Development of Quantitative Real-Time PCR and Loop-Mediated Isothermal Amplification Assays for the Surveillance and Diagnosis of Herpes B Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection and DNA Extraction
2.3. Preparation of the BV DNA Fragments
2.4. qPCR for Validation of Viral DNA Quantity
2.5. LAMP Primer Design
2.6. LAMP Reaction
2.7. Sanger Sequencing to Confirm the Conserved Primer Regions among BV Strains
2.8. BV Phylogenetic Analysis
3. Results
3.1. Development of a Novel qPCR Assay to Detect BV
3.2. Evaluation of the qPCR Assay Using Monkey Specimens
3.3. Development of the LAMP Assay for BV
3.4. Validation of the LAMP Assay Using Monkey Specimens
3.5. Sequence Analyses for the Primer Binding Sites
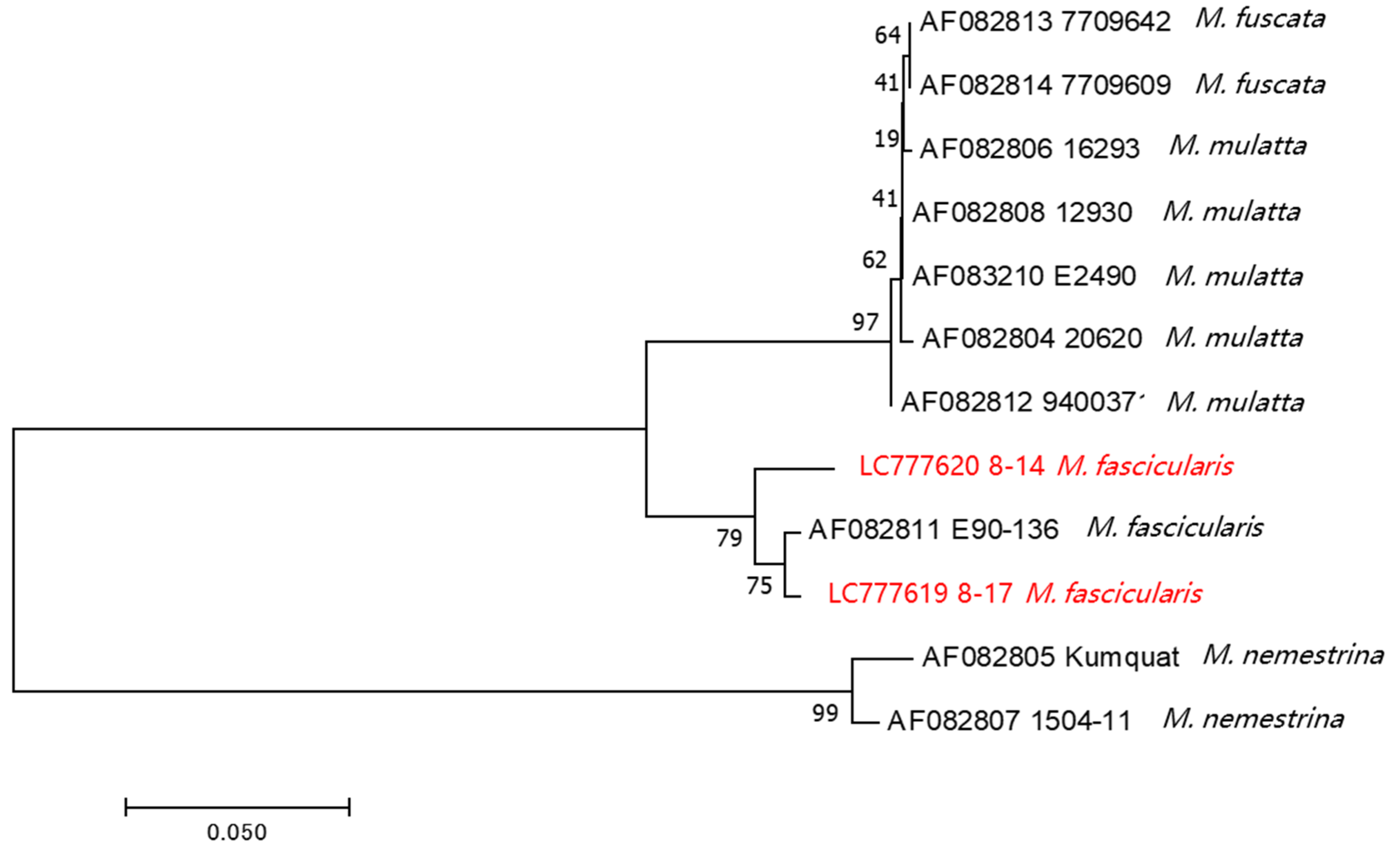
3.6. Phylogenetic Analysis of BV Strains Isolated from Thai Cynomolgus Macaques
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jainkittivong, A.; Langlais, R.P. Herpes B Virus Infection. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1998, 85, 399–403. [Google Scholar] [CrossRef]
- Gumert, M.D. The Common Monkey of Southeast Asia: Long-Tailed Macaque Populations, Ethnophoresy, and Their Occurrence in Human Environments. In Monkeys on the Edge: Ecology and Management of Long-Tailed Macaques and Their Interface with Humans; Fuentes, A., Jones-Engel, L., Gumert, M.D., Eds.; Cambridge Studies in Biological and Evolutionary Anthropology; Cambridge University Press: Cambridge, UK, 2011; pp. 3–44. [Google Scholar] [CrossRef]
- Lee, M.-H.; Rostal, M.K.; Hughes, T.; Sitam, F.; Lee, C.-Y.; Japning, J.; Harden, M.E.; Griffiths, A.; Basir, M.; Wolfe, N.D.; et al. Macacine Herpesvirus 1 in Long-Tailed Macaques, Malaysia, 2009–2011. Emerg. Infect. Dis. J. CDC 2015, 21, 1107–1113. [Google Scholar] [CrossRef]
- Estep, R.D.; Messaoudi, I.; Wong, S.W. Simian Herpesviruses and Their Risk to Humans. Vaccine 2010, 28, B78–B84. [Google Scholar] [CrossRef][Green Version]
- Sato, H.; Arikawa, J.; Furuya, M.; Kitoh, J.; Mannen, K.; Nishimune, Y.; Ohsawa, K.; Serikawa, T.; Shibahara, T.; Watanabe, Y.; et al. Prevalence of Herpes B Virus Antibody in Nonhuman Primates Reared at the National University of Japan. Exp. Anim. 1998, 47, 199–202. [Google Scholar] [CrossRef][Green Version]
- Engel, G.A.; Jones-Engel, L.; Schillaci, M.A.; Suaryana, K.G.; Putra, A.; Fuentes, A.; Henkel, R. Human Exposure to Herpesvirus B–Seropositive Macaques, Bali, Indonesia. Emerg. Infect. Dis. 2002, 8, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D. Record Number of Monkeys Being Used in U.S. Research. Available online: https://www.science.org/content/article/record-number-monkeys-being-used-us-research (accessed on 8 June 2023).
- Wang, W.; Qi, W.; Liu, J.; Du, H.; Zhao, L.; Zheng, Y.; Wang, G.; Pan, Y.; Huang, B.; Feng, Z.; et al. First Human Infection Case of Monkey B Virus Identified in China, 2021. China CDC Wkly. 2021, 3, 632–633. [Google Scholar] [CrossRef] [PubMed]
- Sabin, A.B.; Wright, A.M. Acute ascending myelitis following a monkey bite, with the isolation of a virus capable of reproducing the disease. J. Exp. Med. 1934, 59, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I.; Davenport, D.S.; Stewart, J.A.; Deitchman, S.; Hilliard, J.K.; Chapman, L.E. The B Virus Working Group. Recommendations for Prevention of and Therapy for Exposure to B Virus (Cercopithecine herpesvirus 1). Clin. Infect. Dis. 2002, 35, 1191–1203. [Google Scholar] [CrossRef]
- Arvin, A.; Campadelli-Fiume, G.; Mocarski, E.; Moore, P.S.; Roizman, B.; Whitley, R.; Yamanishi, K. (Eds.) Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Fujima, A.; Ochiai, Y.; Saito, A.; Omori, Y.; Noda, A.; Kazuyama, Y.; Shoji, H.; Tanabayashi, K.; Ueda, F.; Yoshikawa, Y.; et al. Discrimination of Antibody to Herpes B Virus from Antibody to Herpes Simplex Virus Types 1 and 2 in Human and Macaque Sera. J. Clin. Microbiol. 2008, 46, 56–61. [Google Scholar] [CrossRef]
- Hilliard, J.K.; Eberle, R.; Lipper, S.L.; Munoz, R.M.; Weiss, S.A. Herpesvirus Simiae (B Virus): Replication of the Virus and Identification of Viral Polypeptides in Infected Cells. Arch. Virol. 1987, 93, 185–198. [Google Scholar] [CrossRef]
- Perelygina, L.; Patrusheva, I.; Manes, N.; Wildes, M.J.; Krug, P.; Hilliard, J.K. Quantitative Real-Time PCR for Detection of Monkey B Virus (Cercopithecine herpesvirus 1) in Clinical Samples. J. Virol. Methods 2003, 109, 245–251. [Google Scholar] [CrossRef]
- Notomi, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res. 2000, 28, 63e. [Google Scholar] [CrossRef]
- Yoshikawa, R.; Abe, H.; Igasaki, Y.; Negishi, S.; Goto, H.; Yasuda, J. Development and Evaluation of a Rapid and Simple Diagnostic Assay for COVID-19 Based on Loop-Mediated Isothermal Amplification. PLoS Negl. Trop. Dis. 2020, 14, e0008855. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, Y.; Takada, A.; Ebihara, H.; Grolla, A.; Kamo, N.; Feldmann, H.; Kawaoka, Y.; Yasuda, J. Rapid and Simple Detection of Ebola Virus by Reverse Transcription-Loop-Mediated Isothermal Amplification. J. Virol. Methods 2007, 141, 78–83. [Google Scholar] [CrossRef]
- Kurosaki, Y.; Magassouba, N.; Oloniniyi, O.K.; Cherif, M.S.; Sakabe, S.; Takada, A.; Hirayama, K.; Yasuda, J. Development and Evaluation of Reverse Transcription-Loop-Mediated Isothermal Amplification (RT-LAMP) Assay Coupled with a Portable Device for Rapid Diagnosis of Ebola Virus Disease in Guinea. PLoS Negl. Trop. Dis. 2016, 10, e0004472. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, Y.; Magassouba, N.; Bah, H.A.; Soropogui, B.; Doré, A.; Kourouma, F.; Cherif, M.S.; Keita, S.; Yasuda, J. Deployment of a Reverse Transcription Loop-Mediated Isothermal Amplification Test for Ebola Virus Surveillance in Remote Areas in Guinea. J. Infect. Dis. 2016, 214, S229–S233. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, Y.; Grolla, A.; Fukuma, A.; Feldmann, H.; Yasuda, J. Development and Evaluation of a Simple Assay for Marburg Virus Detection Using a Reverse Transcription-Loop-Mediated Isothermal Amplification Method. J. Clin. Microbiol. 2010, 48, 2330–2336. [Google Scholar] [CrossRef]
- Kurosaki, Y.; Martins, D.B.G.; Kimura, M.; dos Catena, A.S.; Borba, M.A.C.S.M.; da Mattos, S.S.; Abe, H.; Yoshikawa, R.; de Lima Filho, J.L.; Yasuda, J. Development and Evaluation of a Rapid Molecular Diagnostic Test for Zika Virus Infection by Reverse Transcription Loop-Mediated Isothermal Amplification. Sci. Rep. 2017, 7, 13503. [Google Scholar] [CrossRef]
- Kurosaki, Y.; Okada, S.; Nakamae, S.; Yasuda, J. A Loop-Mediated Isothermal Amplification Assay for Rapid and Sensitive Detection of Bovine Papular Stomatitis Virus. J. Virol. Methods 2016, 238, 42–47. [Google Scholar] [CrossRef]
- Fukuma, A.; Kurosaki, Y.; Morikawa, Y.; Grolla, A.; Feldmann, H.; Yasuda, J. Rapid Detection of Lassa Virus by Reverse Transcription-Loop-Mediated Isothermal Amplification: Detection of Lassa Virus by RT-LAMP. Microbiol. Immunol. 2011, 55, 44–50. [Google Scholar] [CrossRef]
- Pemba, C.M.; Kurosaki, Y.; Yoshikawa, R.; Oloniniyi, O.K.; Urata, S.; Sueyoshi, M.; Zadeh, V.R.; Nwafor, I.; Iroezindu, M.O.; Ajayi, N.A.; et al. Development of an RT-LAMP Assay for the Detection of Lassa Viruses in Southeast and South-Central Nigeria. J. Virol. Methods 2019, 269, 30–37. [Google Scholar] [CrossRef]
- Oloniniyi, O.K.; Kurosaki, Y.; Miyamoto, H.; Takada, A.; Yasuda, J. Rapid Detection of All Known Ebolavirus Species by Reverse Transcription-Loop-Mediated Isothermal Amplification (RT-LAMP). J. Virol. Methods 2017, 246, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Howson, E.L.A.; Kurosaki, Y.; Yasuda, J.; Takahashi, M.; Goto, H.; Gray, A.R.; Mioulet, V.; King, D.P.; Fowler, V.L. Defining the Relative Performance of Isothermal Assays That Can Be Used for Rapid and Sensitive Detection of Foot-and-Mouth Disease Virus. J. Virol. Methods 2017, 249, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Do, J.; Mahalanabis, M.; Fan, A.; Zhao, L.; Jepeal, L.; Singh, S.K.; Klapperich, C.M. Low Cost Extraction and Isothermal Amplification of DNA for Infectious Diarrhea Diagnosis. PLoS ONE 2013, 8, e60059. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Black, D.H.; Eberle, R. Molecular Evidence for Distinct Genotypes of Monkey B Virus (Herpesvirus Simiae) Which Are Related to the Macaque Host Species. J. Virol. 1998, 72, 9224–9232. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhao, Y.; Ren, X.; Zhou, X.; Zhang, C.; Wan, Z.; Kuang, Y.-Q. Rapid Detection of Monkeypox Virus and Monkey B Virus by a Multiplex Loop-Mediated Isothermal Amplification Assay. J. Infect. 2023, 86, e114–e116. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, J.T.; Wald, A.; Selke, S.; Corey, L.; Magaret, A. The Kinetics of Mucosal Herpes Simplex Virus–2 Infection in Humans: Evidence for Rapid Viral-Host Interactions. J. Infect. Dis. 2011, 204, 554–561. [Google Scholar] [CrossRef]
- Schiffer, J.T.; Mayer, B.T.; Fong, Y.; Swan, D.A.; Wald, A. Herpes Simplex Virus-2 Transmission Probability Estimates Based on Quantity of Viral Shedding. J. R. Soc. Interface 2014, 11, 20140160. [Google Scholar] [CrossRef]
- Oliveira, B.B.; Veigas, B.; Baptista, P.V. Isothermal Amplification of Nucleic Acids: The Race for the Next “Gold Standard”. Front. Sens. 2021, 2, 752600. [Google Scholar] [CrossRef]
- Tamanaha, E.; Zhang, Y.; Tanner, N.A. Profiling RT-LAMP Tolerance of Sequence Variation for SARS-CoV-2 RNA Detection. PLoS ONE 2022, 17, e0259610. [Google Scholar] [CrossRef]
- Bunlungsup, S.; Imai, H.; Hamada, Y.; Matsudaira, K.; Malaivijitnond, S. Mitochondrial DNA and Two Y-Chromosome Genes of Common Long-Tailed Macaques (Macaca fascicularis fascicularis) throughout Thailand and Vicinity. Am. J. Primatol. 2016, 79, e22596. [Google Scholar] [CrossRef] [PubMed]
- Matsudaira, K.; Ishida, T.; Malaivijitnond, S.; Reichard, U.H. Short Dispersal Distance of Males in a Wild White-Handed Gibbon (Hylobates lar) Population. Am. J. Phys. Anthropol. 2018, 167, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Osada, N.; Matsudaira, K.; Hamada, Y.; Malaivijitnond, S. Testing Sex-Biased Admixture Origin of Macaque Species Using Autosomal and X-Chromosomal Genomic Sequences. Genome Biol. Evol. 2020, 13, evaa209. [Google Scholar] [CrossRef]
- Phadphon, P.; Kanthaswamy, S.; Oldt, R.F.; Hamada, Y.; Malaivijitnond, S. Population Structure of Macaca fascicularis aurea, and Their Genetic Relationships with M. f. fascicularis and M. mulatta Determined by 868 RADseq-Derived Autosomal SNPs—A Consideration for Biomedical Research. J. Med. Primatol. 2022, 51, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kolb, A.W.; Brandt, C.R. Genomic Nucleotide-Based Distance Analysis for Delimiting Old World Monkey Derived Herpes Simplex Virus Species. BMC Genom. 2020, 21, 436. [Google Scholar] [CrossRef]
- Fooden, J. Provisional Classifications and Key to Living Species of Macaques (Primates: Macaca). Folia Primatol. Int. J. Primatol. 1976, 25, 225–236. [Google Scholar] [CrossRef]
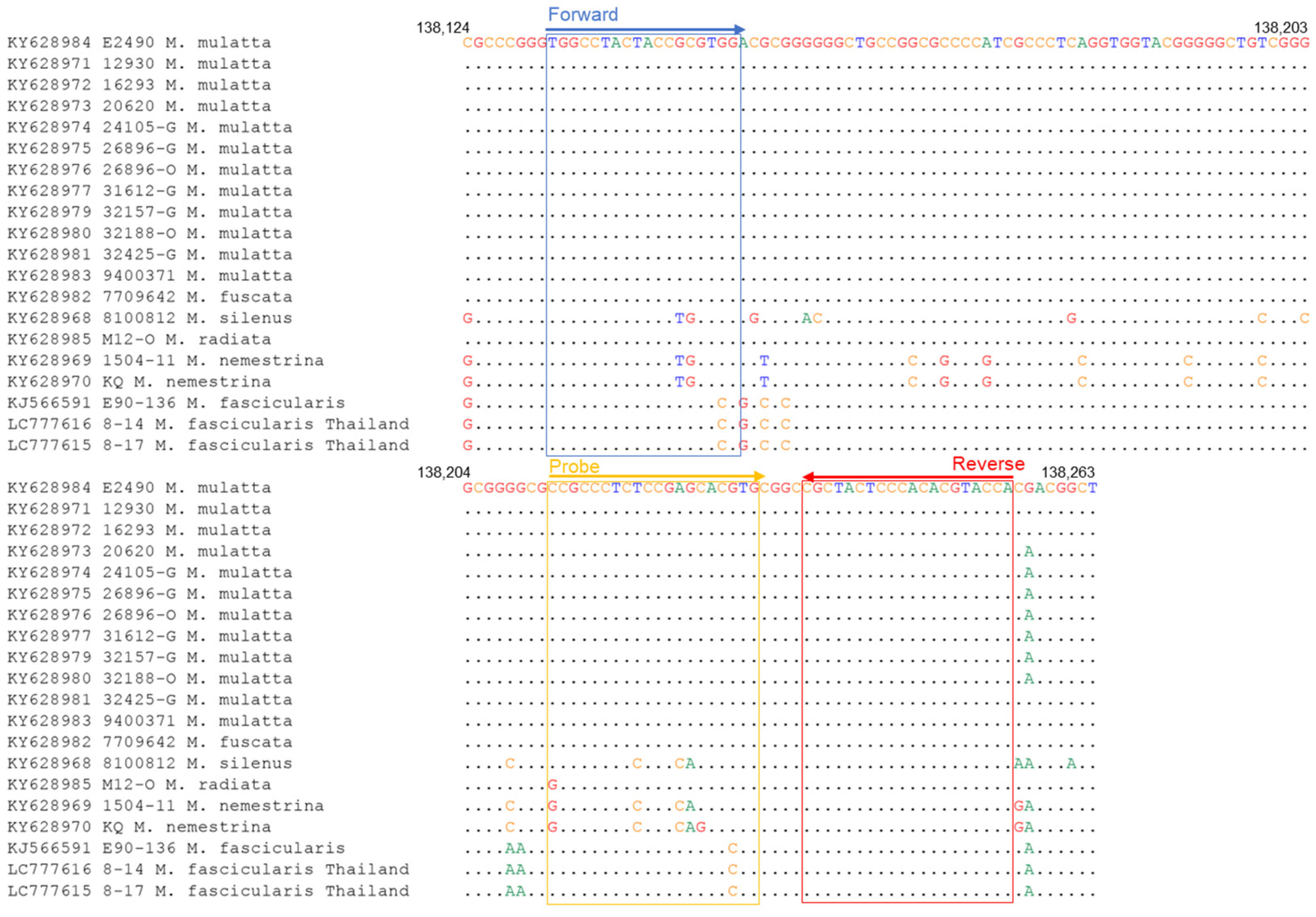

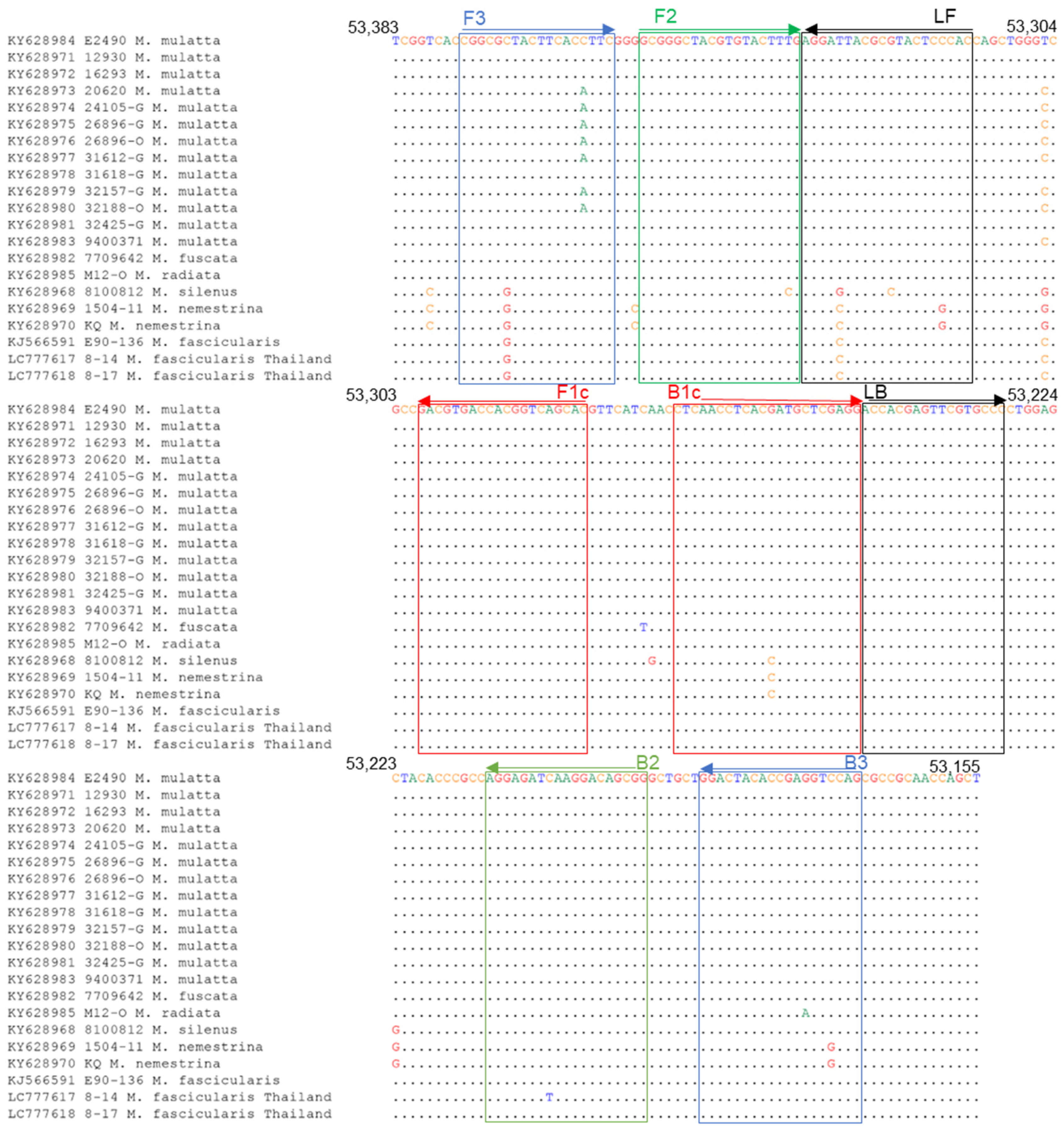
| Primer Set | Primer | Sequence (5′-3′) |
|---|---|---|
| qPCR-gG | BV-qPCR-gG-323_Fw | TGGCCTACTACCGCGTGG |
| BV-qPCR-gG-446_Rv | TGGTACGTGTGGGAGTAGCG | |
| BV-qPCR-gG-403_Probe | CCGCCCTCTCCGAGCACGTG | |
| qPCR-UL29 | BV-qPCR_UL29_Fw | TTGTCCGACTTCTGGTAGGC |
| BV-qPCR_UL29_Rv | AAGGAGATGCGCGTCAAG | |
| BV-qPCR_UL29_Probe | CCGGCRAAGACCACGCGGTT | |
| BV-gG_1st | BV-gG_Fw_1 | GACCCCGCGTACTGCTAC |
| BV-gG_Rv_1 | CCCACCAGGATCTCGTAGTC | |
| BV-gG_nested | BV-gG_Fw_2 | GCCGAYGTCGACAGACAT |
| BV-gG_Rv_1 | CCCACCAGGATCTCGTAGTC | |
| LAMP-UL27 | LAMP_UL27_F3 | CGGCGCTACTTCACCTTC |
| LAMP_UL27_B3 | GGACCTCGGTGTAGTCCAG | |
| LAMP_UL27_FIP | GTGCTGACCGTGGTCACGTCGCGGGCTACGTGTACTTTG | |
| LAMP_UL27_BIP | CTCAACCTCACGATGCTCGAGGCCGCTGTCCTTGATCTCCT | |
| LAMP_UL27_LF | GTGGGAGTACGCGTAATCCT | |
| LAMP_UL27_LB | ACCACGAGTTCGTGCCC |
| DNA | E2490 | E90-136 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qPCR | qPCR-gG | qPCR-UL29 | qPCR-gG | qPCR-UL29 | ||||||||
| No. of DNA Copy | Ct Value | Positive | Ct Value | Positive | Ct Value | Positive | Ct Value | Positive | ||||
| 1 × 106 | 21.16 | 21.18 | 2/2 | 20.68 | 20.37 | 2/2 | 23.16 | 23.01 | 2/2 | 18.99 | 18.96 | 2/2 |
| 1 × 105 | 24.65 | 24.47 | 2/2 | 23.33 | 22.88 | 2/2 | 27.49 | 27.57 | 2/2 | 22.38 | 22.5 | 2/2 |
| 1 × 104 | 27.91 | 27.71 | 2/2 | 26.33 | 26.2 | 2/2 | 30.48 | 30.46 | 2/2 | 25.77 | 26.1 | 2/2 |
| 1 × 103 | 31.21 | 30.93 | 2/2 | 29.76 | 29.7 | 2/2 | 33.28 | 33.76 | 2/2 | 29.36 | 30.31 | 2/2 |
| 1 × 102 | 34.89 | 34.74 | 2/2 | 33.12 | 33.06 | 2/2 | 39.3 | - | 1/2 | 33.17 | 33.3 | 2/2 |
| 1 × 101 | 37.52 | 37.12 | 2/2 | 38.49 | 38.3 | 2/2 | - | - | 0/2 | 38.58 | 38.3 | 2/2 |
| 1 × 100 | - | - | 0/2 | - | - | 0/2 | - | - | 0/2 | - | - | 0/2 |
| Sample ID | qPCR-gG | qPCR-UL29 | Nested PCR-gG | ||
|---|---|---|---|---|---|
| Average of Ct Value | Estimated DNA Copy Number | Average of Ct Value | Estimated DNA Copy Number | ||
| 8-1 | 36.17 | 3.23 | - | - | - |
| 8-2 | 36.93 | 1.99 | - | - | - |
| 8-3 | 36.39 | 2.81 | - | - | - |
| 8-4 | 34.95 | 7.02 | - | - | - |
| 8-5 | 35.78 | 4.14 | - | - | - |
| 8-6 | 38.14 | 0.92 | - | - | - |
| 8-7 | 37 | 1.91 | - | - | - |
| 8-8 | 36.43 | 2.74 | - | - | - |
| 8-9 | 36.67 | 2.35 | - | - | - |
| 8-10 | 37.85 | 0.58 | - | - | - |
| 8-11 | 37.11 | 0.95 | - | - | - |
| 8-12 | 37.58 | 0.69 | - | - | - |
| 8-13 | 37.36 | 0.8 | - | - | - |
| 8-14 | 38.3 | 1.25 | 28 | 12,748 | + |
| 8-15 | 36.95 | 1.06 | - | - | - |
| 8-16 | 36.11 | 1.87 | - | - | - |
| 8-17 | 33 | 15.95 | 24.6 | 177,970 | + |
| 8-18 | 35.14 | 3.56 | - | - | - |
| 8-19 | 35.58 | 2.65 | - | - | - |
| DNA | E2490 | E90-136 | ||
|---|---|---|---|---|
| DNA Copies/ Reaction | Amplification Time (mm:ss) | Amplification Time (mm:ss) | ||
| Run 1 | Run 2 | Run 1 | Run 2 | |
| 5 × 106 | 6:46 | 6:31 | 8:16 | 7:46 |
| 5 × 105 | 7:16 | 7:16 | 9:31 | 9:01 |
| 5 × 104 | 12:01 | 8:16 | 10:31 | 10:16 |
| 5 × 103 | 15:16 | 9:31 | 12:31 | 12:16 |
| 5 × 102 | 15:16 | 11:16 | 19:16 | 16:16 |
| 5 × 101 | 24:16 | 21:31 | 17:46 | 18:16 |
| 5 × 100 | - | - | - | - |
| LAMP Assay | |||
|---|---|---|---|
| No. of Positive Samples | No. of Negative Samples | ||
| qPCR assay | No. of positive samples | 2/97 | 0/97 |
| No. of negative samples | 0/97 | 95/97 | |
| Sample ID | LAMP-UL27 | ||
|---|---|---|---|
| Amplification Time (mm:ss) | Average (mm:ss) | ||
| 8-14 | 12:31 | 13:46 | 13:08 |
| 8-17 | 9:46 | 10:01 | 9:54 |
| DNA | DNA Based on No. 8-14 | |
|---|---|---|
| DNA Copies/Reaction | Amplification Time (mm:ss) | |
| Run 1 | Run 2 | |
| 5 × 106 | 8:31 | 8:46 |
| 5 × 105 | 10:01 | 10:13 |
| 5 × 104 | 11:46 | 11:16 |
| 5 × 103 | 14:01 | 13:46 |
| 5 × 102 | 17:31 | 16:46 |
| 5 × 101 | 22:01 | - |
| 5 × 100 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amano, M.; Sapkanarak, K.; Thbthimthong, W.; Meesawat, S.; Kemthong, T.; Suttisan, N.; Abe, H.; Malaivijitnond, S.; Yasuda, J. Development of Quantitative Real-Time PCR and Loop-Mediated Isothermal Amplification Assays for the Surveillance and Diagnosis of Herpes B Virus Infection. Viruses 2023, 15, 2086. https://doi.org/10.3390/v15102086
Amano M, Sapkanarak K, Thbthimthong W, Meesawat S, Kemthong T, Suttisan N, Abe H, Malaivijitnond S, Yasuda J. Development of Quantitative Real-Time PCR and Loop-Mediated Isothermal Amplification Assays for the Surveillance and Diagnosis of Herpes B Virus Infection. Viruses. 2023; 15(10):2086. https://doi.org/10.3390/v15102086
Chicago/Turabian StyleAmano, Murasaki, Krittiga Sapkanarak, Wipaporn Thbthimthong, Suthirote Meesawat, Taratorn Kemthong, Nutchanat Suttisan, Haruka Abe, Suchinda Malaivijitnond, and Jiro Yasuda. 2023. "Development of Quantitative Real-Time PCR and Loop-Mediated Isothermal Amplification Assays for the Surveillance and Diagnosis of Herpes B Virus Infection" Viruses 15, no. 10: 2086. https://doi.org/10.3390/v15102086
APA StyleAmano, M., Sapkanarak, K., Thbthimthong, W., Meesawat, S., Kemthong, T., Suttisan, N., Abe, H., Malaivijitnond, S., & Yasuda, J. (2023). Development of Quantitative Real-Time PCR and Loop-Mediated Isothermal Amplification Assays for the Surveillance and Diagnosis of Herpes B Virus Infection. Viruses, 15(10), 2086. https://doi.org/10.3390/v15102086






