Immunogenicity and Tolerability of a SARS-CoV-2 TNX-1800, a Live Recombinant Poxvirus Vaccine Candidate, in Syrian Hamsters and New Zealand White Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccine Information
2.2. Ethics Statement
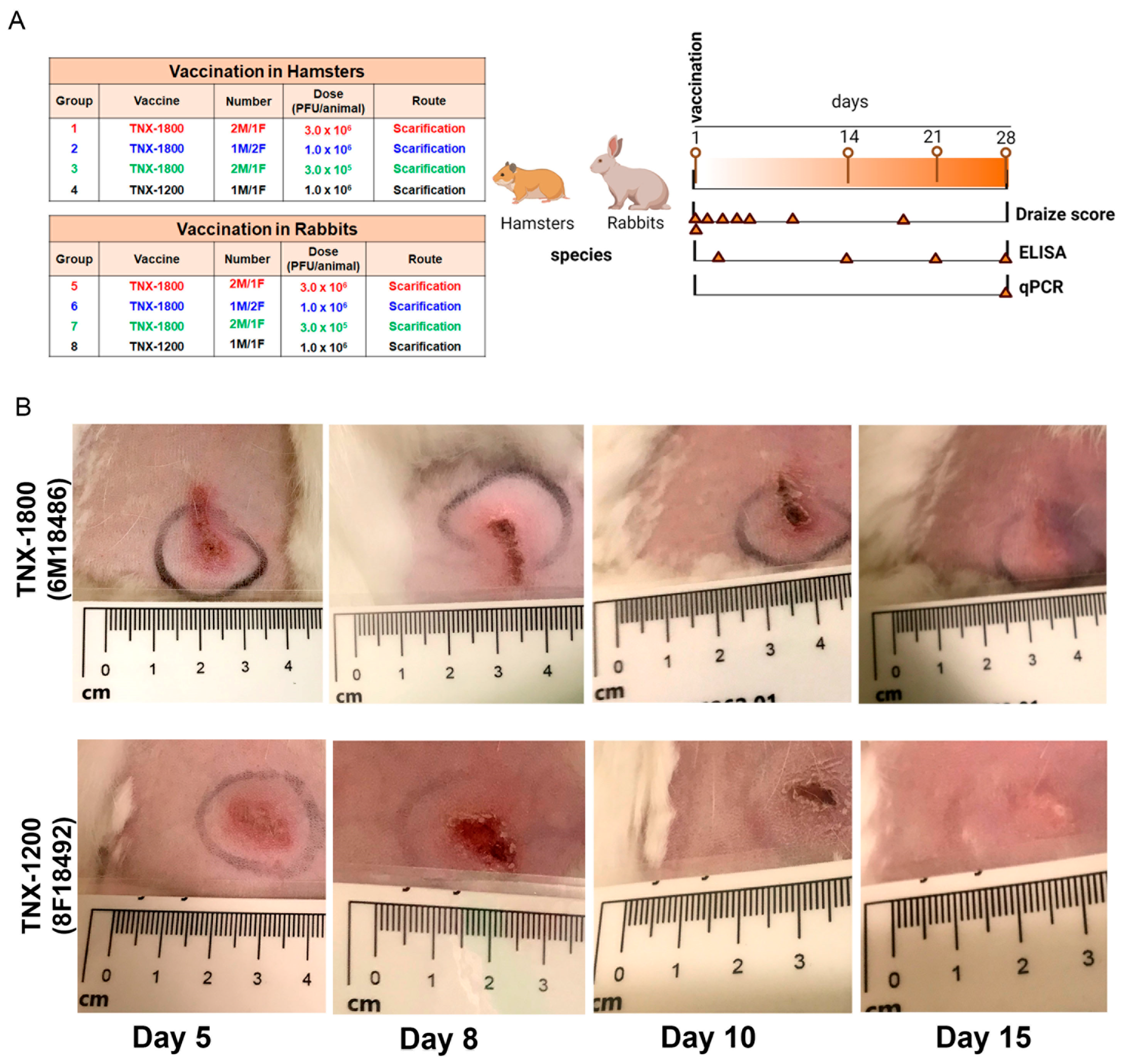
2.3. Study Design and Immunization Procedure
2.4. Body Weight, Lesion Counts, and Draize Scoring
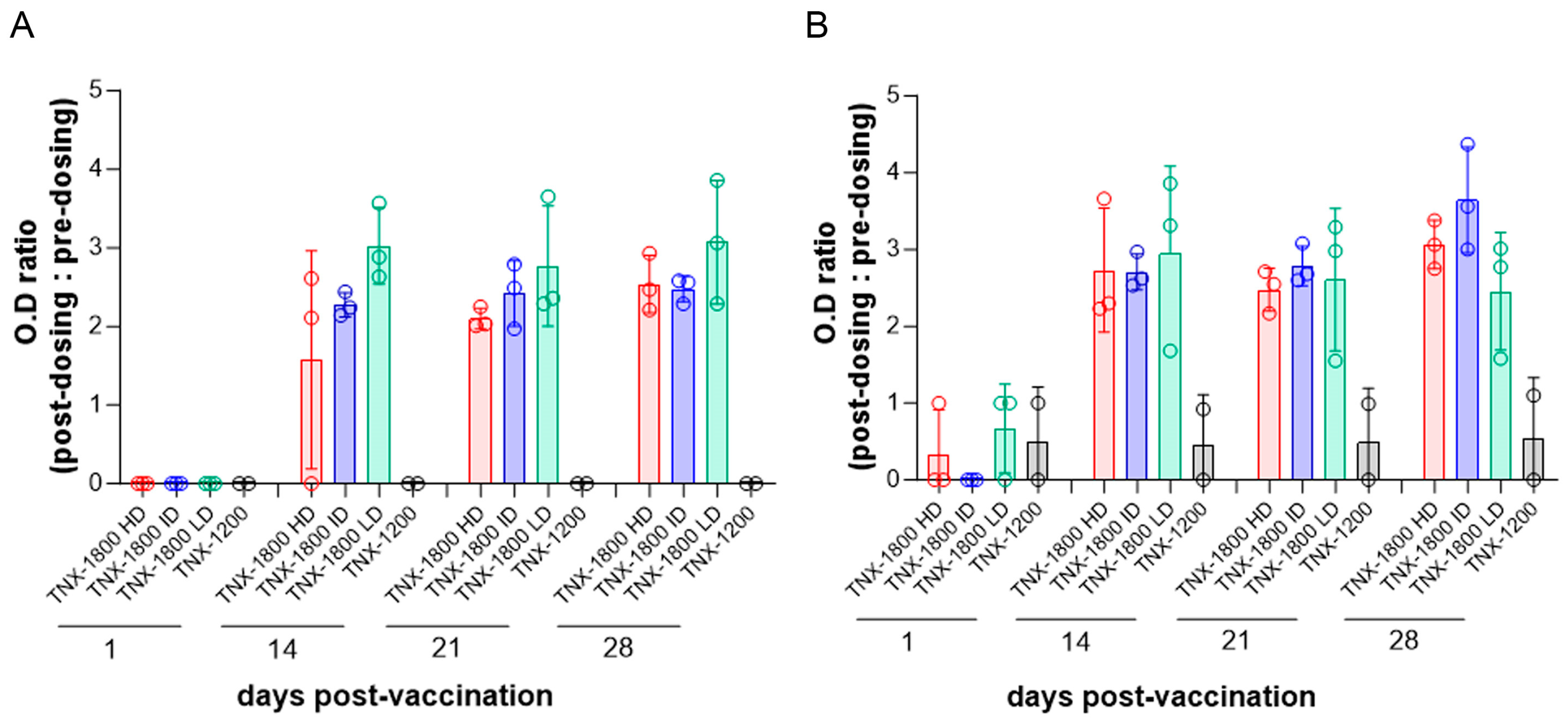
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Viral Load by Quantitative PCR (qPCR) Analysis
2.7. Statistical Analysis
3. Results
3.1. Post-Dosing Clinical Observations and Vaccine Persistence
3.2. TNX-1800 Immunogenicity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Singh, R.; Kaur, J.; Pandey, S.; Sharma, V.; Thakur, L.; Sati, S.; Mani, S.; Asthana, S.; Sharma, T.K.; et al. Wuhan to World: The COVID-19 Pandemic. Front. Cell. Infect. Microbiol. 2021, 11, 596201. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Q.; Cuomo, R.; Purushothaman, V.; Mackey, T. Data Mining and Content Analysis of the Chinese Social Media Platform Weibo During the Early COVID-19 Outbreak: Retrospective Observational Infoveillance Study. JMIR Public Health Surveill. 2020, 6, e18700. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 14.9 Million Excess Deaths Associated with the COVID-19 Pandemic in 2020 and 2021. 2022. Available online: https://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-associated-with-the-covid-19-pandemic-in-2020-and-2021 (accessed on 11 September 2023).
- Havlin, J.; Svorcova, M.; Dvorackova, E.; Lastovicka, J.; Lischke, R.; Kalina, T.; Hubacek, P. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J. Heart Lung Transplant. 2021, 40, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, M.; Niu, X.; Hanson, J.; Jung, K.; Ru, P.; Tu, H.; Jones, D.M.; Vlasova, A.N.; Saif, L.J.; et al. The Cold-Adapted, Temperature-Sensitive SARS-CoV-2 Strain TS11 Is Attenuated in Syrian Hamsters and a Candidate Attenuated Vaccine. Viruses 2022, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Anez, G.; Adelglass, J.M.; Barrat Hernandez, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Guo, X.; Hong, D.; Gong, Q.; Xie, W.; Li, T.; Wang, J.; Chuai, X.; Chiu, S. Duration of humoral immunity from smallpox vaccination and its cross-reaction with Mpox virus. Signal Transduct. Target. Ther. 2023, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Noyce, R.S.; Westfall, L.W.; Fogarty, S.; Gilbert, K.; Mpanju, O.; Stillwell, H.; Esparza, J.; Daugherty, B.; Koide, F.; Evans, D.H.; et al. Single Dose of Recombinant Chimeric Horsepox Virus (TNX-801) Vaccination Protects Macaques from Lethal Monkeypox Challenge. Viruses 2023, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Noyce, R.S.; Lederman, S.; Evans, D.H. Construction of an infectious horsepox virus vaccine from chemically synthesized DNA fragments. PLoS ONE 2018, 13, e0188453. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; de Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA 2021, 325, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: A randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect. Dis. 2022, 22, 196–208. [Google Scholar] [CrossRef]
- Fraser, R.; Orta-Resendiz, A.; Mazein, A.; Dockrell, D.H. Upper respiratory tract mucosal immunity for SARS-CoV-2 vaccines. Trends Mol. Med. 2023, 29, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, R.; Singh, A.; Kumar, S. SARS-CoV-2: Immunity, Challenges with Current Vaccines, and a Novel Perspective on Mucosal Vaccines. Vaccines 2023, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Mohamed, S.; Sanders, E.C.; Gommerman, J.L.; Tal, M.C. Guardians of the oral and nasopharyngeal galaxy: IgA and protection against SARS-CoV-2 infection. Immunol. Rev. 2022, 309, 75–85. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awasthi, M.; Macaluso, A.; Goebel, S.J.; Luea, E.; Noyce, R.S.; Nasar, F.; Daugherty, B.; Bavari, S.; Lederman, S. Immunogenicity and Tolerability of a SARS-CoV-2 TNX-1800, a Live Recombinant Poxvirus Vaccine Candidate, in Syrian Hamsters and New Zealand White Rabbits. Viruses 2023, 15, 2131. https://doi.org/10.3390/v15102131
Awasthi M, Macaluso A, Goebel SJ, Luea E, Noyce RS, Nasar F, Daugherty B, Bavari S, Lederman S. Immunogenicity and Tolerability of a SARS-CoV-2 TNX-1800, a Live Recombinant Poxvirus Vaccine Candidate, in Syrian Hamsters and New Zealand White Rabbits. Viruses. 2023; 15(10):2131. https://doi.org/10.3390/v15102131
Chicago/Turabian StyleAwasthi, Mayanka, Anthony Macaluso, Scott J. Goebel, Erin Luea, Ryan S. Noyce, Farooq Nasar, Bruce Daugherty, Sina Bavari, and Seth Lederman. 2023. "Immunogenicity and Tolerability of a SARS-CoV-2 TNX-1800, a Live Recombinant Poxvirus Vaccine Candidate, in Syrian Hamsters and New Zealand White Rabbits" Viruses 15, no. 10: 2131. https://doi.org/10.3390/v15102131
APA StyleAwasthi, M., Macaluso, A., Goebel, S. J., Luea, E., Noyce, R. S., Nasar, F., Daugherty, B., Bavari, S., & Lederman, S. (2023). Immunogenicity and Tolerability of a SARS-CoV-2 TNX-1800, a Live Recombinant Poxvirus Vaccine Candidate, in Syrian Hamsters and New Zealand White Rabbits. Viruses, 15(10), 2131. https://doi.org/10.3390/v15102131





