Co-Infection of Tomato Brown Rugose Fruit Virus and Pepino Mosaic Virus in Grocery Tomatoes in South Florida: Prevalence and Genomic Diversity
Abstract
:1. Introduction
2. Materials and Methods
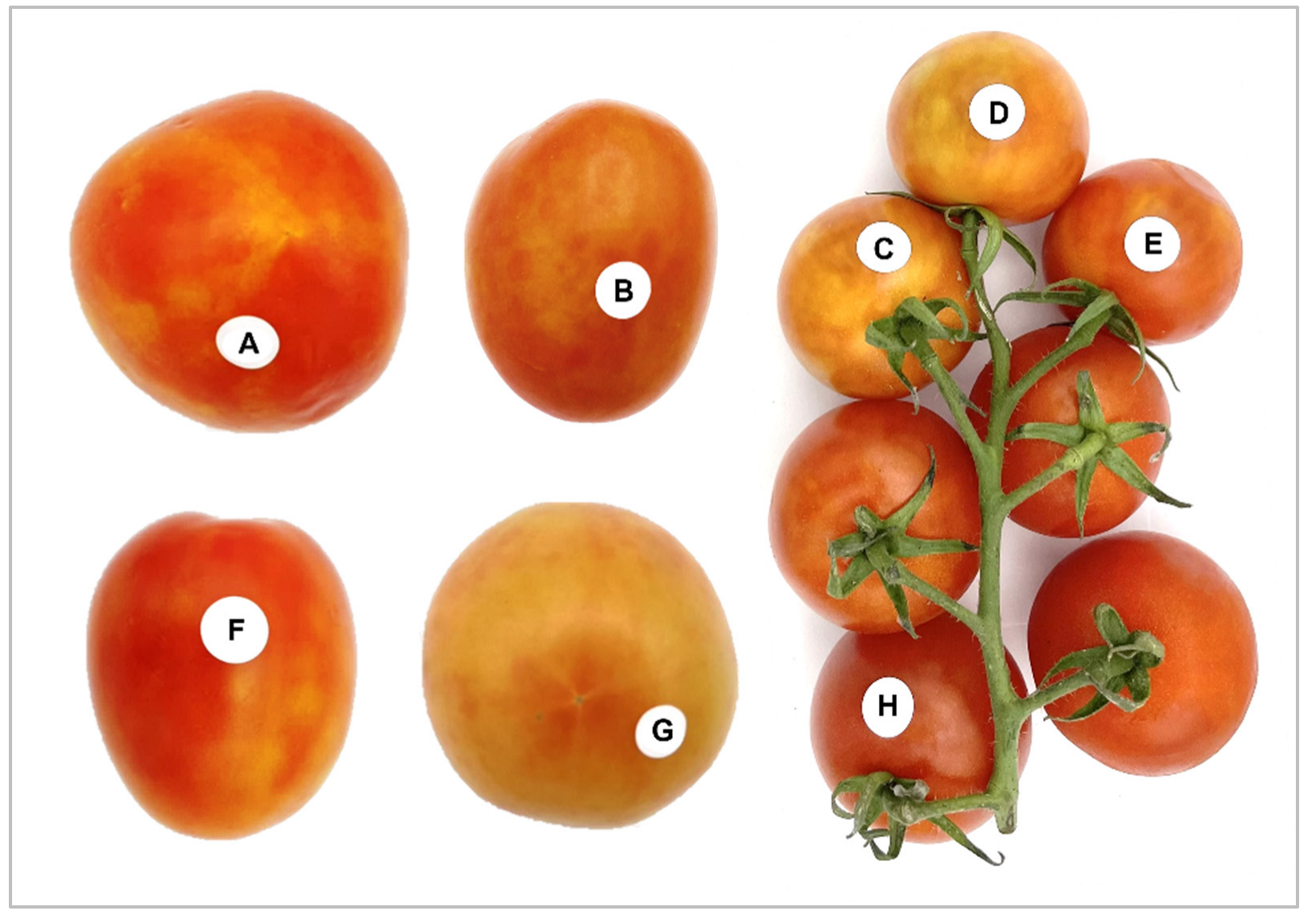
2.1. Sample Collection and Virus Source
2.2. RNA Extraction and Virus Detection
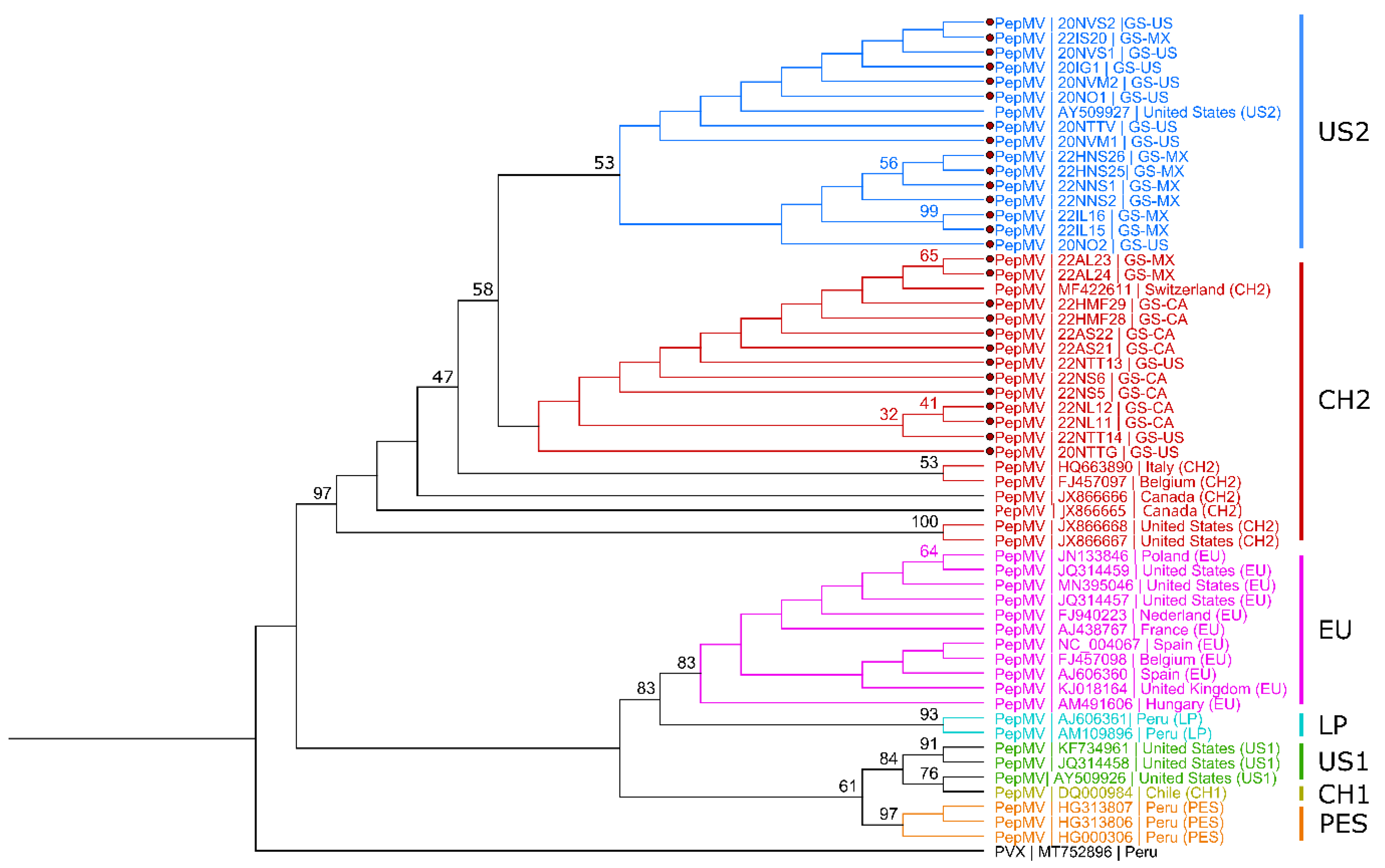
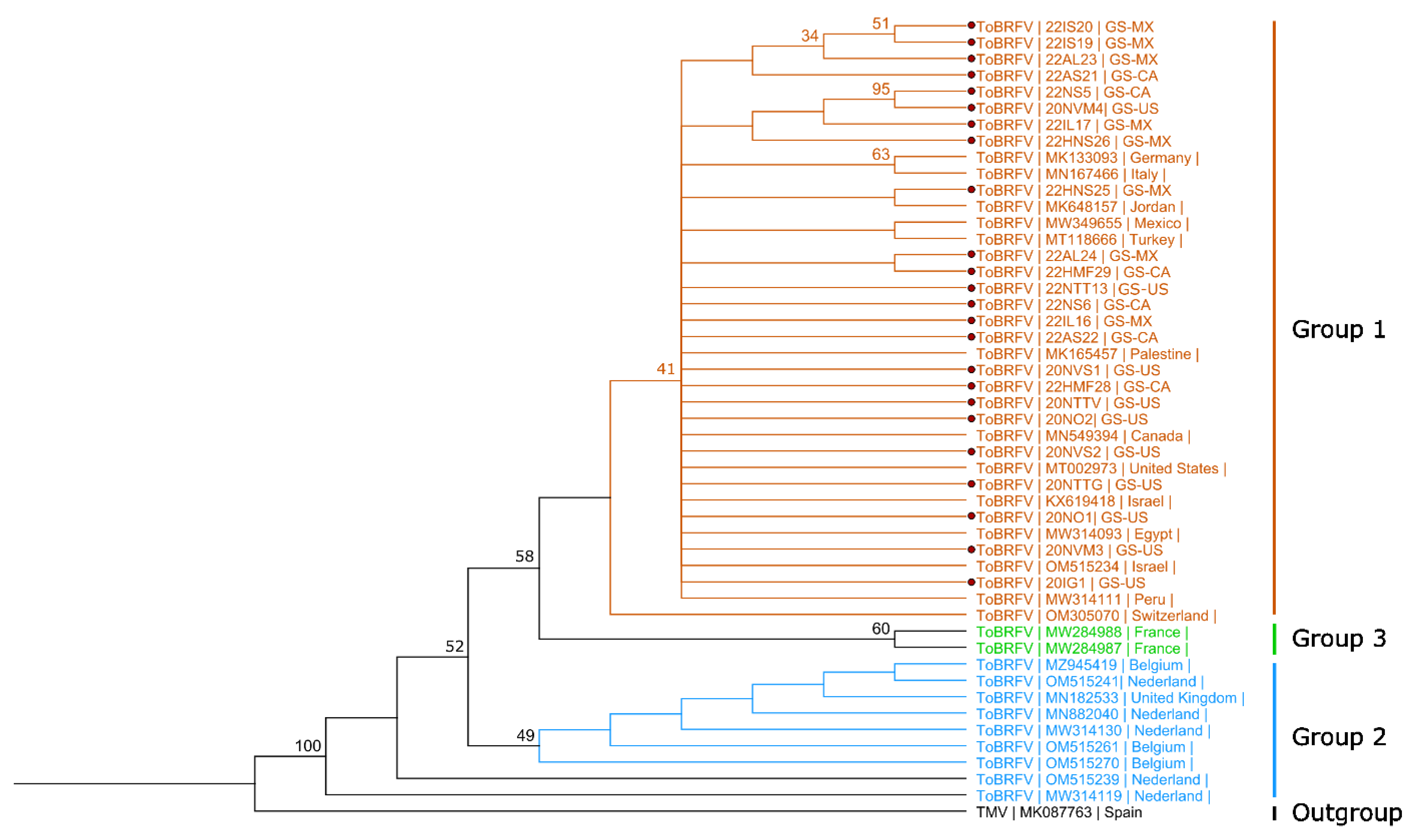
2.3. Sanger Sequencing and Phylogenetic Analysis
3. Results
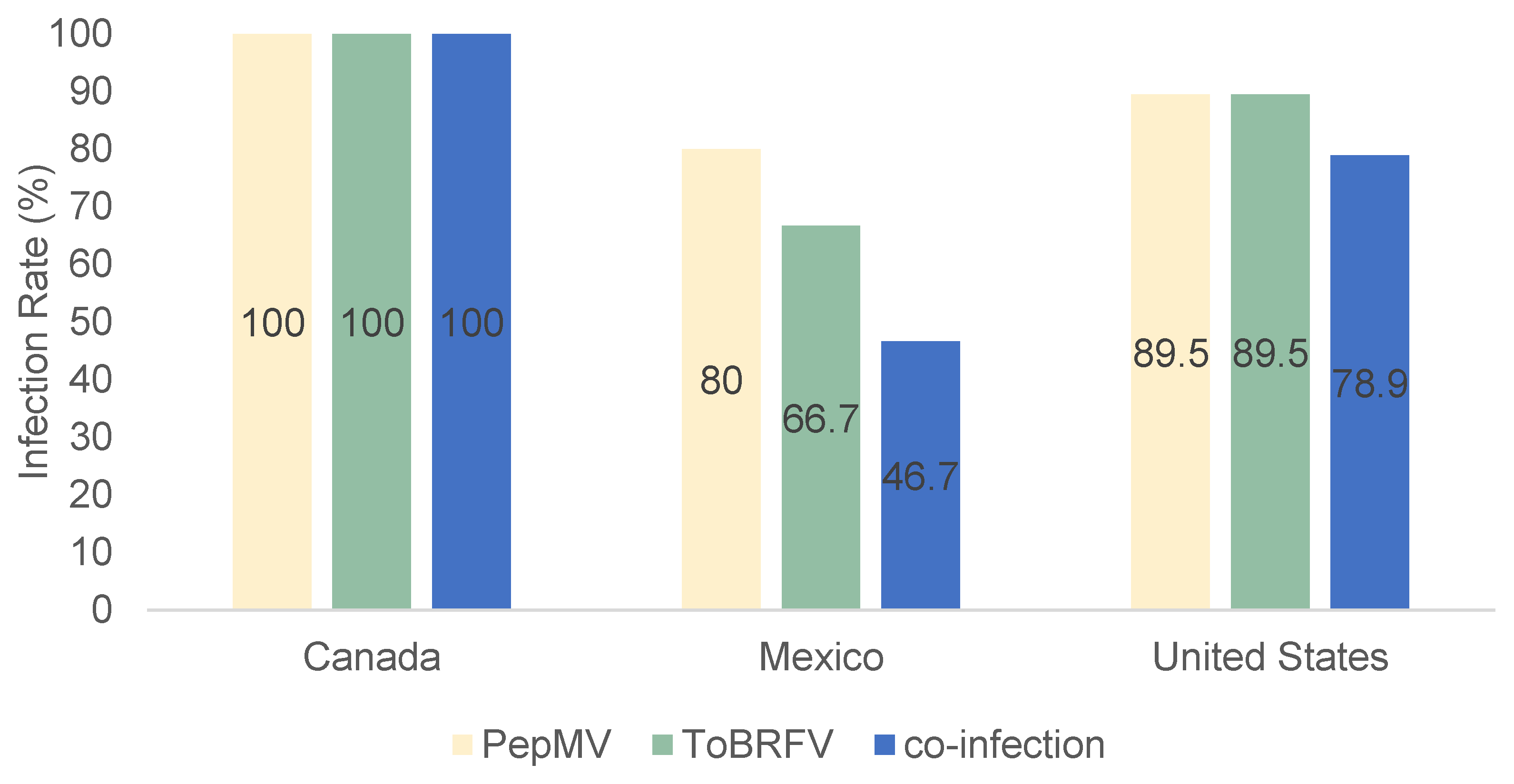
3.1. Tomato Brown Rugose Fruit Virus (ToBRFV) and Pepino Mosaic Virus (PepMV) Detection in Grocery Tomatoes
3.2. Phylogenetic Analysis of Virus Isolates from Grocery Store Tomato Fruits
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, T.J. Resistance at the Tm-2 locus in the tomato to tomato mosaic virus. Euphytica 1980, 29, 189–197. [Google Scholar] [CrossRef]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A.; et al. A New Israeli Tobamovirus Isolate Infects Tomato Plants Harboring Tm-22 Resistance Genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef] [PubMed]
- Hak, H.; Spiegelman, Z. The tomato brown rugose fruit virus movement protein overcomes Tm-22 resistance in tomato while attenuating viral transport. Mol. Plant Microbe Interact. 2021, 34, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Cambrón-Crisantos, J.M.; Rodríguez-Mendoza, J.; Valencia-Luna, J.B.; Rangel, S.A.; de Jesús García-Ávila, C.; López-Buenfil, J.A. First report of Tomato brown rugose fruit virus (ToBRFV) in Michoacan, Mexico. Mex. J. Phytopathol. 2018, 37, 185–192. [Google Scholar]
- Fidan, H.; Sarikaya, P.; Calis, O. First report of Tomato brown rugose fruit virus on tomato in Turkey. New Dis. Rep. 2019, 39, 18. [Google Scholar] [CrossRef]
- Ling, K.-S.; Tian, T.; Gurung, S.; Salati, R.; Gilliard, A. First report of tomato brown rugose fruit virus infecting greenhouse tomato in the US. Plant Dis. 2019, 103, 1439. [Google Scholar] [CrossRef]
- Menzel, W.; Knierim, D.; Winter, S.; Hamacher, J.; Heupel, M. First report of tomato brown rugose fruit virus infecting tomato in Germany. New Dis. Rep. 2019, 39, 2044. [Google Scholar] [CrossRef]
- Skelton, A.; Buxton-Kirk, A.; Ward, R.; Harju, V.; Frew, L.; Fowkes, A.; Long, M.; Negus, A.; Forde, S.; Adams, I. First report of Tomato brown rugose fruit virus in tomato in the United Kingdom. New Dis. Rep. 2019, 40, 12. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, H.; Han, S.; Geng, C.; Tian, Y.; Li, X. First report of Tomato brown rugose fruit virus infecting tomato in China. Plant Dis. 2019, 103, 2973. [Google Scholar] [CrossRef]
- Klap, C.; Luria, N.; Smith, E.; Hadad, L.; Bakelman, E.; Sela, N.; Belausov, E.; Lachman, O.; Leibman, D.; Dombrovsky, A. Tomato Brown Rugose Fruit Virus Contributes to Enhanced Pepino Mosaic Virus Titers in Tomato Plants. Viruses 2020, 12, 879. [Google Scholar] [CrossRef]
- Klap, C.; Luria, N.; Smith, E.; Bakelman, E.; Belausov, E.; Laskar, O.; Lachman, O.; Gal-On, A.; Dombrovsky, A. The potential risk of plant-virus disease initiation by infected tomatoes. Plants 2020, 9, 623. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Koening, R.; Lesemann, D.E. Pepino mosaic virus, a new potexvirus from pepino (Solanum muricatum). Ann. Appl. Biol. 1980, 94, 61–68. [Google Scholar] [CrossRef]
- Wright, D.; Mumford, R. Pepino mosaic Potexvirus (PepMV): First records in tomato in the United Kingdom. In Plant Disease Notice; Central Science Laboratory: York, UK, 1999; Volume 89, p. 400. [Google Scholar]
- Van der Vlugt, R.A.A.; Stijger, C.C.M.M.; Verhoeven, J.T.J.; Lesemann, D.E. First report of Pepino mosaic virus on tomato. Plant Dis. 2000, 84, 103. [Google Scholar] [CrossRef] [PubMed]
- Lesemann, D.-E.; Dalchow, J.; Winter, S.; Pfeilstetter, E. Occurrence of Pepino mosaic virus in European tomato crops: Identification, etiology and epidemiology. Mitt. Biol. Bundesanst. Land. Forstwirtsch. 2000, 376. [Google Scholar]
- French, C.; Bouthillier, M.; Bernardy, M.; Ferguson, G.; Sabourin, M.; Johnson, R.; Masters, C.; Godkin, S.; Mumford, R. First report of Pepino mosaic virus in Canada and the United States. Plant Dis. 2001, 85, 1121. [Google Scholar] [CrossRef] [PubMed]
- Jordá, C.; Perez, A.L.; Martínez-Culebras, P.; Abad, P.; Lacasa, A.; Guerrero, M. First report of Pepino mosaic virus on tomato in Spain. Plant Dis. 2001, 85, 1292. [Google Scholar] [CrossRef] [PubMed]
- Mumford, R.; Metcalfe, E. The partial sequencing of the genomic RNA of a UK isolate of Pepino mosaic virus and the comparison of the coat protein sequence with other isolates from Europe and Peru. Arch. Virol. 2001, 146, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Roggero, P.; Masenga, V.; Lenzi, R.; Coghe, F.; Ena, S.; Winter, S. First report of Pepino mosaic virus in tomato in Italy. Plant Pathol. 2001, 50, 798. [Google Scholar] [CrossRef]
- Mumford, R.A.; Jones, R.A.C. Pepino Mosaic Virus; AAB Descriptions of Plant Viruses 411 No 11 Wellesbourne, UK: Association of Applied Biologists. 2005. Available online: http://www.dpvweb.net/dpv/showadpv.php?dpvno=411 (accessed on 8 December 2022).
- Sempere, R.N.; Gómez-Aix, C.; Ruíz-Ramón, F.; Gómez, P.; Hasiów-Jaroszewska, B.; Sánchez-Pina, M.A. Pepino mosaic virus RNA-dependent RNA polymerase pol domain is a hypersensitive response-like elicitor shared by necrotic and mild isolates. Phytopathology 2016, 106, 395–406. [Google Scholar] [CrossRef]
- Spence, N.; Basham, J.; Mumford, R.; Hayman, G.; Edmondson, R.; Jones, D. Effect of Pepino mosaic virus on the yield and quality of glasshouse-grown tomatoes in the UK. Plant Pathol. 2006, 55, 595–606. [Google Scholar] [CrossRef]
- Hanssen, I.; Paeleman, A.; Vandewoestijne, E.; Van Bergen, L.; Bragard, C.; Lievens, B.; Vanachter, A.; Thomma, B. Pepino mosaic virus isolates and differential symptomatology in tomato. Plant Pathol. 2009, 58, 450–460. [Google Scholar] [CrossRef]
- Davino, S.; Panno, S.; Iacono, G.; Sabatino, L.; D’Anna, F.; Iapichino, G.; Olmos, A.; Scuderi, G.; Rubio, L.; Tomassoli, L.; et al. Genetic variation and evolutionary analysis of Pepino mosaic virus in Sicily: Insights into the dispersion and epidemiology. Plant Pathol. 2017, 66, 368–375. [Google Scholar] [CrossRef]
- Ling, K.S.; Wintermantel, W.M.; Bledsoe, M. Genetic composition of Pepino mosaic virus population in North American greenhouse tomatoes. Plant Dis. 2008, 92, 1683–1688. [Google Scholar] [CrossRef]
- Ling, K.S.; Li, R.; Bledsoe, M. Pepino mosaic virus genotype shift in North America and development of a loop-mediated isothermal amplification for rapid genotype identification. Virol. J. 2013, 10, 117. [Google Scholar] [CrossRef]
- van de Vossenberg, B.T.; Visser, M.; Bruinsma, M.; Koenraadt, H.M.; Westenberg, M.; Botermans, M. Real-time tracking of Tomato brown rugose fruit virus (ToBRFV) outbreaks in the Netherlands using Nextstrain. PLoS ONE 2020, 15, e0234671. [Google Scholar] [CrossRef]
- Abrahamian, P.; Cai, W.; Nunziata, S.O.; Ling, K.-S.; Jaiswal, N.; Mavrodieva, V.A.; Rivera, Y.; Nakhla, M.K. Comparative Analysis of Tomato Brown Rugose Fruit Virus Isolates Shows Limited Genetic Diversity. Viruses 2022, 14, 2816. [Google Scholar] [CrossRef] [PubMed]
- Çelik, A.; Coşkan, S.; Morca, A.F.; Santosa, A.I.; Koolivand, D. Insight into population structure and evolutionary analysis of the emerging tomato brown rugose fruit virus. Plants 2022, 11, 3279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Griffiths, J.S.; Marchand, G.; Bernards, M.A.; Wang, A. Tomato brown rugose fruit virus: An emerging and rapidly spreading plant RNA virus that threatens tomato production worldwide. Mol. Plant Pathol. 2022, 23, 1262–1277. [Google Scholar] [CrossRef]
- Sarkes, A.; Fu, H.; Feindel, D.; Harding, M.; Feng, J. Development and evaluation of a loop-mediated isothermal amplification (LAMP) assay for the detection of Tomato brown rugose fruit virus (ToBRFV). PLoS ONE 2020, 15, e0230403. [Google Scholar] [CrossRef]
- Batuman, O.; Yilmaz, S.; Roberts, P.D.; McAvoy, E.; Hutton, S.F.; Dey, K.; Adkins, S. Tomato Brown Rugose Fruit Virus (ToBRFV): A Potential Threat for Tomato Production in Florida. EDIS 2020, 360, 6. [Google Scholar] [CrossRef]
- Dey, K.; Velez-Climent, M.; Soria, P.; Batuman, O.; Mavrodieva, V.; Wei, G.; Zhou, J.; Adkins, S.; McVay, J. First report of Tomato brown rugose fruit virus infecting tomato in Florida, USA. New Dis. Rep. 2021, 44, e12028. [Google Scholar] [CrossRef]
- USDA National Agricultural Statistics Service (USDA NASS). Quick Stats. Available online: https://quickstats.nass.usda.gov/ (accessed on 12 May 2023).
- Huang, K.M.; Guan, Z.; Hammami, A. The US Fresh Fruit and Vegetable Industry: An Overview of Production and Trade. Agriculture 2022, 1210, 1719. [Google Scholar] [CrossRef]
- USDA. Animal and Plant Health Inspection Service USDA APHIS. 2018. Available online: https://www.aphis.usda.gov/import_export/plants/plant_imports/federal_order/downloads/2020/DA-2020-12.pdf (accessed on 12 July 2023).
- Wylie, S.J.; Li, H.; Sivasithamparam, K.; Jones, M.G. Complete genome analysis of three isolates of narcissus late season yellows virus and two of narcissus yellow stripe virus: Three species or one? Arch. Virol. 2014, 159, 5. [Google Scholar] [CrossRef]
- Mansilla, C.; Sánchez, F.; Ponz, F. The diagnosis of the tomato variant of pepino mosaic virus: An IC-RT-PCR approach. Eur. J. Plant Pathol. 2003, 109, 139–146. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Gibbs, A.J. Tobamovirus classification. In The Plant Viruses; Van Regenmortel, M.H.V., Fraenkel-Conrat, H., Eds.; Springer: Boston, MA, USA, 1986; pp. 167–180. [Google Scholar]
- Duarte, L.M.L.; Alexandre, M.A.V.; Rivas, E.B.; Cattai, M.B.; Soares, R.M.; Harakava, R.; Fernandes, F.M.C. Phylogenetic analysis of Tomato mosaic virus from Hemerocallis sp. and Impatiens hawkeri. Summa Phytopathol. 2007, 33, 409–413. [Google Scholar] [CrossRef]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of Tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE 2019, 14, e0210871. [Google Scholar] [CrossRef]
- Mehle, N.; Bačnik, K.; Bajde, I.; Brodarič, J.; Fox, A.; Gutiérrez-Aguirre, I.; Kitek, M.; Kutnjak, D.; Loh, Y.L.; Vučurović, A.; et al. Tomato brown rugose fruit virus in aqueous environments–Survival and significance of water-mediated transmission. Front. Plant Sci. 2023, 14, 1187920. [Google Scholar] [CrossRef]
- USDA APHIS. Regulatory Options for Tomato Brown Rugose Fruit Virus in the Fruit for Consumption and Plant Propagative Materials (Including Seeds) Pathways. 2023. Available online: https://www.aphis.usda.gov/import_export/plants/plant_imports/process/tobrfv-option-paper-2023.pdf (accessed on 12 July 2023).
- Yilmaz, S.; Hutton, S.F.; Adkins, S.; Batuman, O. Surveys for the resistance-breaking orthotospoviruses in tomato fields in Florida [Poster presentation]. In Proceedings of the APS-Plant Health 2022 Conference, Pittsburgh, PA, USA, 6–10 August 2022. [Google Scholar]
| Primer Name | Sequence (5′-3′) | Target | Amplicon (bp) | Reference |
|---|---|---|---|---|
| ToBRFV-F | GAAGTCCCGATGTCTGTAAGG | partial CP | 842 | [7] |
| ToBRFV-R | GTGCCTACGGATGTGTATGA | |||
| ToBRFV-F5281 ToBRFV-R6308 | AGGACGCAGAAAAGGCAGTT | complete CP | 1028 | This study |
| CCATACACATTTGTCCCGCG | ||||
| F-3666 R-4718 | ATGGTACGAACGGCGGCAG CAATCCTTGATGTGTTTAGCAC | partial RdRp | 1052 | [2] |
| PVX-UniF | ACNTAYGCNGGHTGYCARGG | partial RdRp | 1100 | [39] |
| PVX-UniR | CCATNGTHCCYWANAMCATNAC | |||
| PepMVF | GAGCTGTGGATTCCATCC | partial RdRp | 835 | [39] |
| PepMVR | CAACCTTGTTTAACAAATTGG | |||
| PepMV-F5380 | CACCAATAAATTTAGTTTTAGC | complete CP | 1035 | [11] |
| PepMV-3END | ATTTAGTAGATTTAGATACTAAGG |
| Virus (Strain) | Origin | Host | Sample ID | Accession Number |
|---|---|---|---|---|
| PepMV (CH2) | United States | Solanum lycopersicum L. | 20NTTG * | OP971908 |
| United States | Solanum lycopersicum L. | 22NTT13 * | OP971931 | |
| United States | Solanum lycopersicum L. | 22NTT14 * | OP971932 | |
| Canada | Solanum lycopersicum L. | 22NS5 * | OP971929 | |
| Canada | Solanum lycopersicum L. | 22NS6 * | OP971930 | |
| Canada | Solanum lycopersicum L. | 22NL11 * | OP971925 | |
| Canada | Solanum lycopersicum L. | 22NL12 * | OP971926 | |
| Canada | Solanum lycopersicum L. | 22HMF28 * | OP971918 | |
| Canada | Solanum lycopersicum L. | 22HMF29 * | OP971919 | |
| Canada | Solanum lycopersicum L. | 22AS21 * | OP971916 | |
| Canada | Solanum lycopersicum L. | 22AS22 * | OP971917 | |
| Mexico | Solanum lycopersicum L. | 22AL23 * | OP971914 | |
| Mexico | Solanum lycopersicum L. | 22AL24 * | OP971915 | |
| PepMV (US2) | Mexico | Solanum lycopersicum L. | 22NNS1 * | OP971927 |
| Mexico | Solanum lycopersicum L. | 22NNS2 * | OP971928 | |
| Mexico | Solanum lycopersicum L. | 22HNS25 * | OP971920 | |
| Mexico | Solanum lycopersicum L. | 22HNS26 * | OP971921 | |
| Mexico | Solanum lycopersicum L. | 22IL15 * | OP971922 | |
| Mexico | Solanum lycopersicum L. | 22IL16 * | OP971923 | |
| Mexico | Solanum lycopersicum L. | 22IS20 * | OP971924 | |
| United States | Solanum lycopersicum L. | 20NO1 * | OP971906 | |
| United States | Solanum lycopersicum L. | 20NO2 * | OP971907 | |
| United States | Solanum lycopersicum L. | 20IG1 * | OP971905 | |
| United States | Solanum lycopersicum L. | 20NTTV * | OP971909 | |
| United States | Solanum lycopersicum L. | 20NVM1 * | OP971910 | |
| United States | Solanum lycopersicum L. | 20NVM2 * | OP971911 | |
| United States | Solanum lycopersicum L. | 20NVS1 * | OP971912 | |
| United States | Solanum lycopersicum L. | 20NVS2 * | OP971913 | |
| PepMV (LP) | Peru | Solanum peruvianum L. | NA | AJ606361 |
| Peru | Solanum muricatum L. | NA | AM109896 | |
| PepMV (PES) | Peru | Solanum lycopersicum L. | NA | HG000306 |
| Peru | Solanum peruvianum L. | NA | HG313807 | |
| Peru | Solanum lycopersicum L. | NA | HQ663890 | |
| PepMV (EU) | France | Solanum lycopersicum L. | NA | AJ438767 |
| Spain | Solanum lycopersicum L. | NA | AJ606360 | |
| Solanum lycopersicum L. | NA | NC004067 | ||
| Hungry | Solanum lycopersicum L. | NA | AM491606 | |
| Belgium | Solanum lycopersicum L. | NA | FJ457098 | |
| United Kingdom | Solanum lycopersicum L. | NA | KJ018164 | |
| Netherlands | Solanum lycopersicum L. | NA | FJ940223 | |
| Poland | Solanum lycopersicum L. | NA | JN133846 | |
| United States | Solanum lycopersicum L. | NA | JQ314457 | |
| NA | JQ314459 | |||
| NA | MN395046 | |||
| PepMV (CH2) | Belgium | Solanum lycopersicum L. | NA | FJ457097 |
| Italy | Solanum lycopersicum L. | NA | HQ663890 | |
| Switzerland | Solanum lycopersicum L. | NA | MF422611 | |
| United States | Solanum lycopersicum L. | NA | JX866667 | |
| United States | Solanum lycopersicum L. | NA | JX866668 | |
| Canada | Solanum lycopersicum L. | NA | JX866665 | |
| Canada | Solanum lycopersicum L. | NA | JX866666 | |
| PepMV (CH1) | Chile | Solanum lycopersicum L. | NA | DQ000984 |
| PepMV (US1) | United States | Solanum lycopersicum L. | NA | JQ314458 |
| United States | Solanum lycopersicum L. | NA | KF734961 | |
| United States | Solanum lycopersicum L. | NA | AY509926 | |
| PepMV (US2) | United States | Solanum lycopersicum L. | NA | AY509927 |
| ToBRFV | Canada | Solanum lycopersicum L. | 22NS5 * | OP971902 |
| Canada | Solanum lycopersicum L. | 22NS6 * | OP971903 | |
| United States | Solanum lycopersicum L. | 22NTT13 * | OP971904 | |
| Mexico | Solanum lycopersicum L. | 22IL16 * | OP971896 | |
| Mexico | Solanum lycopersicum L. | 22IL17 * | OP971897 | |
| Mexico | Solanum lycopersicum L. | 22IS19 * | OP971898 | |
| Mexico | Solanum lycopersicum L. | 22IS20 * | OP971899 | |
| Canada | Solanum lycopersicum L. | 22AS21 * | OP971890 | |
| Canada | Solanum lycopersicum L. | 22AS22 * | OP971891 | |
| Mexico | Solanum lycopersicum L. | 22AL23 * | OP971888 | |
| Mexico | Solanum lycopersicum L. | 22AL24 * | OP971889 | |
| Mexico | Solanum lycopersicum L. | 22HNS25 * | OP971894 | |
| Mexico | Solanum lycopersicum L. | 22HNS26 * | OP971895 | |
| Canada | Solanum lycopersicum L. | 22HMF28 * | OP971892 | |
| Canada | Solanum lycopersicum L. | 22HMF29 * | OP971893 | |
| United States | Solanum lycopersicum L. | 20IG1 * | OP971881 | |
| United States | Solanum lycopersicum L. | 20NO1 * | OP971882 | |
| United States | Solanum lycopersicum L. | 20NO2 * | OP971883 | |
| United States | Solanum lycopersicum L. | 20NVS1 * | OP971900 | |
| United States | Solanum lycopersicum L. | 20NVS2 * | OP971901 | |
| United States | Solanum lycopersicum L. | 20NTTG * | OP971884 | |
| United States | Solanum lycopersicum L. | 20NTTV * | OP971885 | |
| United States | Solanum lycopersicum L. | 20NVM3 * | OP971886 | |
| United States | Solanum lycopersicum L. | 20NVM4 * | OP971887 | |
| Jordan | Solanum lycopersicum L. | NA | KT383474 | |
| Israel | Solanum lycopersicum L. | NA | KX619418 | |
| Israel | Solanum lycopersicum L. | NA | OM515237 | |
| Egypt | Solanum lycopersicum L. | NA | MN882030 | |
| Turkey | Solanum lycopersicum L. | NA | MT107885 | |
| Greece | Solanum lycopersicum L. | NA | MN815773 | |
| Italy | Solanum lycopersicum L. | NA | MN167466 | |
| France | Solanum lycopersicum L. | NA | MW284987 | |
| Germany | Solanum lycopersicum L. | NA | MK133095 | |
| Switzerland | Solanum lycopersicum L. | NA | OM305070 | |
| Netherlands | Solanum lycopersicum L. | NA | OM515245 | |
| United Kingdom | Solanum lycopersicum L. | NA | MN182533 | |
| China | Solanum lycopersicum L. | NA | MT018320 | |
| Canada | Solanum lycopersicum L. | NA | MN549394 | |
| Canada | Solanum lycopersicum L. | NA | MN549395 | |
| Canada | Solanum lycopersicum L. | NA | MN549396 | |
| Mexico | Capsicum annuum L. | NA | MW349655 |
| Virus Positive/Total Samples | |||
|---|---|---|---|
| Collection Location | PepMV | ToBRFV | Co-Infection |
| Arcadia | 4/4 | 4/4 | 4/4 |
| Hollywood | 5/6 | 6/6 | 5/6 |
| Immokalee | 9/11 | 9/11 | 7/11 |
| Naples | 29/31 | 26/31 | 24/31 |
| Total | 47/52 | 45/52 | 40/52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, S.; Batuman, O. Co-Infection of Tomato Brown Rugose Fruit Virus and Pepino Mosaic Virus in Grocery Tomatoes in South Florida: Prevalence and Genomic Diversity. Viruses 2023, 15, 2305. https://doi.org/10.3390/v15122305
Yilmaz S, Batuman O. Co-Infection of Tomato Brown Rugose Fruit Virus and Pepino Mosaic Virus in Grocery Tomatoes in South Florida: Prevalence and Genomic Diversity. Viruses. 2023; 15(12):2305. https://doi.org/10.3390/v15122305
Chicago/Turabian StyleYilmaz, Salih, and Ozgur Batuman. 2023. "Co-Infection of Tomato Brown Rugose Fruit Virus and Pepino Mosaic Virus in Grocery Tomatoes in South Florida: Prevalence and Genomic Diversity" Viruses 15, no. 12: 2305. https://doi.org/10.3390/v15122305
APA StyleYilmaz, S., & Batuman, O. (2023). Co-Infection of Tomato Brown Rugose Fruit Virus and Pepino Mosaic Virus in Grocery Tomatoes in South Florida: Prevalence and Genomic Diversity. Viruses, 15(12), 2305. https://doi.org/10.3390/v15122305






