The Time-Dependent Association of Torque Teno Virus Load with the Level of SARS-CoV-2 S1 IgG Antibodies Following COVID-19 Vaccination in Kidney Transplant Recipients
Abstract
:1. Introduction
2. Methods
2.1. Study Participants
2.2. SARS-CoV-2 Spike S1-Specific IgG Antibody Response
2.3. Quantitative TTV PCR
2.4. Statistical Analysis
3. Results
3.1. Associations between Baseline Characteristics and TTV Load at Baseline
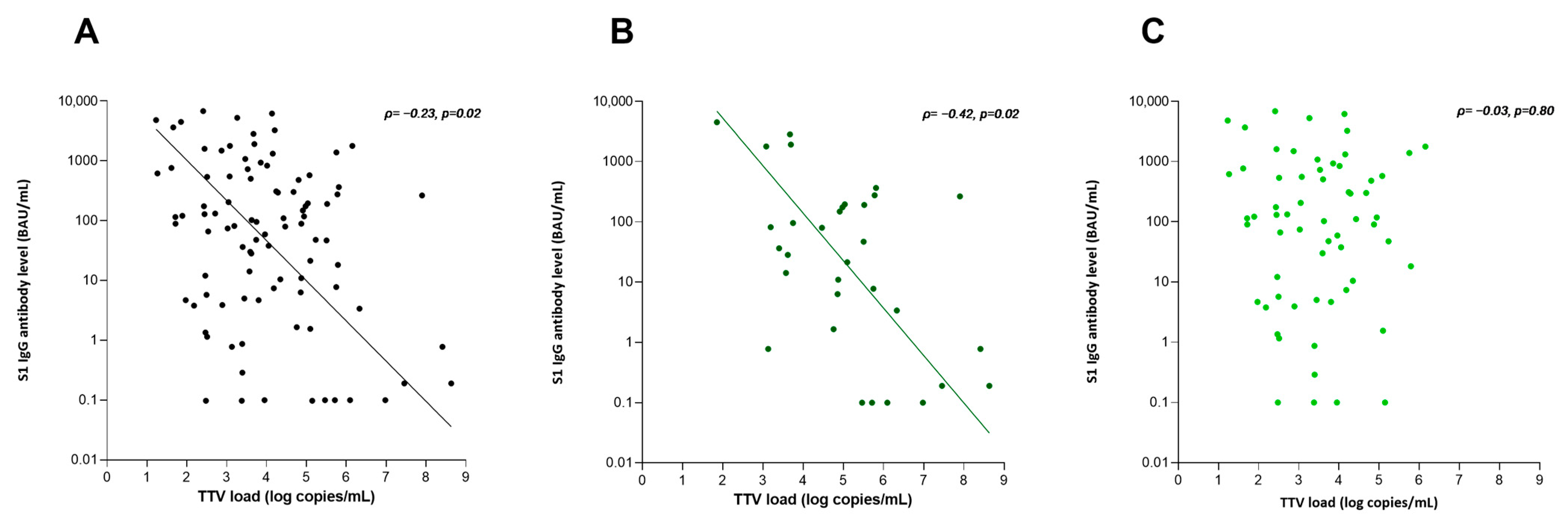
3.2. Association with S1 IgG Antibody Level
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stumpf, J.; Siepmann, T.; Lindner, T.; Karger, C.; Schwöbel, J.; Anders, L.; Faulhaber-Walter, R.; Schewe, J.; Martin, H.; Schirutschke, H.; et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021, 9, 100178. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Lemée, V.; Lamulle, J.; Hanoy, M.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; Le Roy, F.; et al. Antibody and t cell response to SARS-CoV-2 messenger rna bnt162b2 vaccine in kidney transplant recipients and hemodialysis patients. J. Am. Soc. Nephrol. 2021, 32, 2147–2152. [Google Scholar] [CrossRef]
- Sattler, A.; Schrezenmeier, E.; Weber, U.A.; Potekhin, A.; Bachmann, F.; Straub-Hohenbleicher, H.; Budde, K.; Storz, E.; Proß, V.; Bergmann, Y.; et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J. Clin. Investig. 2021, 131, e150175. [Google Scholar] [CrossRef]
- Sanders, J.S.F.; Bemelman, F.J.; Messchendorp, A.L.; Baan, C.C.; van Baarle, D.; van Binnendijk, R.; Diavatopoulos, D.A.; Frölke, S.C.; Geers, D.; GeurtsvanKessel, C.H.; et al. The RECOVAC Immune-response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients With Chronic Kidney Disease, on Dialysis, or Living with a Kidney Transplant. Transplantation 2022, 106, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Kho, M.M.L.; Messchendorp, A.L.; Frölke, S.C.; Imhof, C.; Koomen, V.J.; Malahe, S.R.K.; Vart, P.; Geers, D.; de Vries, R.D.; GeurtsvanKessel, C.H.; et al. Alternative strategies to increase the immunogenicity of COVID-19 vaccines in kidney transplant recipients not responding to two or three doses of an mRNA vaccine (RECOVAC): A randomised clinical trial. Lancet Infect. Dis. 2023, 23, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Meshram, H.S.; Kute, V.; Rane, H.; Dave, R.; Banerjee, S.; Mishra, V.; Chauhan, S. Humoral and cellular response of COVID-19 vaccine among solid organ transplant recipients: A systematic review and meta-analysis. Transpl. Infect. Dis. 2022, 24, e13926. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.S.; Shalabi, H.A.; Alhifany, A.A.; Alotaibi, N.E.; Alnuhait, M.A.; Altheaby, A.R.; Alhazmi, A.Y. Humoral and Cellular Immunity following Five Doses of COVID-19 Vaccines in Solid Organ Transplant Recipients: A Systematic Review and Meta-Analysis. Vaccines 2023, 11, 1166. [Google Scholar] [CrossRef]
- Kulifaj, D.; Tilloy, V.; Scaon, E.; Guerin, E.; Essig, M.; Pichon, N.; Hantz, S.; De Bernardi, A.; Joannes, M.; Barranger, C.; et al. Viral metagenomics analysis of kidney donors and recipients: Torque teno virus genotyping and prevalence. J. Med. Virol. 2020, 92, 3301–3311. [Google Scholar] [CrossRef]
- Strassl, R.; Doberer, K.; Rasoul-Rockenschaub, S.; Herkner, H.; Görzer, I.; Kläger, J.P.; Schmidt, R.; Haslacher, H.; Schiemann, M.; A Eskandary, F.; et al. Torque teno virus for risk stratification of acute biopsyproven alloreactivity in kidney transplant recipients. J. Infect. Dis. 2019, 219, 1934–1939. [Google Scholar] [CrossRef]
- van Rijn, A.L.; Wunderink, H.F.; A Sidorov, I.; de Brouwer, C.S.; Kroes, A.C.; Putter, H.; de Vries, A.P.; I Rotmans, J.; Feltkamp, M.C. Torque teno virus loads after kidney transplantation predict allograft rejection but not viral infection. J. Clin. Virol. 2021, 140, 104871. [Google Scholar] [CrossRef]
- Querido, S.; Adragão, T.; Pinto, I.; Ormonde, C.; Papoila, A.L.; Pessanha, M.A.; Gomes, P.; Ferreira, S.; Figueira, J.M.; Cardoso, C.; et al. Torquetenovirus viral load is associated with anti-spike antibody response in SARS-CoV-2 mRNA BNT162b2 vaccinated kidney transplant patients. Clin. Transplant. 2022, 36, e14825. [Google Scholar] [CrossRef] [PubMed]
- Graninger, M.; Stumpf, J.; Bond, G.; Görzer, I.; Springer, D.N.; Kessel, F.; Kröger, H.; Frank, K.; Tonn, T.; Hugo, C.; et al. Prediction of humoral and cellular immune response to COVID-19 mRNA vaccination by TTV load in kidney transplant recipients and hemodialysis patients. J. Clin. Virol. 2023, 162, 105428. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.; Benotmane, I.; Gallais, F.; Caillard, S.; Fafi-Kremer, S. Torque teno virus viral load predicts SARS-CoV-2 vaccine response in kidney transplant recipients. J. Med. Virol. 2023, 95, e28936. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Baj, A.; Azzi, L.; Novazzi, F.; Maggi, F. TTV viral load as a predictor of antibody response to SARS-CoV-2 vaccination. J. Heart Lung Transplant. 2023, 42, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Messchendorp, A.L.; Sanders, J.-S.; Abrahams, A.C.; Bemelman, F.; Bouwmans, P.; van Den, R.D.; Hilbrands, L.B.; Imhof, C.; Reinders, M.E.J.; Rispens, T.; et al. Incidence and severity of COVID-19 in relation to anti-RBD IgG antibody level after COVID-19 vaccination in kidney transplant recipients. Nephrol. Dial. Transplant. 2023, unpublished. [Google Scholar] [CrossRef]
- Den Hartog, G.; Schepp, R.M.; Kuijer, M.; GeurtsvanKessel, C.; van Beek, J.; Rots, N.; Koopmans, M.P.G.; van der Klis, F.R.M.; van Binnendijk, R.S. SARS-CoV-2-Specific Antibody Detection for Seroepidemiology: A Multiplex Analysis Approach Accounting for Accurate Seroprevalence. J. Infect. Dis. 2020, 222, 1452–1461. [Google Scholar] [CrossRef]
- den Hartog, G.; Vos, E.R.A.; van den Hoogen, L.L.; van Boven, M.; Schepp, R.M.; Smits, G.; van Vliet, J.; Woudstra, L.; Wijmenga-Monsuur, A.J.; van Hagen, C.C.E.; et al. Persistence of antibodies to SARS-CoV-2 in relation to symptoms in a nationwide prospective study. Clin. Infect. Dis. 2021, 73, 2155–2162. [Google Scholar] [CrossRef]
- Geers, D.; Shamier, M.C.; Bogers, S.; den Hartog, G.D.; Gommers, L.; Nieuwkoop, N.N.; Schmitz, K.S.; Rijsbergen, L.C.; van Osch, J.A.T.; Dijkhuizen, E.; et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021, 6, eabj1750. [Google Scholar] [CrossRef]
- Gore, E.J.; Gomes-Neto, A.W.; Wang, L.; Bakker, S.J.L.; Niesters, H.G.M.; de Joode, A.A.E.; Verschuuren, E.A.M.; Westra, J.; Van Leer-Buter, C. Torquetenovirus serum load and long-term outcomes in renal transplant recipients. J. Clin. Med. 2020, 9, 440. [Google Scholar] [CrossRef]
- Görzer, I.; Haloschan, M.; Jaksch, P.; Klepetko, W.; Puchhammer-Stöckl, E. Plasma DNA levels of Torque teno virus and immunosuppression after lung transplantation. J. Heart Lung Transplant. 2014, 33, 320–323. [Google Scholar] [CrossRef]
- Solis, M.; Velay, A.; Gantner, P.; Bausson, J.; Filipputtu, A.; Freitag, R.; Moulin, B.; Caillard, S.; Fafi-Kremer, S. Torquetenovirus viremia for early prediction of graft rejection after kidney transplantation. J. Infect. 2019, 79, 56–60. [Google Scholar] [CrossRef] [PubMed]
- De Vlaminck, I.; Khush, K.K.; Strehl, C.; Kohli, B.; Luikart, H.; Neff, N.F.; Okamoto, J.; Snyder, T.M.; Cornfield, D.N.; Nicolls, M.R.; et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell 2013, 155, 1178. [Google Scholar] [CrossRef] [PubMed]
- Dyer, O. COVID-19: What do we know about XBB.1.5 and should we be worried? BMJ 2023, 382, p1900. [Google Scholar] [CrossRef] [PubMed]
- Hoek, R.A.; Verschuuren, E.A.; de Vries, R.D.; Vonk, J.M.; van Baarle, D.; van der Heiden, M.; van Gemert, J.P.; Gore, E.J.; Niesters, H.G.; Erasmus, M.; et al. High torque tenovirus (TTV) load before first vaccine dose is associated with poor serological response to COVID-19 vaccination in lung transplant recipients. J. Heart Lung Transplant. 2022, 41, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Babel, N.; Hugo, C.; Westhoff, T.H. Vaccination in patients with kidney failure: Lessons from COVID-19. Nat. Rev. Nephrol. 2022, 18, 708–723. [Google Scholar] [CrossRef]
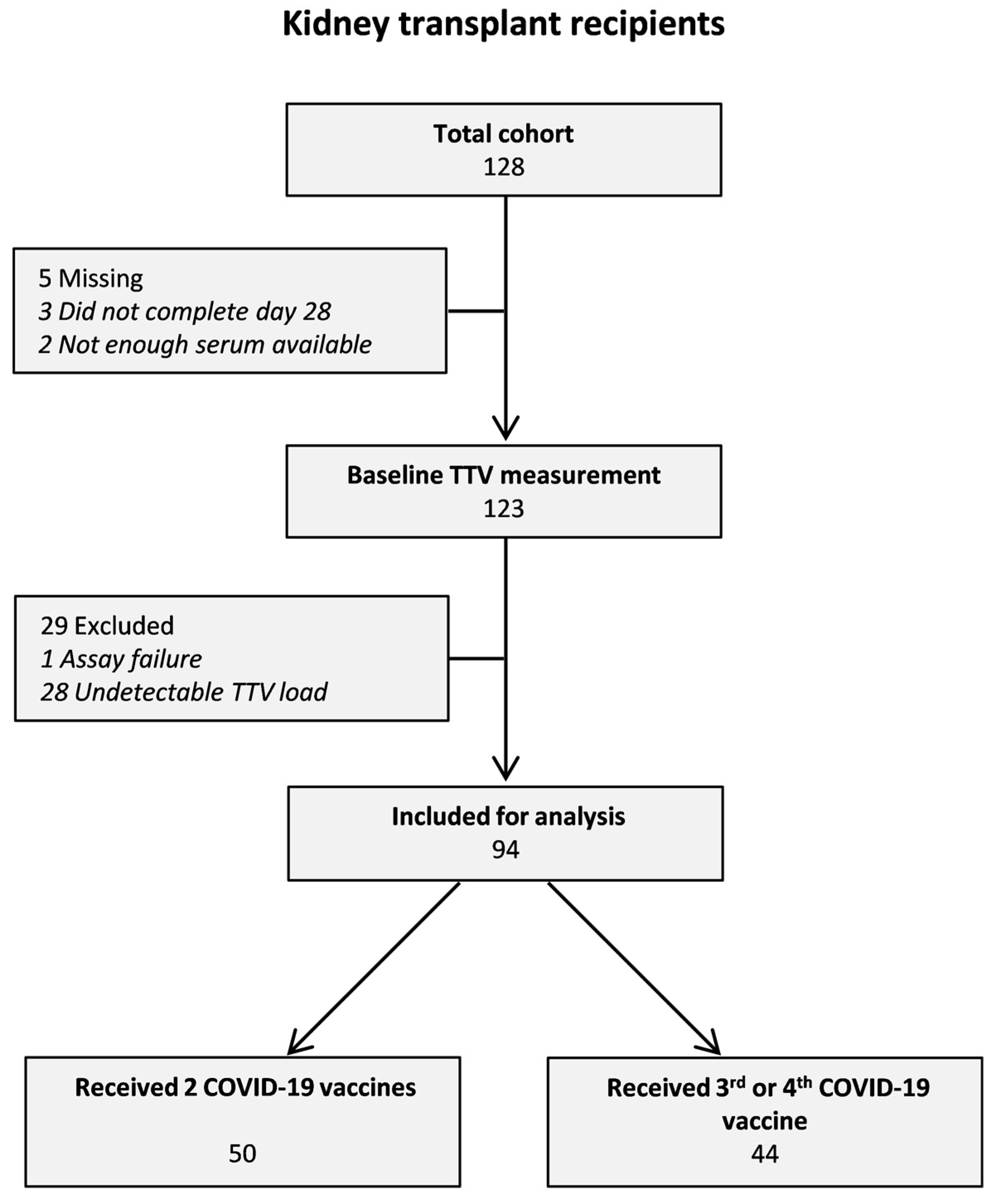
| Total Cohort | TTV-Load | |||
|---|---|---|---|---|
| ≤3.38 | >3.38–<4.75 | ≥4.75 | ||
| (n = 94) | (n = 32) | (n = 31) | (n = 31) | |
| Female, n (%) | 41 (43.6) | 14 (43.8) | 14 (45.2) | 13 (41.9) |
| Caucasian, n (%) | 91 (96.8) | 32 (100) | 30 (96.8) | 29 (93.5) |
| Age (years) | 58.2 ± 12.2 | 58.5 ± 12.9 | 57.5 ± 11.0 | 58.5 ± 13.3 |
| BMI (kg/m2) | 27.2 ± 4.7 | 28.1 ± 5.1 | 26.9 ± 4.5 | 26.6 ± 4.5 |
| Comorbidities, n (%) | ||||
| - Hypertension | 70 (74.5) | 20 (62.5) | 23 (74.2) | 27 (87.1) |
| - Diabetes mellitus | 19 (20.2) | 5 (15.6) | 8 (25.8) | 6 (19.4) |
| - History of malignancy 1 | 10 (10.6) | 3 (9.4) | 5 (16.1) | 2 (6.5) |
| - Auto-immune disease | 8 (8.5) | 3 (9.4) | 2 (6.5) | 3 (9.7) |
| Lymphocytes (109/L) | 1.35 (0.9–2.0) | 1.29 (0.8–1.7) | 1.45 (0.9–2.1) | 1.41 (0.9–2.0) |
| eGFR (mL/min/1.73 m2) | 50.9 ± 18.9 | 46.5 ± 20.3 | 53.8 ± 17.8 | 52.6 ± 18.2 |
| Primary renal diagnosis, n (%) | ||||
| - Immune-mediated disease | 11 (11.7) | 4 (12.5) | 3 (9.7) | 4 (12.9) |
| - Interstitial nephritis | 7 (7.4) | 6 (18.8) | 1 (3.2) | 0 (0.0) |
| - Familial/hereditary renal diseases | 18 (19.1) | 7 (21.9) | 4 (12.9) | 7 (22.6) |
| - Congenital diseases | 9 (9.6) | 4 (12.5) | 3 (9.7) | 2 (6.5) |
| - Vascular diseases | 8 (8.5) | 2 (6.3) | 2 (6.5) | 4 (12.9) |
| - Diabetic kidney disease | 6 (6.4) | 1 (3.1) | 2 (6.5) | 3 (9.7) |
| - Other | 13 (13.8) | 1 (3.1) | 8 (25.8) | 4 (12.9) |
| - Unknown | 22 (23.4) | 7 (21.9) | 8 (25.8) | 7 (22.6) |
| Transplant characteristics | ||||
| - First kidney transplant, n (%) | 83 (88.3) | 27 (84.4) | 28 (90.3) | 28 (90.3) |
| - Time after last transplantation (months) | 41.0 (13.0–85.0) | 63.5 (34.3–114.3) | 45.0 (25.0–88.0) | 13.0 (6.0–54.0) * |
| - Last transplant | ||||
| o Living, n (%) | 62 (66.0) | 24 (75.0) | 20 (64.5) | 18 (58.1) |
| o Pre-emptive, n (%) | 39 (41.5) | 14 (43.8) | 13 (41.9) | 12 (38.7) |
| Immunosuppressive treatment, n (%) | ||||
| - Steroids | 93 (98.9) | 31 (96.9) | 31 (100) | 31 (100) |
| - Mycophenolate mofetil | 84 (89.4) | 28 (87.5) | 28 (90.3) | 28 (90.3) |
| - Calcineurin inhibitor | 89 (94.7) | 28 (87.5) | 31 (100) | 30 (96.8) |
| - Azathioprine | 3 (3.2) | 2 (6.3) | 0 (0.0) | 1 (3.2) |
| - mTOR inhibitor | 2 (2.1) | 0 (0) | 1 (3.2) | 1 (3.2) |
| Tacrolimus trough level (µg/L) | 5.0 (4.3–6.3) | 4.7 (4.2–5.3) | 4.9 (4.1–6.1) | 6.2 (4.9–7.3) ** |
| Number of received COVID-19 vaccinations | ||||
| - 2 | 50 (53.2) | 14 (43.8) | 16 (51.6) | 20 (64.5) |
| - 3 | 33 (35.1) | 15 (46.9) | 11 (35.5) | 7 (22.6) |
| - 4 | 11 (11.7) | 3 (9.4) | 4 (12.9) | 4 (12.9) |
| Time between vaccinations (months) | ||||
| - Between 2nd and 3rd vaccination | 6 (6–7) | - | - | - |
| - Between 3rd and 4th vaccination | 3 (2–3) | - | - | - |
| S1 IgG antibody level after last COVID-19 vaccine (BAU/mL) | 90.0 (5.2–527.8) | 125.3 (5.4–942.0) | 96.5 (14.1–841.0) | 21.4 (0.8–196.4) |
| Univariable | Multivariable | Model 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | St. β | p-Value | B (95% CI) | St. β | p-Value | B (95% CI) | St. β | p-Value | |
| Age (years) | −0.001 (−0.0–0.0) | −0.09 | 0.41 | ||||||
| Female sex | −0.01 (−0.09–0.06) | −0.04 | 0.71 | ||||||
| Time after transplantation (months) | −0.15 (−0.2–−0.1) | −0.44 | <0.001 | −0.16 (−0.2–−0.08) | −0.41 | <0.001 | −0.14 (−0.2–−0.1) | −0.42 | <0.001 |
| Tacrolimus trough level (µg/L) | 0.61 (0.3–1.0) | 0.39 | <0.001 | 0.32 (−0.02–0.6) | 0.20 | 0.06 | |||
| Hypertension (no vs. yes) | 0.10 (0.02–0.18) | 0.25 | 0.02 | 0.08 (−0.0–0.2) | 0.19 | 0.06 | 0.1 (0.01–0.2) | 0.21 | 0.03 |
| Univariable | Multivariable | Model 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B (95% CI) | St. β | p-Value | B (95% CI) | St. β | p-Value | B (95% CI) | St. β | p-Value | |
| TTV load (log copies/mL) | −2.3 (−3.8–−0.8) | −0.30 | 0.004 | −1.55 (−3.2–0.1) | −0.21 | 0.07 | −2.3 (−3.7–−1.0) | −0.30 | <0.001 |
| Hypertension (no vs. yes) | −0.71 (−1.3–−0.08) | −0.20 | 0.03 | −0.22 (−0.8–0.4) | −0.07 | 0.48 | |||
| eGFR (mL/min/1.73 m2) | 0.03 (0.1–0.4) | 0.39 | <0.001 | 0.03 (0.02–0.04) | 0.43 | <0.001 | 0.03 (0.02–0.05) | 0.45 | <0.001 |
| Donor type of last transplant (living vs. deceased donor) | −0.6 (−1.2–−0.02) | −0.21 | 0.04 | −0.42 (−1.0–0.1) | −0.15 | 0.12 | - | ||
| Time after transplantation (months) | 0.62 (0.1–1.1) | 0.24 | 0.02 | 0.44 (−0.2–1.1) | 0.15 | 0.17 | |||
| MMF use (no vs. yes) | −0.77 (−1.7–0.1) | −0.17 | 0.09 | -0.78 (−1.7–0.2) | −0.17 | 0.10 | −1.2 (−2.0–−0.4) | −0.26 | 0.004 |
| Tacrolimus trough level (µg/L) | −3.7 (−6.3–-1.2) | −0.33 | 0.004 | −1.55 (−40–0.9) | −0.14 | 0.21 | |||
| Number of received COVID-19 vaccinations (2 vs. 3 or 4 vaccinations) | 0.60 (0.04–1.1) | 0.22 | 0.04 | 0.53 (−0.03–1.1) | 0.20 | 0.06 | 0.67 (0.2–1.2) | 0.25 | 0.008 |
| Time after Transplantation ≤ 24 Months | Time after Transplantation > 24 Months | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Model 1 | Univariable | Model 1 | |||||||||
| B (95% CI) | St. β | p-Value | B (95% CI) | St. β | p-Value | B (95% CI) | St. β | p-Value | B (95% CI) | St. β | p-Value | |
| TTV load (log copies/mL) | −5.3 (−8.3–−2.3) | −0.55 | 0.001 | −5.3 (−8.3–−2.3) | −0.55 | 0.001 | −0.61 (−2.7–1.5) | −0.08 | 0.55 | |||
| Time after transplantation (months) | 2.08 (0.5–3.7) | 0.44 | 0.01 | −0.15 (−1.3–1.0) | −0.03 | 0.79 | ||||||
| BMI (kg/m2) | 0.10 (−0.01–0.2) | 0.31 | 0.08 | |||||||||
| MMF use (no vs. yes) | −1.61 (−3.3–0.1) | −0.34 | 0.06 | |||||||||
| Tacrolimus trough level (µg/L) | −5.6 (−10–−0.1) | −0.44 | 0.02 | |||||||||
| Donor type of last transplant (living vs. deceased donor) | −0.64 (−1.3–0.7) | −0.23 | 0.07 | −0.62 (−1.2–−0.03) | −0.22 | 0.04 | ||||||
| eGFR (mL/min/1.73 m2) | 0.03 (0.2–0.5) | 0.51 | <0.001 | 0.03 (0.02–0.05) | 0.51 | <0.001 | ||||||
| Number of received COVID-19 vaccinations (2 vs. 3 or 4 vaccinations) | 0.62 (−0.03–1.3) | 0.24 | 0.06 | 0.57 (0.03–1.1) | 0.22 | 0.04 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imhof, C.; Messchendorp, L.; van Baarle, D.; Gansevoort, R.T.; Van Leer-Buter, C.; Sanders, J.-S.F. The Time-Dependent Association of Torque Teno Virus Load with the Level of SARS-CoV-2 S1 IgG Antibodies Following COVID-19 Vaccination in Kidney Transplant Recipients. Viruses 2023, 15, 2189. https://doi.org/10.3390/v15112189
Imhof C, Messchendorp L, van Baarle D, Gansevoort RT, Van Leer-Buter C, Sanders J-SF. The Time-Dependent Association of Torque Teno Virus Load with the Level of SARS-CoV-2 S1 IgG Antibodies Following COVID-19 Vaccination in Kidney Transplant Recipients. Viruses. 2023; 15(11):2189. https://doi.org/10.3390/v15112189
Chicago/Turabian StyleImhof, Céline, Lianne Messchendorp, Debbie van Baarle, Ron T. Gansevoort, Coretta Van Leer-Buter, and Jan-Stephan F. Sanders. 2023. "The Time-Dependent Association of Torque Teno Virus Load with the Level of SARS-CoV-2 S1 IgG Antibodies Following COVID-19 Vaccination in Kidney Transplant Recipients" Viruses 15, no. 11: 2189. https://doi.org/10.3390/v15112189
APA StyleImhof, C., Messchendorp, L., van Baarle, D., Gansevoort, R. T., Van Leer-Buter, C., & Sanders, J.-S. F. (2023). The Time-Dependent Association of Torque Teno Virus Load with the Level of SARS-CoV-2 S1 IgG Antibodies Following COVID-19 Vaccination in Kidney Transplant Recipients. Viruses, 15(11), 2189. https://doi.org/10.3390/v15112189






