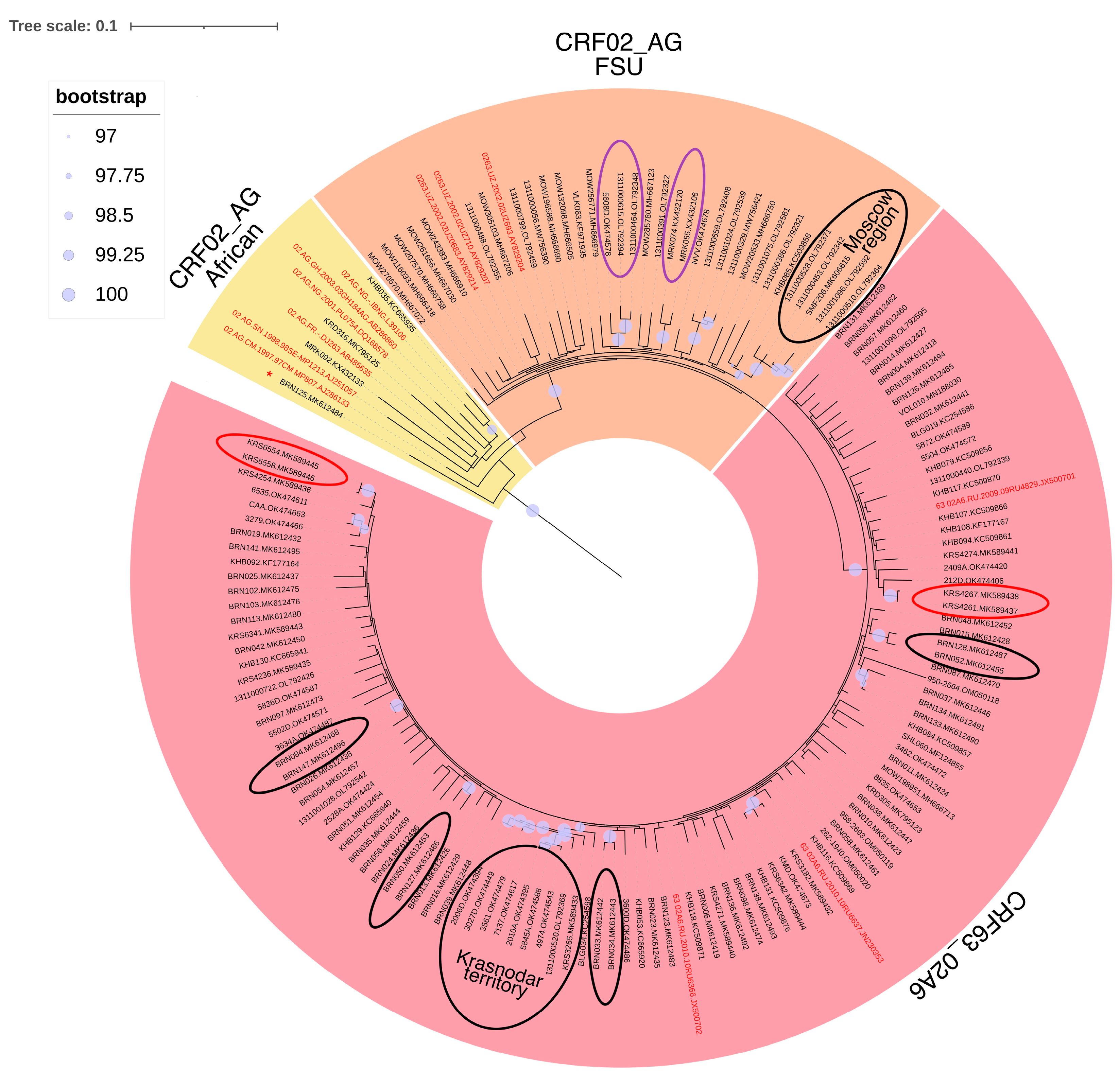
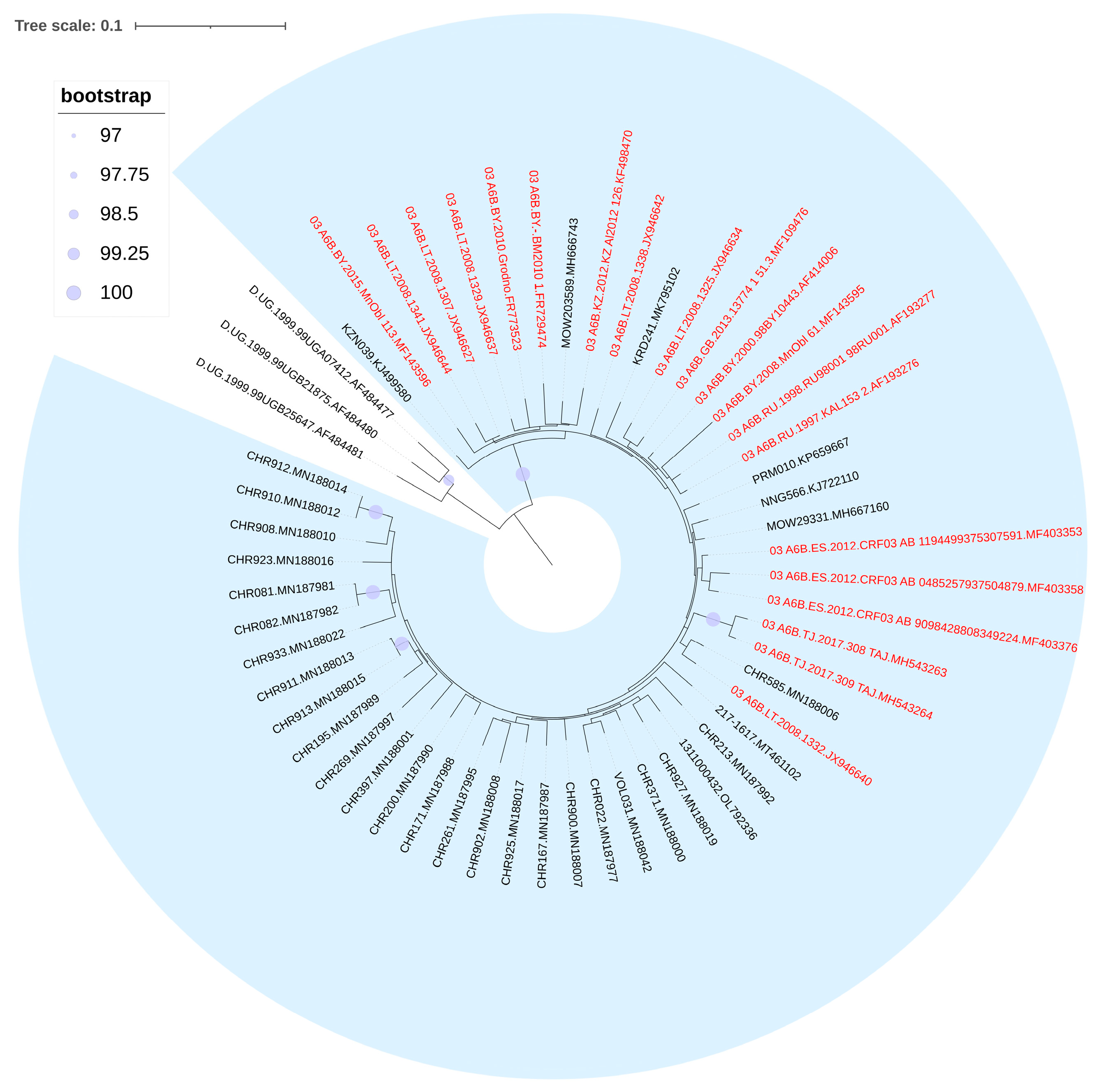
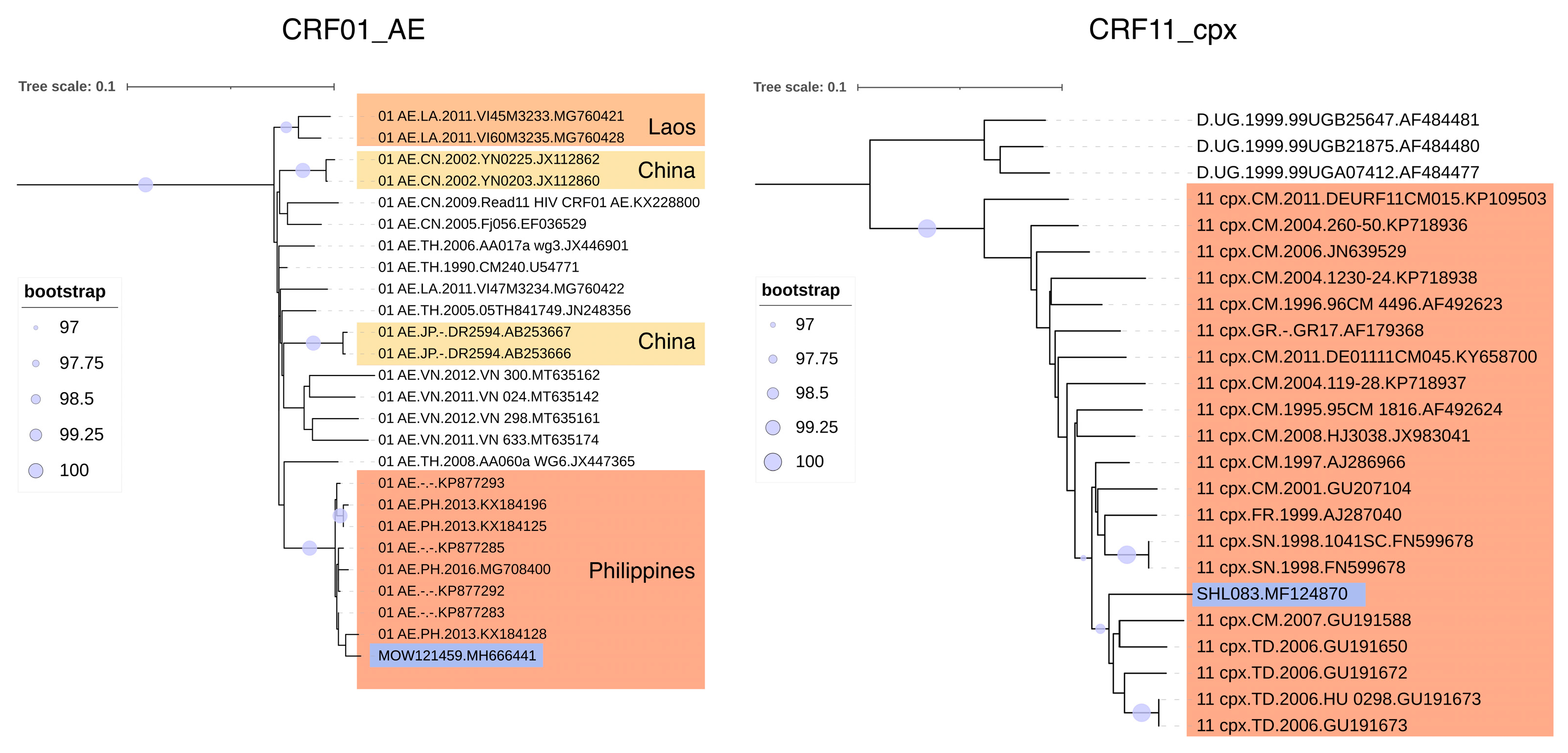
3.3. Phylogenetic Analysis of HIV-1 Recombinant Forms
3.4. URFs Found in Russia
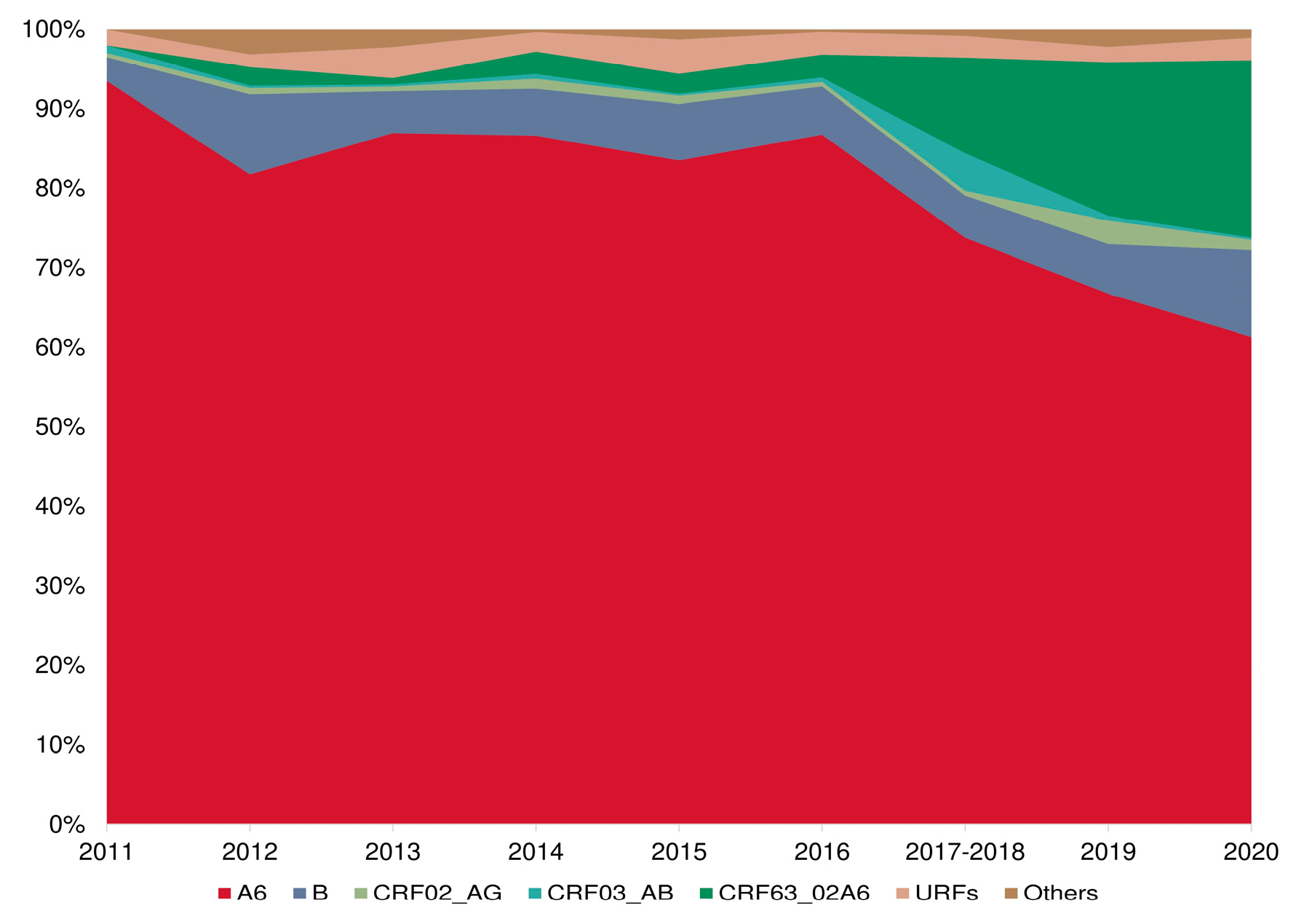
3.5. Prevalence of HIV-1 Recombinant Forms
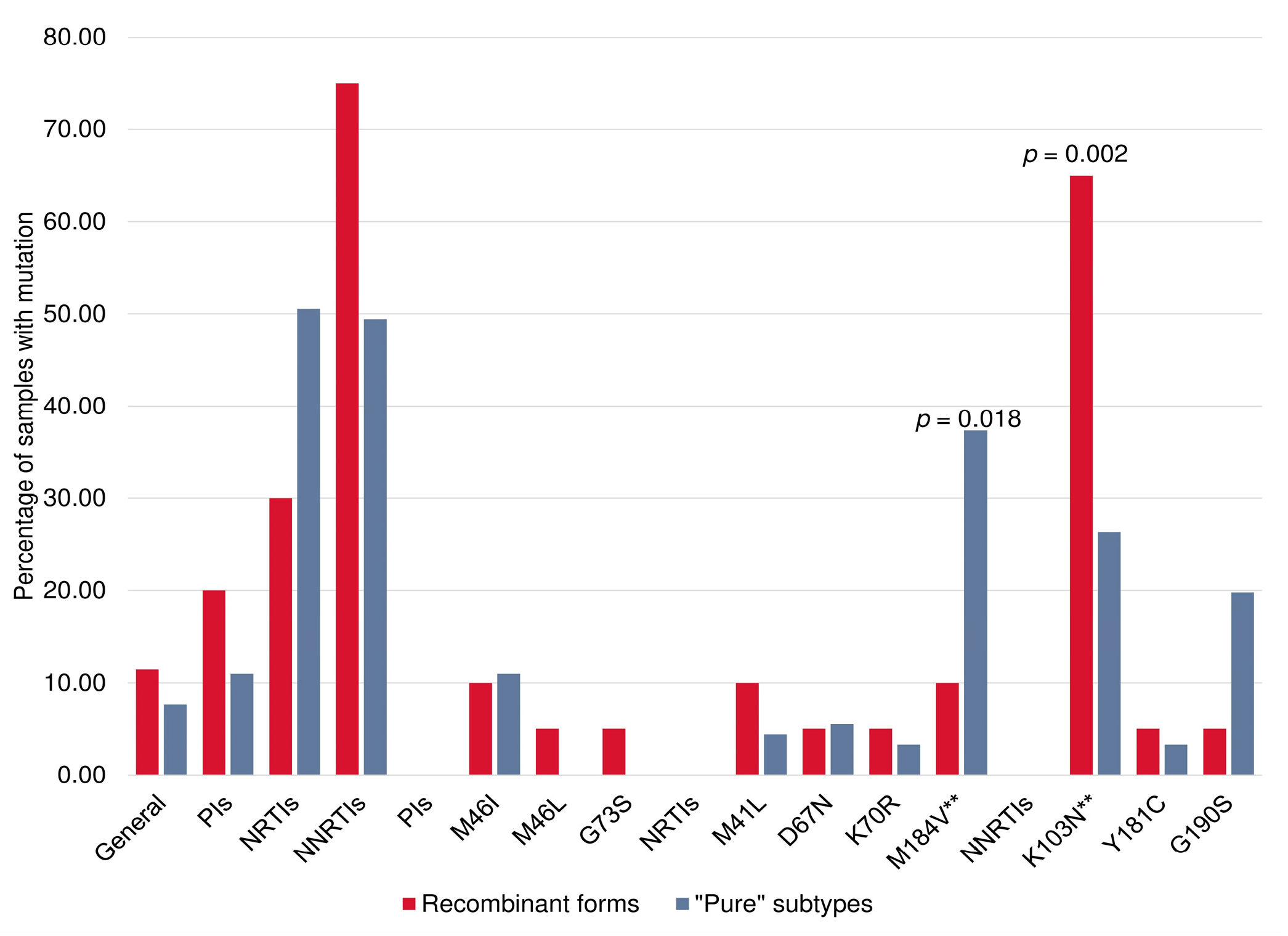
3.6. PrimDR and DRMs between HIV-1 “Pure” Subtypes and Recombinant Forms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. HIV and AIDS. Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 1 September 2023).
- Pokrovsky, V.V.; Ladnaya, N.N.; Sokolov, E.V. HIV Infection. Newsletter No. 47. Available online: http://www.hivrussia.info/wp-content/uploads/2023/05/Byulleten-47-VICH-infektsiya-za-2021-g.pdf (accessed on 1 September 2023).
- The Joint United Nations Programme on HIV/AIDS (UNAIDS). Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 1 September 2023).
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2022 (2021 Data). Available online: https://www.ecdc.europa.eu/sites/default/files/documents/2022-Annual_HIV_Report_final.pdf (accessed on 1 September 2023).
- Yamaguchi, J.; Vallari, A.; McArthur, C.; Sthreshley, L.; Cloherty, G.A.; Berg, M.G.; Rodgers, M.A. Brief Report: Complete Genome Sequence of CG-0018a-01 Establishes HIV-1 Subtype L. J. Acquir. Immune Defic. Syndr. 2020, 83, 319–322. [Google Scholar] [CrossRef]
- Bobkova, M.R. HIV Drug Resistance; Chelovek: Moscow, Russia, 2014; ISBN 978-5-906131-42-3. (In Russian) [Google Scholar]
- Bbosa, N.; Kaleebu, P.; Ssemwanga, D. HIV Subtype Diversity Worldwide. Curr. Opin. HIV AIDS 2019, 14, 153–160. [Google Scholar] [CrossRef]
- Bobkov, A.F.; Kazennova, E.V.; Selimova, L.M.; Ladnaia, N.N.; Bobkova, M.R.; Kravchenko, A.V. HIV-1 Subtypes in Russia in 1987–1988. Zhurnal Mikrobiol. Epidemiol. I Immunobiol. 1999, 1, 43–45. (In Russian) [Google Scholar]
- Pokrovsky, V.V. HIV Infection Comes. Ter. Arkh. 2004, 79, 9–14. (In Russian) [Google Scholar]
- Bobkov, A.F.; Kazennova, E.V.; Selimova, L.M.; Khanina, T.A.; Bobkova, M.P.; Zaznobova, N.A.; Rakina, I.N.; Zarubin, S.N.; Malykh, L.P.; Chernysheva, A.S.; et al. Molecular and Epidemiologic Characteristics of HIV-Infection Outbreak in the Irkutsk Region. Zhurnal Mikrobiol. Epidemiol. I Immunobiol. 2001, 4, 18–20. (In Russian) [Google Scholar]
- Lapovok, I.A.; Lopatukhin, A.E.; Kireev, D.E.; Kazennova, E.V.; Lebedev, A.V.; Bobkova, M.R.; Kolomeets, A.N.; Turbina, G.I.; Shipulin, G.A.; Ladnaya, N.N.; et al. Molecular Epidemiological Analysis of HIV-1 Variants Circulating in Russia in 1987–2015. Ter. Arkh. 2017, 89, 44–49. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Hemelaar, J.; Elangovan, R.; Yun, J.; Dickson-Tetteh, L.; Fleminger, I.; Kirtley, S.; Williams, B.; Gouws-Williams, E.; Ghys, P.D.; Abimiku, A.G.; et al. Global and Regional Molecular Epidemiology of HIV-1, 1990–2015: A Systematic Review, Global Survey, and Trend Analysis. Lancet Infect. Dis. 2019, 19, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Pasechnik, O.A.; Blokh, A.I. The Prevalence of HIV Recombinant Forms in Russia and Countries of the CIS: Systematic Review and Metaanalysis. Russ. J. Infect. Immun. 2018, 8, 127–138. (In Russian) [Google Scholar] [CrossRef]
- Liitsola, K.; Tashkinova, I.; Laukkanen, T.; Korovina, G.; Smolskaja, T.; Momot, O.; Mashkilleyson, N.; Chaplinskas, S.; Brummer-Korvenkontio, H.; Vanhatalo, J.; et al. HIV-1 Genetic Subtype A/B Recombinant Strain Causing an Explosive Epidemic in Injecting Drug Users in Kaliningrad. AIDS 1998, 12, 1907–1919. [Google Scholar] [CrossRef]
- Baryshev, P.B.; Bogachev, V.V.; Gashnikova, N.M. HIV-1 Genetic Diversity in Russia: CRF63_02A1, a New HIV Type 1 Genetic Variant Spreading in Siberia. AIDS Res. Hum. Retroviruses 2014, 30, 592–597. [Google Scholar] [CrossRef]
- Leite, T.C.N.F.; Campos, D.P.; Coelho, A.B.; Teixeira, S.L.M.; Veloso, V.; Morgado, M.G.; Guimarães, M.L. Impact of HIV-1 Subtypes on AIDS Progression in a Brazilian Cohort. AIDS Res. Hum. Retroviruses 2017, 33, 41–48. [Google Scholar] [CrossRef]
- Li, X.; Xue, Y.; Zhou, L.; Lin, Y.; Yu, X.; Wang, X.; Zhen, X.; Zhang, W.; Ning, Z.; Yue, Q.; et al. Evidence That HIV-1 CRF01_AE Is Associated with Low CD4+T Cell Count and CXCR4 Co-Receptor Usage in Recently Infected Young Men Who Have Sex with Men (MSM) in Shanghai, China. PLoS ONE 2014, 9, e89462. [Google Scholar] [CrossRef] [PubMed]
- Tarosso, L.F.; Sanabani, S.S.; Ribeiro, S.P.; Sauer, M.M.; Tomiyama, H.I.; Sucupira, M.C.; Diaz, R.S.; Sabino, E.C.; Kalil, J.; Kallas, E.G. Short Communication: HIV Type 1 Subtype BF Leads to Faster CD4+ T Cell Loss Compared to Subtype B. AIDS Res. Hum. Retroviruses 2014, 30, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Althaus, C.L.; Bonhoeffer, S. Stochastic Interplay between Mutation and Recombination during the Acquisition of Drug Resistance Mutations in Human Immunodeficiency Virus Type 1. J. Virol. 2005, 79, 13572–13578. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Rodríguez, A.; Crandall, K.A.; Posada, D. Recombination Favors the Evolution of Drug Resistance in HIV-1 during Antiretroviral Therapy. Infect. Genet. Evol. 2007, 7, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Kouyos, R.D.; Fouchet, D.; Bonhoeffer, S. Recombination and Drug Resistance in HIV: Population Dynamics and Stochasticity. Epidemics 2009, 1, 58–69. [Google Scholar] [CrossRef]
- Immonen, T.T.; Conway, J.M.; Romero-Severson, E.O.; Perelson, A.S.; Leitner, T. Recombination Enhances HIV-1 Envelope Diversity by Facilitating the Survival of Latent Genomic Fragments in the Plasma Virus Population. PLoS Comput. Biol. 2015, 11, e1004625. [Google Scholar] [CrossRef]
- Applied Biosystems. ViroSeqTM HIV-1 Genotyping System. Version 2. User’s Manual. Available online: https://tools.thermofisher.com/content/sfs/manuals/cms_041134.pdf (accessed on 1 September 2023).
- Larsson, A. AliView: A Fast and Lightweight Alignment Viewer and Editor for Large Datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Struck, D.; Lawyer, G.; Ternes, A.M.; Schmit, J.C.; Bercoff, D.P. COMET: Adaptive Context-Based Modeling for Ultrafast HIV-1 Subtype Identification. Nucleic Acids Res. 2014, 42, e144. [Google Scholar] [CrossRef]
- Shafer, R.W. Rationale and Uses of a Public HIV Drug-Resistance Database. J. Infect. Dis. 2006, 194 (Suppl. 1), S51–S58. [Google Scholar] [CrossRef]
- Pineda-Peña, A.C.; Faria, N.R.; Imbrechts, S.; Libin, P.; Abecasis, A.B.; Deforche, K.; Gómez-López, A.; Camacho, R.J.; De Oliveira, T.; Vandamme, A.M. Automated Subtyping of HIV-1 Genetic Sequences for Clinical and Surveillance Purposes: Performance Evaluation of the New REGA Version 3 and Seven Other Tools. Infect. Genet. Evol. 2013, 19, 337–348. [Google Scholar] [CrossRef]
- Schultz, A.K.; Bulla, I.; Abdou-Chekaraou, M.; Gordien, E.; Morgenstern, B.; Zoulim, F.; Dény, P.; Stanke, M. JpHMM: Recombination Analysis in Viruses with Circular Genomes Such as the Hepatitis B Virus. Nucleic Acids Res. 2012, 40, W193–W198. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and Analysis of Recombination Patterns in Virus Genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods. 2012, 9, 772. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Gifford, R.J.; Liu, T.F.; Rhee, S.Y.; Kiuchi, M.; Hue, S.; Pillay, D.; Shafer, R.W. The Calibrated Population Resistance Tool: Standardized Genotypic Estimation of Transmitted HIV-1 Drug Resistance. Bioinformatics 2009, 25, 1197–1198. [Google Scholar] [CrossRef] [PubMed]
- Glauser, M.P.; Francioli, P. Clinical and Epidemiological Survey of Acquired Immune Deficiency Syndrome in Europe. Eur. J. Clin. Microbiol. 1984, 3, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, C.; Thakallapalli, R.; Eskild, A.; De Ronde, A. Genetic Analysis Reveals Epidemiologic Patterns in the Spread of Human Immunodeficiency Virus. Am. J. Epidemiol. 2000, 152, 814–822. [Google Scholar] [CrossRef]
- Lukashov, V.V.; Kuiken, C.L.; Vlahov, D.; Coutinho, R.A.; Goudsmit, J. Evidence for HIV Type 1 Strains of U.S. Intravenous Drug Users as Founders of AIDS Epidemic among Intravenous Drug Users in Northern Europe. AIDS Res. Hum. Retroviruses 1996, 12, 1179–1183. [Google Scholar] [CrossRef]
- Thomson, M.M.; Nájera, R. Increasing HIV-1 Genetic Diversity in Europe. J. Infect. Dis. 2007, 196, 1120–1124. [Google Scholar] [CrossRef]
- Junqueira, D.M.; Almeida, S.E. HIV-1 subtype B: Traces of a pandemic. Virology 2016, 495, 173–184. [Google Scholar] [CrossRef]
- Ladnaia, N.N.; Pokrovsky, V.V.; Sokolova, E.V.; Chekryzhova, D.G.; Kirzhanova, V.V. Prevalence of Human Immune Deficiency Virus Infection in the Territories of the Russian Federation in 2021. Epidemiol. Infect. Dis. Cur. Items 2022, 12, 12–18. (In Russian) [Google Scholar] [CrossRef]
- Carr, J.K.; Nadai, Y.; Eyzaguirre, L.; Saad, M.D.; Khakimov, M.M.; Yakubov, S.K.; Birx, D.L.; Graham, R.R.; Wolfe, N.D.; Earhart, K.C.; et al. Outbreak of a West African Recombinant of HIV-1 in Tashkent, Uzbekistan. J. Acquir. Immune Defic. Syndr. 2005, 39, 570–575. [Google Scholar] [PubMed]
- Lapovok, I.A.; Saleeva, D.V.; Kirichenko, A.A.; Murzakova, A.V.; Lopatukhin, A.E.; Kireev, D.E. The Study of Dual HIV Infection Prevalence in Russia. Infekc. Bolezn. 2020, 18, 138–148. (In Russian) [Google Scholar] [CrossRef]
- Smith, D.M.; Wong, J.K.; Hightower, G.K.; Ignacio, C.C.; Koelsch, K.K.; Petropoulos, C.J.; Richman, D.D.; Little, S.J. HIV Drug Resistance Acquired through Superinfection. AIDS 2005, 19, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Federal State Statistics Service (Rosstat). Demographics. Available online: https://rosstat.gov.ru/folder/12781 (accessed on 1 September 2023).
- Bobkova, M.R. Current Status of HIV-1 Diversity and Drug Resistance Monitoring in the Former USSR. AIDS Rev. 2013, 15, 204–212. [Google Scholar] [PubMed]
- World Health Organization. HIV Drug Resistance. Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-drug-resistance (accessed on 1 September 2023).
- Zhang, Y.; Luo, Y.; Li, Y.; Zhang, Y.; Wu, W.; Peng, H.; Han, L.; Chen, Y.; Ruan, L.; Yang, R. Genetic Diversity, Complicated Recombination, and Deteriorating Drug Resistance Among HIV-1-Infected Individuals in Wuhan, China. AIDS Res. Hum. Retroviruses 2021, 37, 246–251. [Google Scholar] [CrossRef]
- Deng, X.; Liu, J.; Li, J.; Yang, B.; Shu, Y.; Zhang, M.; Dong, X. Prevalence of HIV-1 Drug-Resistance Genotypes Among Unique Recombinant Forms from Yunnan Province, China in 2016–2017. AIDS Res. Hum. Retroviruses 2020, 36, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, V.V. HIV Infection and AIDS: A National Guide. Brief Edition; GEOTAR-Media: Moscow, Russia, 2021; ISBN 978-5-9704-6468-7. [Google Scholar]
- Bogachev, V.V. Molecular and Epidemiological Features of the Spread of HIV Infection in the Novosibirsk Region in 2008–2012; Koltsovo: Novosibirsk, Russia, 2014. (In Russian) [Google Scholar]
- Sanaubarova, A.; Pujol-Hodge, E.; Dzissyuk, N.; Lemey, P.; Vermund, S.H.; Leigh Brown, A.J.; Ali, S. High-Level Drug-Resistant Mutations among HIV-1 Subtype A6 and CRF02_AG in Kazakhstan. Viruses 2023, 15, 1407. [Google Scholar] [CrossRef]
- Nicastri, E.; Sarmati, L.; d’Ettorre, G.; Palmisano, L.; Parisi, S.G.; Uccella, I.; Rianda, A.; Concia, E.; Vullo, V.; Vella, S.; et al. Replication capacity, biological phenotype, and drug resistance of HIV strains isolated from patients failing antiretroviral therapy. J. Med. Virol. 2003, 69, 1–6. [Google Scholar] [CrossRef]
- Wang, J.; Bambara, R.A.; Demeter, L.M.; Dykes, C. Reduced fitness in cell culture of HIV-1 with nonnucleoside reverse transcriptase inhibitor-resistant mutations correlates with relative levels of reverse transcriptase content and RNase H activity in virions. J. Virol. 2010, 84, 9377–9389. [Google Scholar] [CrossRef]
- Metzner, K.J.; Leemann, C.; Di Giallonardo, F.; Grube, C.; Scherrer, A.U.; Braun, D.; Kuster, H.; Weber, R.; Guenthard, H.F. Reappearance of minority K103N HIV-1 variants after interruption of ART initiated during primary HIV-1 infection. PLoS ONE 2011, 6, e21734. [Google Scholar] [CrossRef] [PubMed]
- Poon, A.F.Y.; Ndashimye, E.; Avino, M.; Gibson, R.; Kityo, C.; Kyeyune, F.; Nankya, I.; Quiñones-Mateu, M.E.; ARTS, E.J.; Paton, N.I.; et al. First-Line HIV Treatment Failures in Non-B Subtypes and Recombinants: A Cross-Sectional Analysis of Multiple Populations in Uganda. AIDS Res. Ther. 2019, 16, 3. [Google Scholar] [CrossRef]
- Ministry of Health of the Russian Federation. Clinical Recommendations: HIV Infection in Adults. 2020. Available online: https://cr.minzdrav.gov.ru/recomend/79_1?ysclid=lp7bi9178g336959806 (accessed on 20 November 2023). (In Russian)
- International Treatment Preparedness Coalition Eastern Europe and Central Asia. The First Long-Term Contract for a Drug for the Treatment of HIV in Russia. 2021. Available online: https://itpc-eeca.org/2021/03/12/pervyj-dolgosrochnyj-kontrakt-na-preparat-dlja-lechenija-vich-v-rossii/?fbclid=IwAR0FFOaB-8rQJjYU8QFymADElDeKuavLWzM8dVwBmQQevldTZvLaba9bjJI (accessed on 20 November 2023). (In Russian).
- Cutrell, A.G.; Schapiro, J.M.; Perno, C.F.; Kuritzkes, D.R.; Quercia, R.; Patel, P.; Polli, J.W.; Dorey, D.; Wang, Y.; Wu, S.; et al. Exploring predictors of HIV-1 virologic failure to long-acting cabotegravir and rilpivirine: A multivariable analysis. AIDS 2021, 35, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, C.; Storto, A.; Soulié, C.; Ferré, V.M.; Wirden, M.; Joly, V.; Lambert-Niclot, S.; Palich, R.; Morand-Joubert, L.; Landman, R.; et al. Prevalence of genotypic baseline risk factors for cabotegravir + rilpivirine failure among ARV-naive patients. J. Antimicrob. Chemother. 2021, 76, 2983–2987. [Google Scholar] [CrossRef]

| Amplification Protocols | Primers | Nucleotide Sequence of Primers (5′→3′) | |
|---|---|---|---|
| A (single PR-RT fragment) | 1 round | RP1S RP1A | GAAAAAGGGCTGTTGGAAATGTGGAA AAATTTAGGAGTCTTTCCCCATATTACTATGC |
| 2 round | PROS2 RTOA | GCTAATTTTTTAGGGAAGATCTGGCCTT TGCCTCTGTTAATTGTTTTACATCATTAGTGTG | |
| B (separate fragments encoding PR and part of RT) | 1 round | POM (PR) R2726 (PR) F2491 (RT) RT2A (RT) | CCCTAGGAAAAAGGGCTGTTGGA TGGAGTATTGTATGGATTTTCAGGC CCCCTGTCAACATAATTGGA TCTGTATATCATTGACAGTCCAGC |
| 2 round | F2111(PR) polR1 (PR) RT1A (RT) R3271 (RT) | CAAAGGGAGGCCAGGAAATTT TCTCTTCTGTTAATGGCCATTGTTTAA GTTGACTCAGCTTGGTTGTAC ACTGTCCATTTGTCAGGATG | |
| Year | Sex | Median Age | Risk Factor for HIV-1 Infection | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male, N (%) | Female, N (%) | Heterosexual, N (%) | IDU, N (%) | MSM, N (%) | MTCT, N (%) | Unknown, N (%) | Other, N (%) | |||
| 2011 | 107 (53.5) | 93 (46.5) | 32.0 [23.6–40.4] | 88 (44.0) | 99 (49.5) | 5 (2.5) | 3 (1.5) | 4 (2.0) | 1 (0.5) | 200 |
| 2012 | 309 (48.7) | 326 (51.3) | 33.0 [23.3–42.7] | 271 (42.7) | 306 (48.2) | 9 (1.4) | 34 (5.3) | 14 (2.2) | 1 (0.2) | 635 |
| 2013 | 183 (50.8) | 177 (49.2) | 34.0 [23.7–44.3] | 189 (52.5) | 134 (37.2) | 10 (2.8) | 10 (2.8) | 15 (4.2) | 2 (0.5) | 360 |
| 2014 | 177 (55.1) | 144 (44.9) | 35.0 [24.3–45.7] | 156 (48.6) | 122 (38.0) | 15 (4.7) | 16 (5.0) | 9 (2.8) | 3 (0.9) | 321 |
| 2015 | 237 (60.2) | 157 (39.8) | 35.0 [24.2–45.8] | 200 (50.8) | 147 (37.3) | 18 (4.6) | 22 (5.6) | 6 (1.5) | 1 (0.2) | 394 |
| 2016 | 206 (59.4) | 141 (40.6) | 36. [25.3–46.7] | 198 (57.1) | 112 (32.3) | 10 (2.9) | 20 (5.8) | 4 (1.1) | 3 (0.9) | 347 |
| 2017 | 235 (58.8) | 165 (41.2) | 36.0 [26.2–45.8] | 223 (55.8) | 147 (36.8) | 18 (4.5) | 6 (1.5) | 5 (1.2) | 1 (0.2) | 400 |
| 2018 | 60 (56.1) | 47 (43.9) | 34.0 [23.0–45.0] | 60 (56.1) | 29 (27.1) | 13 (12.2) | 1 (0.9) | 3 (2.8) | 1 (0.9) | 107 |
| 2019 | 164 (62.4) | 99 (37.6) | 37.0 [26.2–47.8] | 168 (63.9) | 64 (24.3) | 28 (10.6) | 1 (0.4) | 1 (0.4) | 1 (0.4) | 263 |
| 2020 | 97 (64.2) | 54 (35.8) | 39.0 [29.4–48.6] | 89 (58.9) | 36 (23.8) | 22 (14.6) | - | 4 (2.7) | - | 151 |
| Total | 1775 (55.9) | 1403 (44.1) | 35.0 [24.6–45.4] | 1642 (51.7) | 1196 (37.6) | 148 (4.7) | 113 (3.6) | 65 (2.0) | 14 (0.4) | 3178 |
| Genetic Variant | Transmission Risk Groups | |||||
|---|---|---|---|---|---|---|
| Heterosexual, N (%) | IDU, N (%) | MSM, N (%) | MTCT, N (%) | Other, N (%) | Unknown, N (%) | |
| A1 | 1 (0.1) | 0 | 0 | 0 | 0 | 0 |
| A6 | 1385 (84.3) | 1010 (84.4) | 62 (41.9) | 104 (92.0) | 10 (71.4) | 62 (95.5) |
| B | 97 (5.9) | 55 (4.6) | 73 (49.3) | 1 (0.9) | 0 | 1 (1.5) |
| C | 13 (0.8) | 8 (0.7) | 0 | 0 | 0 | 1 (1.5) |
| CRF01_AE | 1 (0.1) | 0 | 0 | 0 | 0 | 0 |
| CRF02_AG/ CRF63_02A6 | 65 (3.9) | 77 (6.4) | 3 (2.0) | 3 (2.7) | 3 (21.4) | 0 |
| CRF03_AB | 24 (1.5) | 12 (1,0) | 0 | 0 | 0 | 0 |
| CRF11_cpx | 1 (0.1) | 0 | 0 | 0 | 0 | 0 |
| D | 1 (0.1) | 0 | 0 | 0 | 0 | 0 |
| F1 | 0 | 0 | 0 | 1 (0.9) | 0 | 0 |
| G | 12 (0.7) | 1 (0,1) | 5 (3.4) | 0 | 0 | 0 |
| URFs | 42 (2.5) | 33 (2.8) | 5 (3.4) | 4 (3.5) | 1 (7.2) | 1 (1.5) |
| Total | 1642 | 1196 | 148 | 113 | 14 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonova, A.; Kazennova, E.; Lebedev, A.; Ozhmegova, E.; Kuznetsova, A.; Tumanov, A.; Bobkova, M. Recombinant Forms of HIV-1 in the Last Decade of the Epidemic in the Russian Federation. Viruses 2023, 15, 2312. https://doi.org/10.3390/v15122312
Antonova A, Kazennova E, Lebedev A, Ozhmegova E, Kuznetsova A, Tumanov A, Bobkova M. Recombinant Forms of HIV-1 in the Last Decade of the Epidemic in the Russian Federation. Viruses. 2023; 15(12):2312. https://doi.org/10.3390/v15122312
Chicago/Turabian StyleAntonova, Anastasiia, Elena Kazennova, Aleksey Lebedev, Ekaterina Ozhmegova, Anna Kuznetsova, Aleksandr Tumanov, and Marina Bobkova. 2023. "Recombinant Forms of HIV-1 in the Last Decade of the Epidemic in the Russian Federation" Viruses 15, no. 12: 2312. https://doi.org/10.3390/v15122312
APA StyleAntonova, A., Kazennova, E., Lebedev, A., Ozhmegova, E., Kuznetsova, A., Tumanov, A., & Bobkova, M. (2023). Recombinant Forms of HIV-1 in the Last Decade of the Epidemic in the Russian Federation. Viruses, 15(12), 2312. https://doi.org/10.3390/v15122312






