Clade 2.3.4.4b H5N8 Subtype Avian Influenza Viruses Were Identified from the Common Crane Wintering in Yunnan Province, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Genomic Segment Amplification Eight AIV Segments
2.3. Next-Generation Sequencing
2.4. Phylogenetic Analysis and Molecular Characterization
3. Results
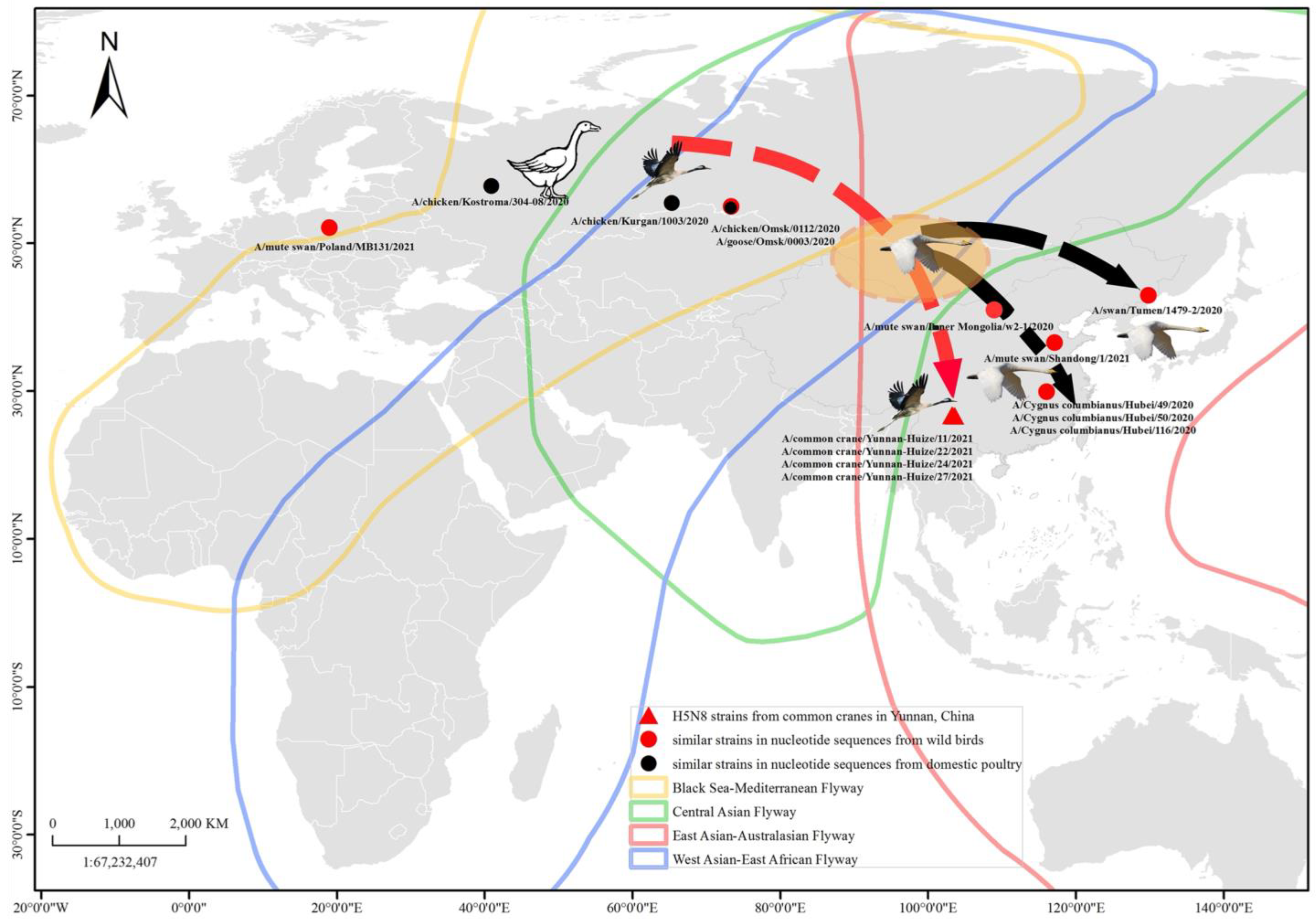
3.1. Virus Determination
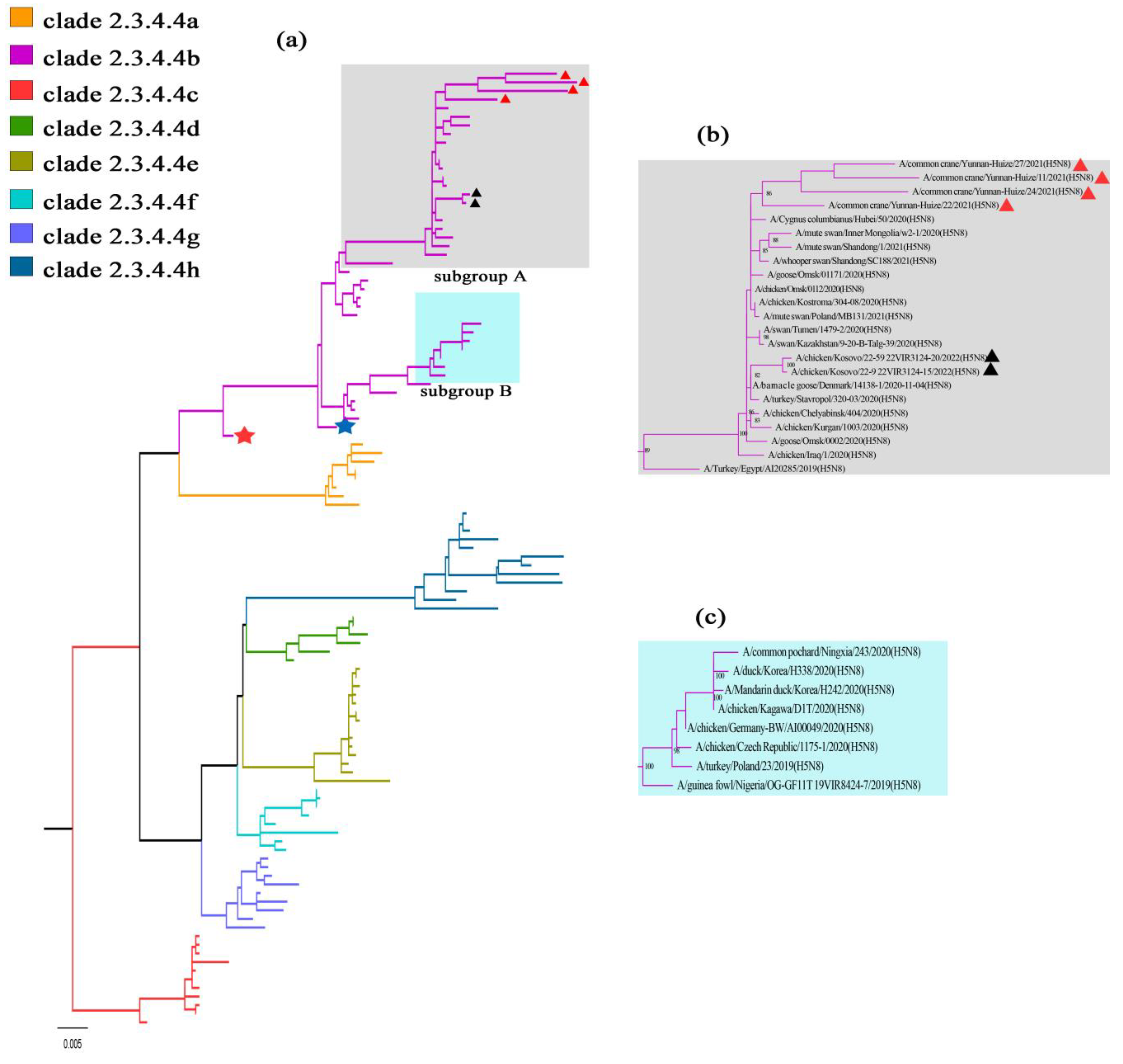
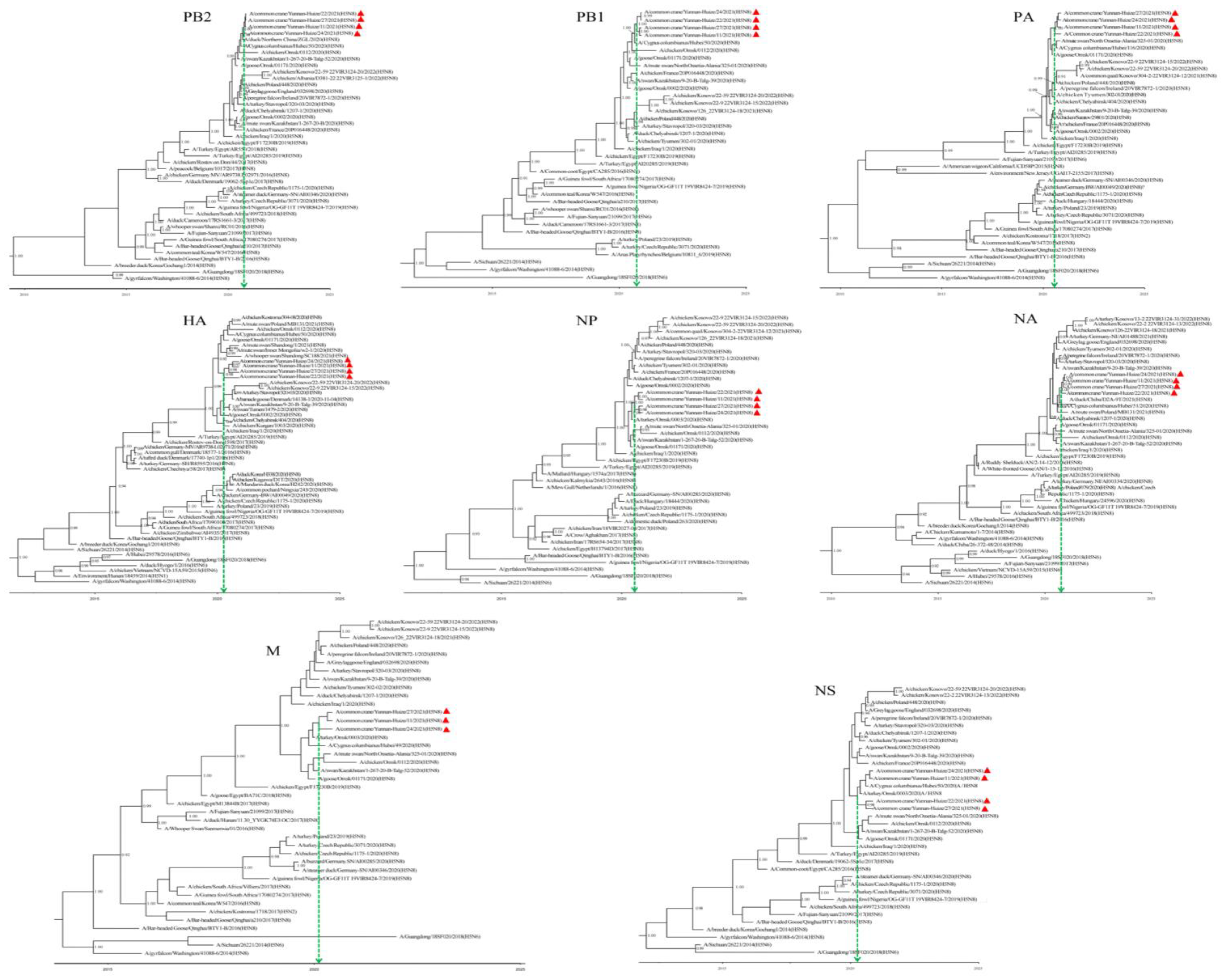
3.2. Phylogenetic Characteristics
3.3. Amino Acid Sequence Characteristics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olsen, B.; Munster, V.J.; Wallensten, A.; Waldenström, J.; Osterhaus, A.D.; Fouchier, R.A. Global patterns of influenza a virus in wild birds. Science 2006, 312, 384–388. [Google Scholar] [CrossRef]
- Röhm, C.; Horimoto, T.; Kawaoka, Y.; Süss, J.; Webster, R.G. Do hemagglutinin genes of highly pathogenic avian influenza viruses constitute unique phylogenetic lineages? Virology 1995, 209, 664–670. [Google Scholar] [CrossRef][Green Version]
- Webster, R.G.; Peiris, M.; Chen, H.; Guan, Y. H5N1 outbreaks and enzootic influenza. Emerg. Infect. Dis. 2006, 12, 3–8. [Google Scholar] [CrossRef]
- Nunez, I.A.; Ross, T.M. A review of H5Nx avian influenza viruses. Ther. Adv. Vaccines Immunother. 2019, 7, 2515135518821625. [Google Scholar]
- Yamaji, R.; Saad, M.D.; Davis, C.T.; Swayne, D.E.; Wang, D.; Wong, F.Y.K.; McCauley, J.W.; Peiris, J.S.M.; Webby, R.J.; Fouchier, R.A.M.; et al. Pandemic potential of highly pathogenic avian influenza clade 2.3.4.4 A(H5) viruses. Rev. Med. Virol. 2020, 30, e2099. [Google Scholar]
- Smith, G.J.; Donis, R.O. Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013–2014. Influenza Other Respir. Viruses 2015, 9, 271–276. [Google Scholar]
- WHO/OIE/FAO, Revised and updated nomenclature for highly pathogenic avian influenza A (H5N1) viruses. Influenza Other Respir. Viruses 2014, 8, 384–388. [CrossRef]
- WHO. Antigenic and genetic characteristics of zoonotic influenza A viruses and development of candidate vaccine viruses for pandemic preparedness. Wkly Epidemiol. Rec. 2022, 97, 550–565. [Google Scholar]
- Li, Y.; Liu, H.J.; Li, X.; Lv, X.R.; Chai, H.L. Global epidemiology of H5N8 subtype highly pathogenic avian inflenza virus. Acta Microbiol. Sin. 2022, 62, 10–23. [Google Scholar]
- Lee, Y.J.; Kang, H.M.; Lee, E.K.; Song, B.M.; Jeong, J.; Kwon, Y.K.; Kim, H.R.; Lee, K.J.; Hong, M.S.; Jang, I.; et al. Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerg. Infect. Dis. 2014, 20, 1087–1089. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Bi, Y.; Sun, J.; Wong, G.; Liu, D.; Li, L.; Liu, J.; Chen, Q.; Wang, H.; et al. Highly Pathogenic Avian Influenza A(H5N8) Virus in Wild Migratory Birds, Qinghai Lake, China. Emerg. Infect. Dis. 2017, 23, 637–641. [Google Scholar] [CrossRef]
- Lee, D.H.; Sharshov, K.; Swayne, D.E.; Kurskaya, O.; Sobolev, I.; Kabilov, M.; Alekseev, A.; Irza, V.; Shestopalov, A. Novel Reassortant Clade 2.3.4.4 Avian Influenza A(H5N8) Virus in Wild Aquatic Birds, Russia, 2016. Emerg. Infect. Dis. 2017, 23, 359–360. [Google Scholar]
- Śmietanka, K.; Świętoń, E.; Kozak, E.; Wyrostek, K.; Tarasiuk, K.; Tomczyk, G.; Konopka, B.; Welz, M.; Domańska-Blicharz, K.; Niemczuk, K. Highly Pathogenic Avian Influenza H5N8 in Poland in 2019–2020. J. Vet. Res. 2020, 64, 469–476. [Google Scholar] [CrossRef]
- Zhao, K.; Gu, M.; Zhong, L.; Duan, Z.; Zhang, Y.; Zhu, Y.; Zhao, G.; Zhao, M.; Chen, Z.; Hu, S.; et al. Characterization of three H5N5 and one H5N8 highly pathogenic avian influenza viruses in China. Vet. Microbiol. 2013, 163, 351–357. [Google Scholar] [CrossRef]
- Lycett, S.J.; Bodewes, R.; Pohlmann, A.; Banks, J.; Banyai, K.; Boni, M.F.; Bouwstra, R.; Breed, A.C.; Brown, I.H.; Chen, H.; et al. Role for migratory wild birds in the global spread of avian influenza H5N8. Science 2016, 354, 213–217. [Google Scholar]
- Antigua, K.J.C.; Choi, W.S.; Baek, Y.H.; Song, M.S. The Emergence and Decennary Distribution of Clade 2.3.4.4 HPAI H5Nx. Microorganisms 2019, 7, 156. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, S.; Yin, X.; Li, Z.; Lan, Z.; Xire, L.; Wang, Z.; Xie, Y.; Peng, C.; Li, J.; et al. Comparative Antigenicity and Pathogenicity of Two Distinct Genotypes of Highly Pathogenic Avian Influenza Viruses (H5N8) From Wild Birds in China, 2020–2021. Front. Microbiol. 2022, 13, 893253. [Google Scholar] [CrossRef]
- Cui, P.; Zeng, X.; Li, X.; Li, Y.; Shi, J.; Zhao, C.; Qu, Z.; Wang, Y.; Guo, J.; Gu, W.; et al. Genetic and biological characteristics of the globally circulating H5N8 avian influenza viruses and the protective efficacy offered by the poultry vaccine currently used in China. Sci. China Life Sci. 2022, 65, 795–808. [Google Scholar] [CrossRef]
- Xiong, J.; Zhou, H.; Fan, L.; Zhu, G.; Li, Y.; Chen, G.; Zhang, J.; Li, J.; Zheng, H.; Feng, W.; et al. Emerging highly pathogenic avian influenza (H5N8) virus in migratory birds in Central China, 2020. Emerg. Microbes Infect. 2021, 10, 1503–1506. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Z.X.; Xue, X.Y.; Wang, W.H.; Han, L.X.; Dai, H.Y.; Gao-Yin, L.I.; Duan, B.F.; Zhang, W.D.; Liu, Q. Analysis of genetic characteristics of H9N2 subtype avian influenza viruses in the plateau wetlands of Lashihai, Yunnan. Chin. J. Prev. Vet. Med. 2022, 44, 250–256. [Google Scholar]
- Huang, X.; Madan, A. CAP3: A DNA sequence assembly program. Genome Res. 1999, 9, 868–877. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, I.; Sharshov, K.; Dubovitskiy, N.; Kurskaya, O.; Alekseev, A.; Leonov, S.; Yushkov, Y.; Irza, V.; Komissarov, A.; Fadeev, A.; et al. Highly Pathogenic Avian Influenza A(H5N8) Virus Clade 2.3.4.4b, Western Siberia, Russia, 2020. Emerg. Infect. Dis. 2021, 27, 2224–2227. [Google Scholar]
- Yehia, N.; Naguib, M.M.; Li, R.; Hagag, N.; El-Husseiny, M.; Mosaad, Z.; Nour, A.; Rabea, N.; Hasan, W.M.; Hassan, M.K.; et al. Multiple introductions of reassorted highly pathogenic avian influenza viruses (H5N8) clade 2.3.4.4b causing outbreaks in wild birds and poultry in Egypt. Infect. Genet. Evol. 2018, 58, 56–65. [Google Scholar]
- Hanna, A.; Banks, J.; Marston, D.A.; Ellis, R.J.; Brookes, S.M.; Brown, I.H. Genetic Characterization of Highly Pathogenic Avian Influenza (H5N8) Virus from Domestic Ducks, England, November 2014. Emerg. Infect. Dis. 2015, 21, 879–882. [Google Scholar] [CrossRef]
- Luczo, J.M.; Stambas, J.; Durr, P.A.; Michalski, W.P.; Bingham, J. Molecular pathogenesis of H5 highly pathogenic avian influenza: The role of the haemagglutinin cleavage site motif. Rev. Med. Virol. 2015, 25, 406–430. [Google Scholar] [CrossRef]
- Herfst, S.; Schrauwen, E.J.A.; Linster, M.; Chutinimitkul, S.; de Wit, E.; Munster, V.J.; Sorrell, E.M.; Bestebroer, T.M.; Burke, D.F.; Smith, D.J.; et al. Airborne Transmission of Influenza A/H5N1 Virus Between Ferrets. Science 2012, 336, 1534–1541. [Google Scholar] [CrossRef]
- Xu, C.; Hu, W.-B.; Xu, K.; He, Y.-X.; Wang, T.-Y.; Chen, Z.; Li, T.-X.; Liu, J.-H.; Buchy, P.; Sun, B. Amino acids 473V and 598P of PB1 from an avian-origin influenza A virus contribute to polymerase activity, especially in mammalian cells. J. Gen. Virol. 2012, 93 Pt 3, 531–540. [Google Scholar] [CrossRef]
- Valley-Omar, Z.; Cloete, A.; Pieterse, R.; Walaza, S.; Salie-Bassier, Y.; Smith, M.; Govender, N.; Seleka, M.; Hellferscee, O.; Mtshali, P.S.; et al. Human surveillance and phylogeny of highly pathogenic avian influenza A(H5N8) during an outbreak in poultry in South Africa, 2017. Influenza Other Respir. Viruses 2020, 14, 266–273. [Google Scholar] [CrossRef]
- Song, J.; Xu, J.; Shi, J.; Li, Y.; Chen, H. Synergistic Effect of S224P and N383D Substitutions in the PA of H5N1 Avian Influenza Virus Contributes to Mammalian Adaptation. Sci. Rep. 2015, 5, 10510. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Yamada, S.; Fukuyama, S.; Murakami, S.; Zhao, D.; Uraki, R.; Watanabe, T.; Tomita, Y.; Macken, C.; Neumann, G.; et al. Virulence-Affecting Amino Acid Changes in the PA Protein of H7N9 Influenza A Viruses. J. Virol. 2014, 88, 3127–3134. [Google Scholar] [CrossRef]
- Hulse-Post, D.J.; Franks, J.; Boyd, K.; Salomon, R.; Hoffmann, E.; Yen, H.L.; Webby, R.J.; Walker, D.; Nguyen, T.D.; Webster, R.G. Molecular changes in the polymerase genes (PA and PB1) associated with high pathogenicity of H5N1 influenza virus in mallard ducks. J. Virol. 2007, 81, 8515–8524. [Google Scholar] [CrossRef]
- Fan, S.; Deng, G.; Song, J.; Tian, G.; Suo, Y.; Jiang, Y.; Guan, Y.; Bu, Z.; Kawaola, Y.; Chen, H. Two amino acid residues in the matrix protein M1 contribute to the virulence difference of H5N1 avian influenza viruses in mice. Virology 2009, 384, 28–32. [Google Scholar] [CrossRef]
- Jiao, P.; Tian, G.; Li, Y.; Deng, G.; Jiang, Y.; Liu, C.; Liu, W.; Bu, Z.; Kawaoka, Y.; Chen, H. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J. Virol. 2008, 82, 1146–1154. [Google Scholar] [CrossRef]
- Tada, T.; Suzuki, K.; Sakurai, Y.; Kubo, M.; Okada, H.; Itoh, T.; Tsukamoto, K. NP Body Domain and PB2 Contribute to Increased Virulence of H5N1 Highly Pathogenic Avian Influenza Viruses in Chickens. J. Virol. 2011, 85, 1834–1846. [Google Scholar] [CrossRef]
- BirdLife International. Species Factsheet: Grus Grus. Available online: http://datazone.birdlife.org/species/factsheet/common-crane-grus-grus/text (accessed on 24 October 2022).
- Li, X.; Yang, F. Population Numbers and Distribution of Black-necked Cranes (Grus nigricollis) in the Yungui Gaoyuan Plateau. Chin. J. Zool. 2003, 33, 43–45. [Google Scholar]
- Zhao, Z.J.; Cai, R.; Peng, C.; Zhong, X.Y.; Dao, M.B. Number and distribution of cranes and waterbirds at Dashanbao Black-necked Cranes National Nature Reserve, China during the 2013 wintering period. Zool. Res. 2014, 35 (Suppl. S1), 215–218. [Google Scholar]
- Liu, H. Migration routes and stopovers of Common Crane wintering in Caohai National Nature Reserves. Master’s Thesis, Southwest Forestry University, Kunming, China, 2018. [Google Scholar]
- Li, S.; Meng, W.; Liu, D.; Yang, Q.; Chen, L.; Dai, Q.; Ma, T.; Gao, R.; Ru, W.; Li, Y.; et al. Migratory Whooper Swans Cygnus cygnus Transmit H5N1 Virus between China and Mongolia: Combination Evidence from Satellite Tracking and Phylogenetics Analysis. Sci. Rep. 2018, 8, 7049. [Google Scholar] [CrossRef]
- Lloren, K.K.S.; Lee, T.; Kwon, J.J.; Song, M.-S. Molecular Markers for Interspecies Transmission of Avian Influenza Viruses in Mammalian Hosts. Int. J. Mol. Sci. 2017, 18, 2706. [Google Scholar] [CrossRef]
- Pyankova, O.G.; Susloparov, I.M.; Moiseeva, A.A.; Kolosova, N.P.; Onkhonova, G.S.; Danilenko, A.V.; Vakalova, E.V.; Shendo, G.L.; Nekeshina, N.N.; Noskova, L.N.; et al. Isolation of clade 2.3.4.4b A(H5N8), a highly pathogenic avian influenza virus, from a worker during an outbreak on a poultry farm, Russia, December 2020. Euro Surveill. 2021, 26, 2100439. [Google Scholar]
- Suttie, A.; Deng, Y.M.; Greenhill, A.R.; Dussart, P.; Horwood, P.F.; Karlsson, E.A. Inventory of molecular markers affecting biological characteristics of avian influenza A viruses. Virus Genes 2019, 55, 739–768. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Subbarao, K.; Cox, N.J.; Guo, Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: Similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 1999, 261, 15–19. [Google Scholar]
- Matsuoka, Y.; Swayne, D.E.; Thomas, C.; Rameix-Welti, M.A.; Naffakh, N.; Warnes, C.; Altholtz, M.; Donis, R.; Subbarao, K. Neuraminidase stalk length and additional glycosylation of the hemagglutinin influence the virulence of influenza H5N1 viruses for mice. J. Virol. 2009, 83, 4704–4708. [Google Scholar] [CrossRef] [PubMed]
| Gene Segment 1 | Mean tMRCA | 95% HPD (Highest Posterior Density) | Posterior Probability |
|---|---|---|---|
| PB2 | October 2020 | September 2020, November 2020 | 1.0000 |
| PB1 | September 2020 | August 2020, October 2020 | 0.9988 |
| PA | July 2020 | May 2020, October 2020 | 1.0000 |
| HA | April 2020 | November 2019, August 2020 | 0.9855 |
| NP | July 2020 | April 2020, September 2020 | 1.0000 |
| NA | October 2020 | July 2020, November 2020 | 0.7117 |
| MP | August 2020 | June 2020, November 2020 | 0.7530 |
| NS | April 2020 | October 2019, June 2020 | 0.7253 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Xue, X.; Zhang, Z.; Wu, M.J.; Ji, J.; Wang, W.; Yin, H.; Li, S.; Dai, H.; Duan, B.; et al. Clade 2.3.4.4b H5N8 Subtype Avian Influenza Viruses Were Identified from the Common Crane Wintering in Yunnan Province, China. Viruses 2023, 15, 38. https://doi.org/10.3390/v15010038
Yang Q, Xue X, Zhang Z, Wu MJ, Ji J, Wang W, Yin H, Li S, Dai H, Duan B, et al. Clade 2.3.4.4b H5N8 Subtype Avian Influenza Viruses Were Identified from the Common Crane Wintering in Yunnan Province, China. Viruses. 2023; 15(1):38. https://doi.org/10.3390/v15010038
Chicago/Turabian StyleYang, Qinhong, Xiaoyan Xue, Zhenxing Zhang, Ming J. Wu, Jia Ji, Wei Wang, Hongbin Yin, Suhua Li, Hongyang Dai, Bofang Duan, and et al. 2023. "Clade 2.3.4.4b H5N8 Subtype Avian Influenza Viruses Were Identified from the Common Crane Wintering in Yunnan Province, China" Viruses 15, no. 1: 38. https://doi.org/10.3390/v15010038
APA StyleYang, Q., Xue, X., Zhang, Z., Wu, M. J., Ji, J., Wang, W., Yin, H., Li, S., Dai, H., Duan, B., Liu, Q., & Song, J. (2023). Clade 2.3.4.4b H5N8 Subtype Avian Influenza Viruses Were Identified from the Common Crane Wintering in Yunnan Province, China. Viruses, 15(1), 38. https://doi.org/10.3390/v15010038





