Influenza A and B Viruses in Fine Aerosols of Exhaled Breath Samples from Patients in Tropical Singapore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Recruitment and Ethics Statement
2.2. Sample Processing
2.3. Quantification of Influenza Viral Load
2.4. Influenza Virus Culture
2.5. Statistical Analyses
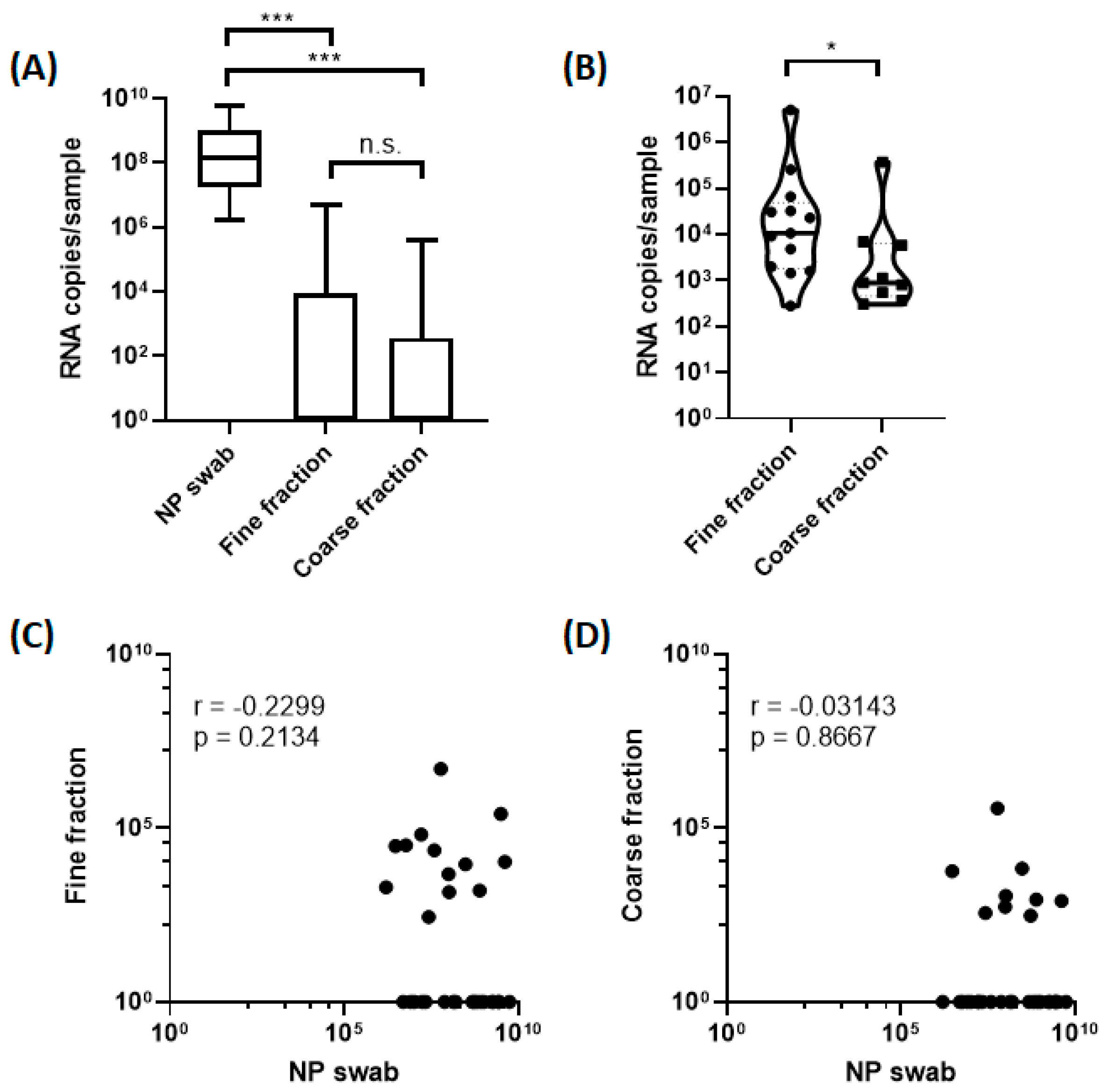
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019, 7, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, W.G.; Blachere, F.M.; Thewlis, R.E.; Vishnu, A.; Davis, K.A.; Cao, G.; Palmer, J.E.; Clark, K.E.; Fisher, M.A.; Khakoo, R.; et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS ONE 2010, 5, e15100. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, W.E.; Swett, K.; Leng, I.; Peters, T.R. Exposure to influenza virus aerosols during routine patient care. J. Infect. Dis. 2013, 207, 1037–1046. [Google Scholar] [CrossRef]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- Aiello, A.E.; Coulborn, R.M.; Aragon, T.J.; Baker, M.G.; Burrus, B.B.; Cowling, B.J.; Duncan, A.; Enanoria, W.; Fabian, M.P.; Ferng, Y.H.; et al. Research findings from nonpharmaceutical intervention studies for pandemic influenza and current gaps in the research. Am. J. Infect. Control 2010, 38, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Fabian, P.; McDevitt, J.J.; DeHaan, W.H.; Fung, R.O.; Cowling, B.J.; Chan, K.H.; Leung, G.M.; Milton, D.K. Influenza virus in human exhaled breath: An observational study. PLoS ONE 2008, 3, e2691. [Google Scholar] [CrossRef] [PubMed]
- Cowling, B.J.; Ip, D.K.; Fang, V.J.; Suntarattiwong, P.; Olsen, S.J.; Levy, J.; Uyeki, T.M.; Leung, G.M.; Peiris, J.S.M.; Chotpitayasunondh, T.; et al. Aerosol transmission is an important mode of influenza A virus spread. Nat. Commun. 2013, 4, 1935. [Google Scholar] [CrossRef]
- Tellier, R. Aerosol transmission of influenza A virus: A review of new studies. J. R. Soc. Interface 2009, 6, S783–S790. [Google Scholar] [CrossRef]
- Yan, J.; Grantham, M.; Pantelic, J.; Bueno de Mesquita, P.J.; Albert, B.; Liu, F.; Ehrman, S.; Milton, D.K.; EMIT Consortium. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. USA 2018, 115, 1081–1086. [Google Scholar] [CrossRef]
- Leung, N.H.L.; Chu, D.K.W.; Shiu, E.Y.C.; Chan, K.H.; McDevitt, J.J.; Hau, B.J.P.; Yen, H.L.; Li, Y.; Ip, D.K.M.; Peiris, J.S.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef]
- Short, K.R.; Richard, M.; Verhagen, J.H.; van Riel, D.; Schrauwen, E.J.; van den Brand, J.M.; Mänz, B.; Bodewes, R.; Herfst, S. One health, multiple challenges: The inter-species transmission of influenza A virus. One Health 2015, 1, 1–13. [Google Scholar] [CrossRef]
- Harrington, W.N.; Kackos, C.M.; Webby, R.J. The evolution and future of influenza pandemic preparedness. Exp. Mol. Med. 2021, 53, 737–749. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of respiratory viral infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Chan, P.K.; Mok, H.Y.; Lee, T.C.; Chu, I.M.; Lam, W.Y.; Sung, J.J. Seasonal influenza activity in Hong Kong and its association with meteorological variations. J. Med. Virol. 2009, 81, 1797–1806. [Google Scholar] [CrossRef]
- Tan, A.L.; Virk, R.K.; Tambyah, P.A.; Inoue, M.; Lim, E.A.; Chan, K.W.; Chelvi, C.S.; Ooi, S.T.; Chua, C.; Tan, B.H. Surveillance and clinical characterization of influenza in a university cohort in Singapore. PLoS ONE 2015, 10, e0119485. [Google Scholar] [CrossRef]
- McDevitt, J.J.; Koutrakis, P.; Ferguson, S.T.; Wolfson, J.M.; Fabian, M.P.; Martins, M.; Pantelic, J.; Milton, D.K. Development and performance evaluation of an exhaled-breath bioaerosol collector for influenza virus. Aerosol Sci. Technol. 2013, 47, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Morawska, L.; Milton, D.K. It is time to address airborne transmission of Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2311–2313. [Google Scholar] [CrossRef] [PubMed]
- Milton, D.K.; Fabian, M.P.; Cowling, B.J.; Grantham, M.L.; McDevitt, J.J. Influenza virus aerosols in human exhaled breath: Particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013, 9, e1003205. [Google Scholar] [CrossRef] [PubMed]
- Ivan, F.X.; Zhou, X.; Lau, S.H.; Rashid, S.; Teo, J.S.M.; Lee, H.K.; Koay, E.S.; Chan, K.P.; Leo, Y.S.; Chen, M.I.C.; et al. Molecular insights into evolution, mutations and receptor-binding specificity of influenza A and B viruses from outpatients and hospitalized patients in Singapore. Int. J. Infect. Dis. 2020, 90, 84–96. [Google Scholar] [CrossRef]
- Chamseddine, A.; Soudani, N.; Kanafani, Z.; Alameddine, I.; Dbaibo, G.; Zaraket, H.; El-Fadel, M. Detection of influenza virus in air samples of patient rooms. J. Hosp. Infect. 2021, 108, 33–42. [Google Scholar] [CrossRef]
- Blachere, F.M.; Lindsley, W.G.; Pearce, T.A.; Anderson, S.E.; Fisher, M.; Khakoo, R.; Meade, B.J.; Lander, O.; Davis, S.; Thewlis, R.E.; et al. Measurement of airborne influenza virus in a hospital emergency department. Clin. Infect. Dis. 2009, 48, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, W.G.; Blachere, F.M.; Davis, K.A.; Pearce, T.A.; Fisher, M.A.; Khakoo, R.; Davis, S.M.; Rogers, M.E.; Thewlis, R.E.; Posada, J.A.; et al. Distribution of airborne influenza virus and respiratory syncytial virus in an urgent care medical clinic. Clin. Infect. Dis. 2010, 50, 693–698. [Google Scholar] [CrossRef]
- Alford, R.H.; Kasel, J.A.; Gerone, P.J.; Knight, V. Human influenza resulting from aerosol inhalation. Proc. Soc. Exp. Biol. Med. 1966, 122, 800–804. [Google Scholar] [CrossRef] [PubMed]
- van Elden, L.J.; Nijhuis, M.; Schipper, P.; Schuurman, R.; van Loon, A.M. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J. Clin. Microbiol. 2001, 39, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Haslbeck, K.; Schwarz, K.; Hohlfeld, J.M.; Seume, J.R.; Koch, W. Submicron droplet formation in the human lung. J. Aerosol Sci. 2010, 41, 429–438. [Google Scholar] [CrossRef]
- Bueno de Mesquita, P.J.; Nguyen-Van-Tam, J.; Killingley, B.; Enstone, J.; Lambkin-Williams, R.; Gilbert, A.S.; Mann, A.; Forni, J.; Yan, J.; Pantelic, J.; et al. Influenza A (H3) illness and viral aerosol shedding from symptomatic naturally infected and experimentally infected cases. Influenza Other Respir. Viruses 2021, 15, 154–163. [Google Scholar] [CrossRef]
- Hsu, C.H.; Chen, H.P.; Chen, P.L.; Chan, Y.J. Detection of influenza and non-influenza respiratory viruses in lower respiratory tract specimens among hospitalized adult patients and analysis of the clinical outcome. J. Microbiol. Immunol. Infect. 2022, 55, 820–828. [Google Scholar] [CrossRef]
- Dong, Z.; Ma, J.; Qiu, J.; Ren, Q.; Shan, Q.; Duan, X.; Li, G.; Zuo, Y.Y.; Qi, Y.; Liu, Y.; et al. Airborne fine particles drive H1N1 viruses deep into the lower respiratory tract and distant organs. Sci. Adv. 2023, 9, eadf2165. [Google Scholar] [CrossRef]
- Lednicky, J.A.; Lauzard, M.; Fan, Z.H.; Jutla, A.; Tilly, T.B.; Gangwar, M.; Usmani, M.; Shankar, S.N.; Mohamed, K.; Eiguren-Fernandez, A.; et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 2020, 100, 476–482. [Google Scholar] [CrossRef]
- Adenaiye, O.O.; Lai, J.; Bueno de Mesquita, P.J.; Hong, F.; Youssefi, S.; German, J.; Tai, S.H.S.; Albert, B.; Schanz, M.; Weston, S.; et al. Infectious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in exhaled aerosols and efficacy of masks during early mild infection. Clin. Infect. Dis. 2022, 75, e241–e248. [Google Scholar] [CrossRef]
- Coleman, K.K.; Tay, D.J.W.; Tan, K.S.; Ong, S.W.X.; Than, T.S.; Koh, M.H.; Chin, Y.Q.; Nasir, H.; Mak, T.M.; Chu, J.J.H.; et al. Viral load of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in respiratory aerosols emitted by patients with coronavirus disease 2019 (COVID-19) while breathing, talking, and singing. Clin. Infect. Dis. 2022, 74, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Ong, S.W.X.; Koh, M.H.; Tay, D.J.W.; Aw, D.Z.H.; Nah, Y.W.; Abdullah, M.R.B.; Coleman, K.K.; Milton, D.K.; Chu, J.J.H.; et al. SARS-CoV-2 Omicron variant shedding during respiratory activities. Int. J. Infect. Dis. 2023, 131, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Singanayagam, A.; Goonawardane, N.; Moshe, M.; Sweeney, F.P.; Sukhova, K.; Killingley, B.; Kalinova, M.; Mann, A.J.; Catchpole, A.P.; et al. Viral emissions into the air and environment after SARS-CoV-2 human challenge: A phase 1, open label, first-in-human study. Lancet Microbe 2023, 4, e579–e590. [Google Scholar] [CrossRef] [PubMed]
- Soo, R.J.J.; Chiew, C.J.; Ma, S.; Pung, R.; Lee, V. Decreased influenza incidence under COVID-19 control measures, Singapore. Emerg. Infect. Dis. 2020, 26, 1933–1935. [Google Scholar] [CrossRef]
- Archer, J.; McCarthy, L.P.; Symons, H.E.; Watson, N.A.; Orton, C.M.; Browne, W.J.; Harrison, J.; Moseley, B.; Philip, K.E.J.; Calder, J.D.; et al. Comparing aerosol number and mass exhalation rates from children and adults during breathing, speaking and singing. Interface Focus 2022, 12, 20210078. [Google Scholar] [CrossRef] [PubMed]
| Total Subjects (n = 31) | |
|---|---|
| Parameter | |
| Age (years) Median Range | 23 19–54 |
| Temperature (°C) | |
| Median Range | 38.2 36.4–39.8 |
| Male (n (%)) | 20 (64.5%) |
| Onset of symptoms since (n (%)) 1 day ago 2 days ago 3 days ago | 18 (58.1%) 11 (35.5%) 2 (6.5%) |
| Symptoms (n (%)) Runny nose Sore throat Headache Sweats or chills Cough | 27 (87.1%) 26 (83.9%) 27 (87.1%) 28 (90.3%) 27 (87.1%) |
| Influenza vaccination in past 10 years (n (%)) | 12 (38.7%) |
| Infected with influenza type A (n (%)) * | 24 (77.4%) |
| Infected with influenza type B (n (%)) * | 7 (22.6%) |
| Virus culture positives (n (%)) Nasopharyngeal sample Fine aerosol fraction | 28 (90.3%) 9 (29.0%) |
| Sample ID | Influenza Type a | NP Swab b | Fine Fraction c | Coarse Fraction c | Virus Culture Positive |
|---|---|---|---|---|---|
| G2-5.1 * (R) | A (H3N2) | 2.95 × 106 | 3.05 × 104 | 5.78 × 103 | NP |
| G2-6.1 | A (H3N2) | 1.03 × 107 | - | - | NP and F |
| G2-7.1 (R) | B (Victoria) | 5.95 × 107 | 5.01 × 106 | 3.73 × 105 | NP and F |
| G2-8.1 (R) | A (H3N2) | 4.92 × 106 | - | - | NP |
| G2-9.1 (R) | A (H3N2) | 9.94 × 107 | 4.76 × 103 | 5.47 × 102 | NP |
| G2-10.1 | A (H3N2) | 1.36 × 108 | - | - | NP and F |
| G2-14.1 * (R) | B (Victoria) | 1.62 × 107 | 6.58 × 104 | - | NP and F |
| G2-15.1 * | B (Victoria) | 1.71 × 109 | - | - | NP and F |
| G2-19.1 | A (H1N1) | 1.93 × 109 | - | - | NP |
| G2-20.1 (R) | A (H1N1) | 5.92 × 106 | 3.21 × 104 | - | NP |
| G2-22.1 | A (H1N1) | 5.52 × 109 | - | - | NP |
| G2-23.1 (R) | A (H1N1) | 7.96 × 106 | - | - | NP |
| G2-24.1 * | B (Yamagata) | 8.27 × 108 | - | - | NP |
| G2-25.1* | A (H1N1) | 7.63 × 107 | - | - | NP |
| G2-26.1 * (R) | A (H3N2) | 5.35 × 108 | - | 3.03 × 102 | NP |
| G2-27.1 * | A (H1N2) | 2.75 × 109 | - | - | NP |
| G2-29.1 * | A (H3N2) | 4.76 × 108 | - | - | NP |
| G2-31.1 | A (H3N2) | 2.14 × 107 | - | - | NP |
| G2-34.1 * (R) | B (Yamagata) | 3.85 × 107 | 2.28 × 104 | - | NP |
| G2-36.1 | B (Yamagata) | 1.56 × 108 | - | - | NP and F |
| G2-37.1 * | A | 1.03 × 109 | - | - | NP and F |
| G2-41.1 * | A | 2.67 × 109 | - | - | NP and F |
| G2-42.1 | A | 6.46 × 108 | - | - | NP |
| G2-43.1 (R) | B (Yamagata) | 4.05 × 109 | 1.07 × 104 | 8.02 × 102 | NP and F |
| G2-44.1 * (R) | A (H1N1) | 3.15 × 109 | 2.56 × 105 | - | NP |
| G2-49.1 (R) | A | 2.65 × 107 | 2.78 × 102 | 3.62 × 102 | - |
| G2-51.1 (R) | A (H3N2) | 3.04 × 108 | 9.08 × 103 | 6.85 × 103 | NP |
| G2-52.1 (R) | A (H3N2) | 1.70 × 107 | - | - | - |
| G2-53.1 (R) | A | 1.02 × 108 | 1.44 × 103 | 1.13 × 103 | - |
| G2-63.1 (R) | A (H3N2) | 7.66 × 108 | 1.60 × 103 | 8.86 × 102 | NP |
| G2-72.1 | A | 1.58 × 106 | 1.99 × 103 | - | NP |
| Variable | Detected (n = 14) a | Not Detected (n = 17) b | p Value |
|---|---|---|---|
| Age | 23 (19–28 years) | 23 (19–54 years) | 0.42 |
| Temperature | 38.1 (36.4–39.7 °C) | 38.4 (37.3–39.8 °C) | 0.15 |
| Male | 11 (78.6%) | 9 (52.9%) | 0.26 |
| Days since symptom onset | 1 (1–3 days) | 1 (1–3 days) | >0.99 |
| Symptoms | |||
| Runny nose | 13 (92.9%) | 14 (82.4%) | 0.61 |
| Sore throat | 12 (85.7%) | 14 (82.4%) | >0.99 |
| Headache | 12 (85.7%) | 15 (88.2%) | >0.99 |
| Sweats or chills | 12 (85.7%) | 16 (94.1%) | 0.58 |
| Cough | 13 (92.9%) | 14 (82.4%) | 0.61 |
| Influenza vaccination in past 10 years | 5 (35.7%) | 7 (41.2%) | >0.99 |
| Infected with influenza type A | 10 (71.4%) | 14 (82.4%) | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, V.T.K.; Tay, D.J.W.; Chen, M.I.C.; Tang, J.W.; Milton, D.K.; Tham, K.W. Influenza A and B Viruses in Fine Aerosols of Exhaled Breath Samples from Patients in Tropical Singapore. Viruses 2023, 15, 2033. https://doi.org/10.3390/v15102033
Chow VTK, Tay DJW, Chen MIC, Tang JW, Milton DK, Tham KW. Influenza A and B Viruses in Fine Aerosols of Exhaled Breath Samples from Patients in Tropical Singapore. Viruses. 2023; 15(10):2033. https://doi.org/10.3390/v15102033
Chicago/Turabian StyleChow, Vincent T. K., Douglas Jie Wen Tay, Mark I. C. Chen, Julian W. Tang, Donald K. Milton, and Kwok Wai Tham. 2023. "Influenza A and B Viruses in Fine Aerosols of Exhaled Breath Samples from Patients in Tropical Singapore" Viruses 15, no. 10: 2033. https://doi.org/10.3390/v15102033
APA StyleChow, V. T. K., Tay, D. J. W., Chen, M. I. C., Tang, J. W., Milton, D. K., & Tham, K. W. (2023). Influenza A and B Viruses in Fine Aerosols of Exhaled Breath Samples from Patients in Tropical Singapore. Viruses, 15(10), 2033. https://doi.org/10.3390/v15102033







