The Bovine Seminal Plasma Protein PDC-109 Possesses Pan-Antiviral Activity
Abstract
:1. Introduction
2. Materials and Methods
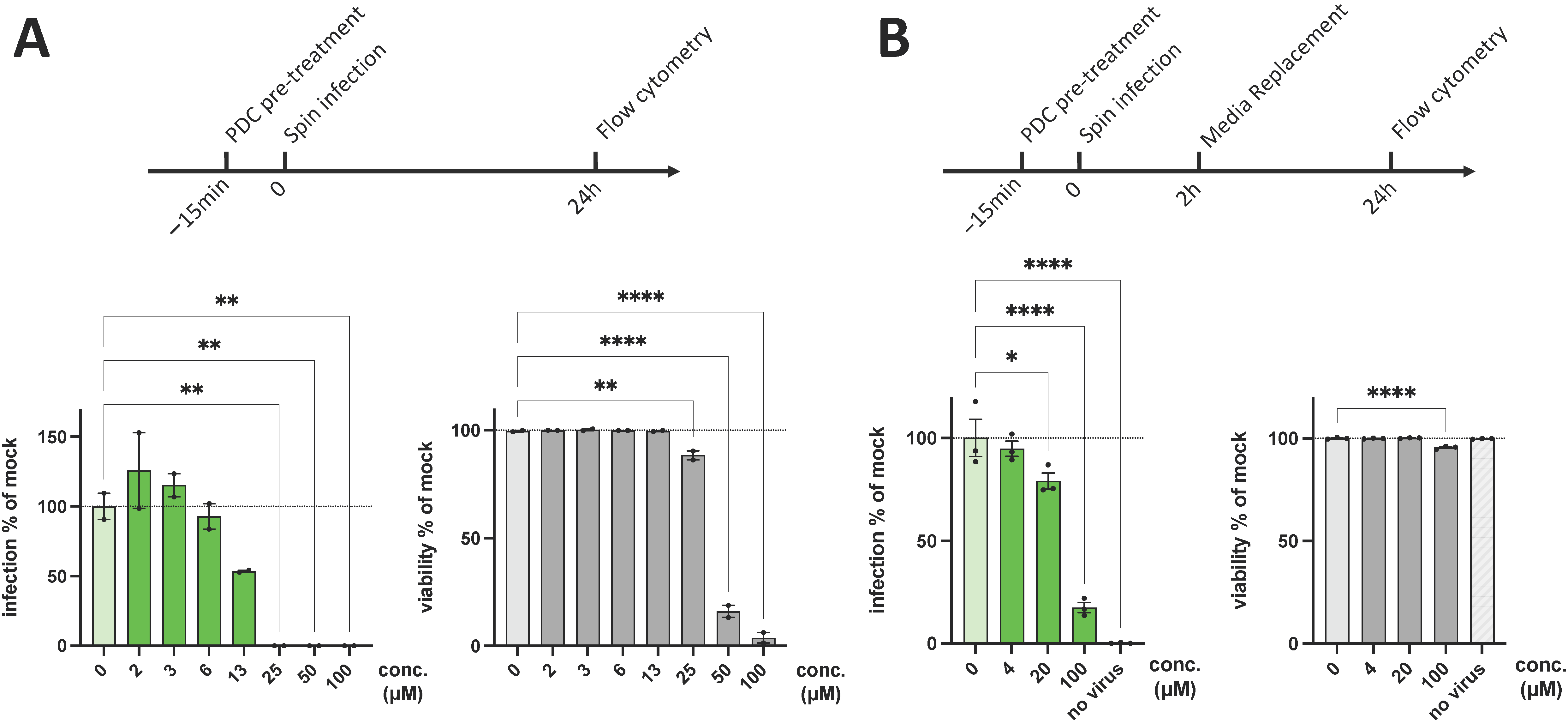
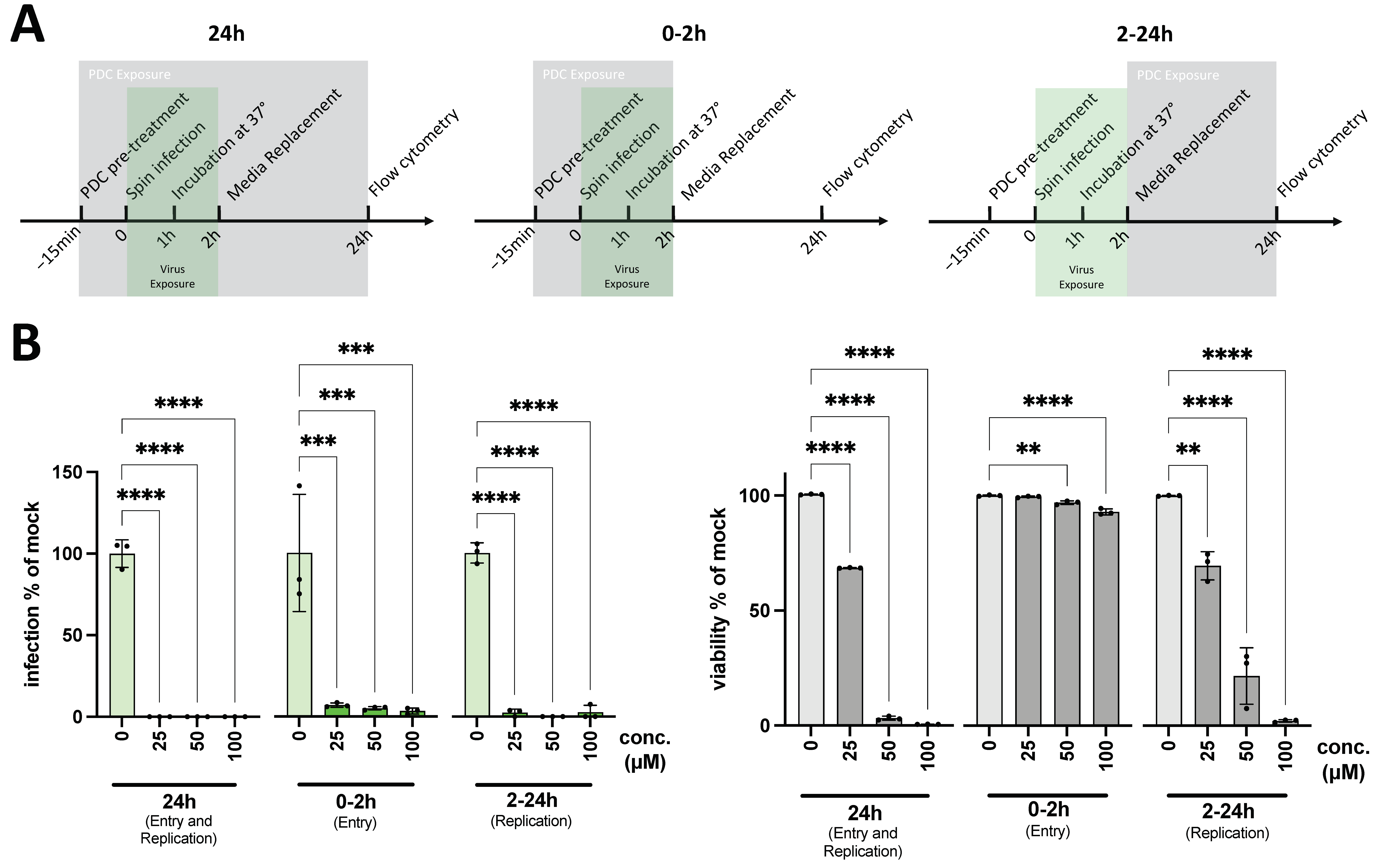
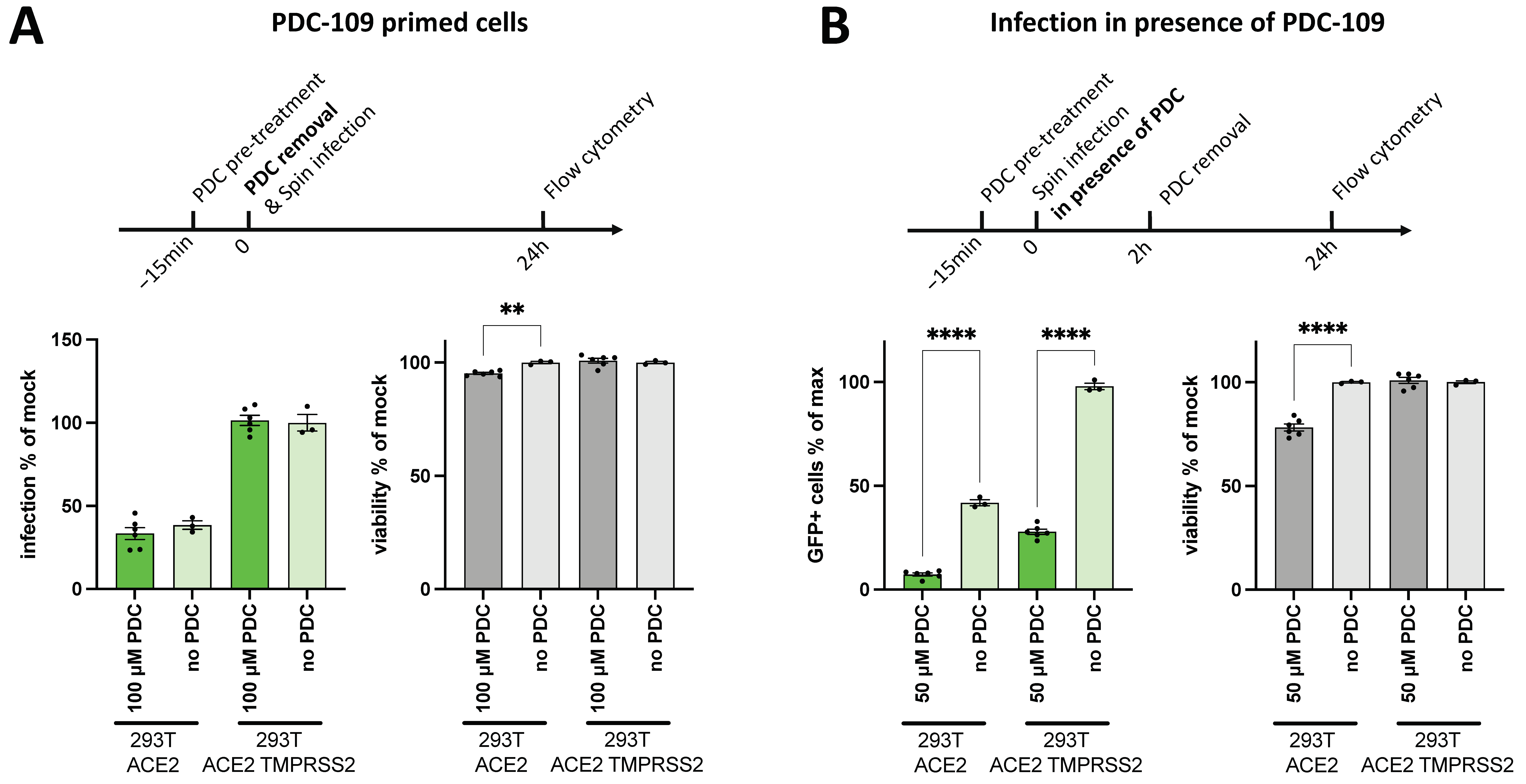
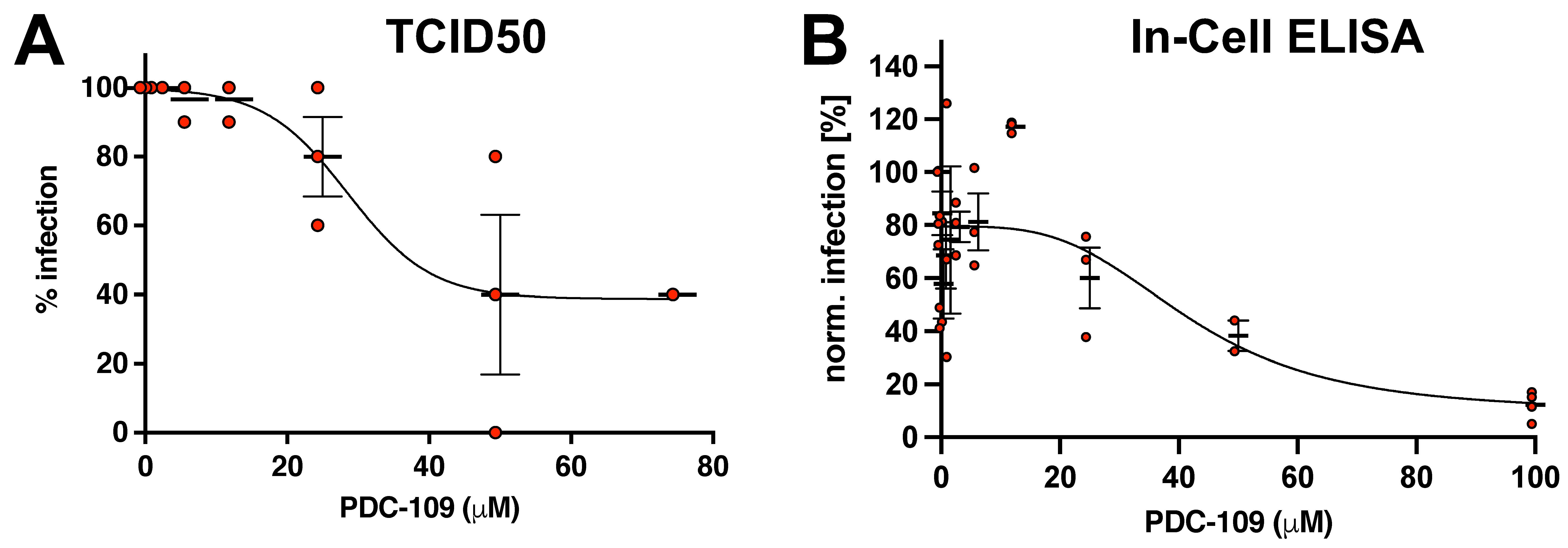
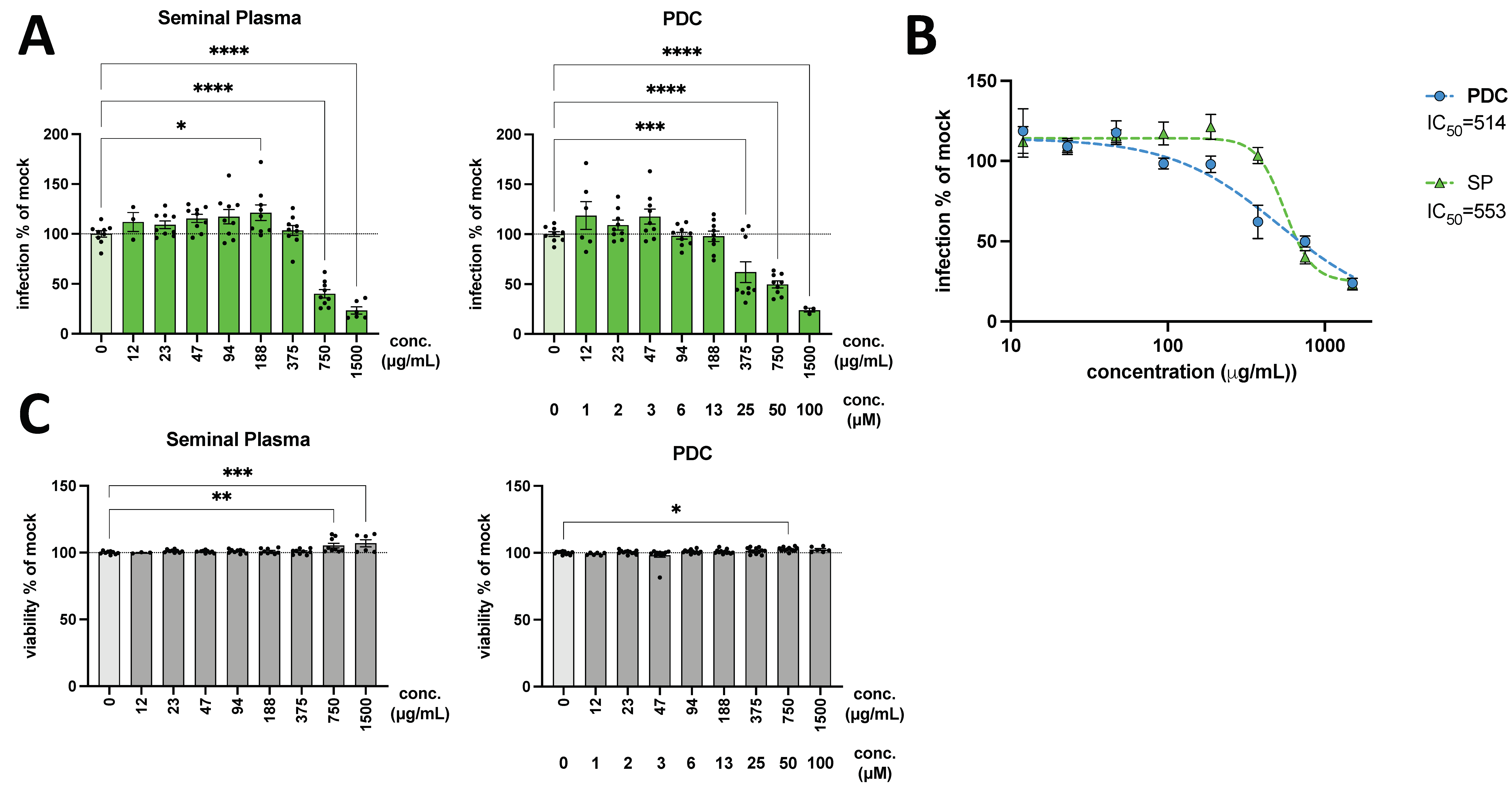
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dejucq, N.; Jégou, B. Viruses in the Mammalian Male Genital Tract and Their Effects on the Reproductive System. Microbiol. Mol. Biol. Rev. 2001, 65, 208–231. [Google Scholar] [CrossRef] [PubMed]
- Lippold, S.; Braun, B.; Krüger, F.; Harms, M.; Müller, J.A.; Groß, R.; Münch, J.; von Einem, J. Natural Inhibitor of Human Cytomegalovirus in Human Seminal Plasma. J. Virol. 2019, 93, e01855-18. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, W.; Gong, M.; Wang, F.; Wu, H.; Liu, W.; Gao, Y.; Liu, B.; Chen, S.; Lu, W.; et al. Characterization of an Antiviral Component in Human Seminal Plasma. Front. Immunol. 2021, 12, 580454. [Google Scholar] [CrossRef] [PubMed]
- Münch, J.; Rücker, E.; Ständker, L.; Adermann, K.; Goffinet, C.; Schindler, M.; Wildum, S.; Chinnadurai, R.; Rajan, D.; Specht, A.; et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 2007, 131, 1059–1071. [Google Scholar] [CrossRef]
- Bouvet, J.P.; Grésenguet, G.; Bélec, L. Vaginal pH neutralization by semen as a cofactor of HIV transmission. Clin. Microbiol. Infect. 1997, 3, 19–23. [Google Scholar] [CrossRef]
- Nijmeijer, B.M.; Bermejo-Jambrina, M.; Kaptein, T.M.; Ribeiro, C.M.S.; Wilflingseder, D.; Geijtenbeek, T.B.H. HIV-1 subverts the complement system in semen to enhance viral transmission. Mucosal Immunol. 2021, 14, 743–750. [Google Scholar] [CrossRef]
- Martellini, J.A.; Cole, A.L.; Venkataraman, N.; Quinn, G.A.; Svoboda, P.; Gangrade, B.K.; Pohl, J.; Sørensen, O.E.; Cole, A.M. Cationic polypeptides contribute to the anti-HIV-1 activity of human seminal plasma. FASEB J. 2009, 23, 3609–3618. [Google Scholar] [CrossRef]
- Malm, J.; Sørensen, O.; Persson, T.; Frohm-Nilsson, M.; Johansson, B.; Bjartell, A.; Lilja, H.; Ståhle-Bäckdahl, M.; Borregaard, N.; Egesten, A. The Human Cationic Antimicrobial Protein (hCAP-18) Is Expressed in the Epithelium of Human Epididymis, Is Present in Seminal Plasma at High Concentrations, and Is Attached to Spermatozoa. Infect. Immun. 2000, 68, 4297–4302. [Google Scholar] [CrossRef]
- Doncel, G.F.; Joseph, T.; Thurman, A.R. Role of Semen in HIV-1 Transmission: Inhibitor or facilitator? Am. J. Reprod. Immunol. 2011, 65, 292–301. [Google Scholar] [CrossRef]
- Christopher-Hennings, J.; Nelson, E.A.; Althouse, G.C.; Lunney, J. Comparative antiviral and proviral factors in semen and vaccines for preventing viral dissemination from the male reproductive tract and semen. Anim. Heal. Res. Rev. 2008, 9, 59–69. [Google Scholar] [CrossRef]
- Sabatte, J.; Faigle, W.; Ceballos, A.; Morelle, W.; Rodríguez Rodrígues, C.; Remes Lenicov, F.; Thépaut, M.; Fieschi, F.; Malchiodi, E.; Fernández, M.; et al. Semen Clusterin Is a Novel DC-SIGN Ligand. J. Immunol. 2011, 187, 5299–5309. [Google Scholar] [CrossRef]
- Kim, K.-A.; Yolamanova, M.; Zirafi, O.; Roan, N.R.; Standker, L.; Forssmann, W.-G.; Burgener, A.; Dejucq-Rainsford, N.; Hahn, B.H.; Shaw, G.M.; et al. Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI. Retrovirology 2010, 7, 55. [Google Scholar] [CrossRef]
- Madison, M.N.; Roller, R.J.; Okeoma, C.M. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology 2014, 11, 102. [Google Scholar] [CrossRef]
- Welke, R.W.; Haralampiev, I.; Schröter, F.; Braun, B.C.; Herrmann, A.; Sieben, C.; Müller, P. Inhibition of influenza virus activity by the bovine seminal plasma protein PDC-109. Eur. Biophys. J. 2019, 48, 503–511. [Google Scholar] [CrossRef]
- Fan, J.; Lefebvre, J.; Manjunath, P. Bovine seminal plasma proteins and their relatives: A new expanding superfamily in mammals. Gene 2006, 375, 63–74. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L. Insights into structure-function correlations of ungulate seminal plasma proteins. Soc. Reprod. Fertil. Suppl. 2007, 65, 201–215. [Google Scholar]
- Ekhlasi-Hundrieser, M.; Müller, P.; Töpfer-Petersen, E. Male Secretory Proteins Sperm Tools for Fertilisation. In Biology of Male Germ Cells; Shaker Publisher GmbH: Düren, Germany, 2009; pp. 173–210. ISBN 978-3-8322-7685-0. [Google Scholar]
- Rodríguez-Martínez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal Plasma Proteins: What Role Do They Play? Am. J. Reprod. Immunol. 2011, 66, 11–22. [Google Scholar] [CrossRef]
- Caballero, I.; Parrilla, I.; Almiñana, C.; del Olmo, D.; Roca, J.; Martínez, E.; Vázquez, J. Seminal Plasma Proteins as Modulators of the Sperm Function and Their Application in Sperm Biotechnologies. Reprod. Domest. Anim. 2012, 47, 12–21. [Google Scholar] [CrossRef]
- Gwathmey, T.M.; Ignotz, G.G.; Mueller, J.L.; Manjunath, P.; Suarez, S.S. Bovine Seminal Plasma Proteins PDC-109, BSP-A3, and BSP-30-kDa Share Functional Roles in Storing Sperm in the Oviduct1. Biol. Reprod. 2006, 75, 501–507. [Google Scholar] [CrossRef]
- Gasset, M.; Magdaleno, L.; Calvete, J.J. Biophysical Study of the Perturbation of Model Membrane Structure Caused by Seminal Plasma Protein PDC-109. Arch. Biochem. Biophys. 2000, 374, 241–247. [Google Scholar] [CrossRef]
- Desnoyers, L.; Manjunath, P. Major proteins of bovine seminal plasma exhibit novel interactions with phospholipid. J. Biol. Chem. 1992, 267, 10149–10155. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Anbazhagan, V.; Pratap, T.V.; Marsh, D.; Swamy, M.J. Membrane Insertion and Lipid-Protein Interactions of Bovine Seminal Plasma Protein PDC-109 Investigated by Spin-Label Electron Spin Resonance Spectroscopy. Biophys. J. 2001, 81, 2215–2225. [Google Scholar] [CrossRef]
- Greube, A.; Müller, K.; Töpfer-Petersen, E.; Herrmann, A.; Müller, P. Influence of the bovine seminal plasma protein PDC-109 on the physical state of membranes. Biochemistry 2001, 40, 8326–8334. [Google Scholar] [CrossRef] [PubMed]
- Sankhala, R.S.; Swamy, M.J. The Major Protein of Bovine Seminal Plasma, PDC-109, Is a Molecular Chaperone. Biochemistry 2010, 49, 3908–3918. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Varela, P.F.; Sanz, L.; Romero, A.; Mann, K.; Töpfer-Petersen, E. A Procedure for the Large-Scale Isolation of Major Bovine Seminal Plasma Proteins. Protein Expr. Purif. 1996, 8, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Leblond, E.; Desnoyers, L.; Manjunath, P. Phosphorylcholine-binding proteins from the seminal fluids of different species share antigenic determinants with the major proteins of bovine seminal plasma. Mol. Reprod. Dev. 1993, 34, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Berger Rentsch, M.; Zimmer, G. A Vesicular Stomatitis Virus Replicon-Based Bioassay for the Rapid and Sensitive Determination of Multi-Species Type I Interferon. PLoS ONE 2011, 6, e25858. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Mü, M.A.; Drosten, C.; Pö, S.; Krü, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor Article SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 1–10. [Google Scholar] [CrossRef]
- Roost, H.P.; Bachmann, M.F.; Haag, A.; Kalinke, U.; Pliska, V.; Hengartner, H.; Zinkernagel, R.M. Early high-affinity neutralizing anti-viral IgG responses without further overall improvements of affinity. Proc. Natl. Acad. Sci. USA 1995, 92, 1257–1261. [Google Scholar] [CrossRef]
- Hoffmann, M.; Wu, Y.J.; Gerber, M.; Berger-Rentsch, M.; Heimrich, B.; Schwemmle, M.; Zimmer, G. Fusion-active glycoprotein G mediates the cytotoxicity of vesicular stomatitis virus M mutants lacking host shut-off activity. J. Gen. Virol. 2010, 91, 2782–2793. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Schöler, L.; Le-Trilling, V.T.K.; Eilbrecht, M.; Mennerich, D.; Anastasiou, O.E.; Krawczyk, A.; Herrmann, A.; Dittmer, U.; Trilling, M. A Novel In-Cell ELISA Assay Allows Rapid and Automated Quantification of SARS-CoV-2 to Analyze Neutralizing Antibodies and Antiviral Compounds. Front. Immunol. 2020, 11, 573526. [Google Scholar] [CrossRef]
- Manjunath, P.; Bergeron, A.; Lefebvre, J.; Fan, J. Seminal plasma proteins: Functions and interaction with protective agents during semen preservation. Soc. Reprod. Fertil. Suppl. 2007, 65, 217–228. [Google Scholar] [PubMed]
- Bedford, J.M. The Functions—or Not—of Seminal Plasma? Biol. Reprod. 2015, 92, 1–3. [Google Scholar] [CrossRef]
- Carette, J.E.; Raaben, M.; Wong, A.C.; Herbert, A.S.; Obernosterer, G.; Mulherkar, N.; Kuehne, A.I.; Kranzusch, P.J.; Griffin, A.M.; Ruthel, G.; et al. Ebola virus entry requires the cholesterol transporter Niemann–Pick C1. Nature 2011, 477, 340–343. [Google Scholar] [CrossRef]
- Jae, L.T.; Raaben, M.; Herbert, A.S.; Kuehne, A.I.; Wirchnianski, A.S.; Soh, T.K.; Stubbs, S.H.; Janssen, H.; Damme, M.; Saftig, P.; et al. Lassa virus entry requires a trigger-induced receptor switch. Science 2014, 344, 1506–1510. [Google Scholar] [CrossRef]
- Jangra, R.K.; Herbert, A.S.; Li, R.; Jae, L.T.; Kleinfelter, L.M.; Slough, M.M.; Barker, S.L.; Guardado-Calvo, P.; Román-Sosa, G.; Dieterle, M.E.; et al. Protocadherin-1 is essential for cell entry by New World hantaviruses. Nature 2018, 563, 559–563. [Google Scholar] [CrossRef]
- Kondratowicz, A.S.; Lennemann, N.J.; Sinn, P.L.; Davey, R.A.; Hunt, C.L.; Moller-Tank, S.; Meyerholz, D.K.; Rennert, P.; Mullins, R.F.; Brindley, M.; et al. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc. Natl. Acad. Sci. USA 2011, 108, 8426–8431. [Google Scholar] [CrossRef]
- Raaben, M.; Jae, L.T.; Herbert, A.S.; Kuehne, A.I.; Stubbs, S.H.; Chou, Y.; Blomen, V.A.; Kirchhausen, T.; Dye, J.M.; Brummelkamp, T.R.; et al. NRP2 and CD63 Are Host Factors for Lujo Virus Cell Entry. Cell Host Microbe. 2017, 22, 688–696.e5. [Google Scholar] [CrossRef]
- Riblett, A.M.; Blomen, V.A.; Jae, L.T.; Altamura, L.A.; Doms, R.W.; Brummelkamp, T.R.; Wojcechowskyj, J.A. A Haploid Genetic Screen Identifies Heparan Sulfate Proteoglycans Supporting Rift Valley Fever Virus Infection. J. Virol. 2016, 90, 1414–1423. [Google Scholar] [CrossRef]
- Salazar-García, M.; Acosta-Contreras, S.; Rodríguez-Martínez, G.; Cruz-Rangel, A.; Flores-Alanis, A.; Patiño-López, G.; Luna-Pineda, V.M. Pseudotyped Vesicular Stomatitis Virus-Severe Acute Respiratory Syndrome-Coronavirus-2 Spike for the Study of Variants, Vaccines, and Therapeutics Against Coronavirus Disease 2019. Front. Microbiol. 2022, 12, 817200. [Google Scholar] [CrossRef]
- Ng, D.L.; Goldgof, G.M.; Shy, B.R.; Levine, A.G.; Balcerek, J.; Bapat, S.P.; Prostko, J.; Rodgers, M.; Coller, K.; Pearce, S.; et al. SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood. Nat. Commun. 2020, 11, 4698. [Google Scholar] [CrossRef] [PubMed]
- Oguntuyo, K.Y.; Stevens, C.S.; Hung, C.T.; Ikegame, S.; Acklin, J.A.; Kowdle, S.S.; Carmichael, J.C.; Chiu, H.P.; Azarm, K.D.; Haas, G.D.; et al. Quantifying Absolute Neutralization Titers against SARS-CoV-2 by a Standardized Virus Neutralization Assay Allows for Cross-Cohort Comparisons of COVID-19 Sera. MBio 2021, 12, e02492-20. [Google Scholar] [CrossRef]
- Borena, W.; Bánki, Z.; Bates, K.; Winner, H.; Riepler, L.; Rössler, A.; Pipperger, L.; Theurl, I.; Falkensammer, B.; Ulmer, H.; et al. Persistence of immunity to SARS-CoV-2 over time in the ski resort Ischgl. EBioMedicine 2021, 70, 103534. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, J.; Li, Q.; Hu, H.; Lu, J.; Chen, Z. Advances in Neutralization Assays for SARS-CoV-2. Scand. J. Immunol. 2021, 94, e13088. [Google Scholar] [CrossRef]
- Finkelshtein, D.; Werman, A.; Novick, D.; Barak, S.; Rubinstein, M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 2013, 110, 7306–7311. [Google Scholar] [CrossRef] [PubMed]
- De Petro, G.; Barlati, S.; Vartio, T.; Vaheri, A. Transformation-enhancing activity of gelatin-binding fragments of fibronectin. Proc. Natl. Acad. Sci. USA 1981, 78, 4965–4969. [Google Scholar] [CrossRef] [PubMed]
- Atkin, K.E.; Brentnall, A.S.; Harris, G.; Bingham, R.J.; Erat, M.C.; Millard, C.J.; Schwarz-Linek, U.; Staunton, D.; Vakonakis, I.; Campbell, I.D.; et al. The Streptococcal Binding Site in the Gelatin-binding Domain of Fibronectin Is Consistent with a Non-linear Arrangement of Modules. J. Biol. Chem. 2010, 285, 36977–36983. [Google Scholar] [CrossRef]
- Larson, C.L.; Samuelson, D.R.; Eucker, T.P.; O’Loughlin, J.L.; Konkel, M.E. The fibronectin-binding motif within FlpA facilitates Campylobacter jejuni adherence to host cell and activation of host cell signaling. Emerg. Microbes Infect. 2013, 2, 1–12. [Google Scholar] [CrossRef]
- Kobayashi, K.; Koike, S. Cellular receptors for enterovirus A71. J. Biomed. Sci. 2020, 27, 23. [Google Scholar] [CrossRef]
- Keski-Oja, J.; Hautanen, A.; Julkunen, I. Fibronectin and Viral Pathogenesis. Clin. Infect. Dis. 1987, 9, S404–S411. [Google Scholar] [CrossRef]
- Pande, H.; Terramani, T.; Tressel, T.; Churchill, M.A.; Hawkins, G.G.; Zaia, J.A. Altered expression of fibronectin gene in cells infected with human cytomegalovirus. J. Virol. 1990, 64, 1366–1369. [Google Scholar] [CrossRef]
- Müller, P.; Erlemann, K.-R.; Müller, K.; Calvete, J.J.; Töpfer-Petersen, E.; Marienfeld, K.; Herrmann, A. Biophysical characterization of the interaction of bovine seminal plasma protein PDC-109 with phospholipid vesicles. Eur. Biophys. J. 1998, 27, 33–41. [Google Scholar] [CrossRef]
- Le Guillou, J.; Ropers, M.-H.; Gaillard, C.; David-Briand, E.; van Leeuwen-Ibarrola, J.; Desherces, S.; Schmitt, E.; Bencharif, D.; Amirat-Briand, L.; Anton, M.; et al. Sequestration of bovine seminal plasma proteins by different assemblies of phosphatidylcholine: A new technical approach. Colloids Surf. B Biointerfaces 2016, 140, 523–530. [Google Scholar] [CrossRef]
- Müller, P.; Greube, A.; Töpfer-Petersen, E.; Herrmann, A. Influence of the bovine seminal plasma protein PDC-109 on cholesterol in the presence of phospholipids. Eur. Biophys. J. 2002, 31, 438–447. [Google Scholar] [CrossRef]
- Moreau, R.; Frank, P.G.; Perreault, C.; Marcel, Y.L.; Manjunath, P. Seminal plasma choline phospholipid-binding proteins stimulate cellular cholesterol and phospholipid efflux. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 1999, 1438, 38–46. [Google Scholar] [CrossRef]
- Moreau, R.; Thérien, I.; Lazure, C.; Manjunath, P. Type II Domains of BSP-A1/-A2 Proteins: Binding Properties, Lipid Efflux, and Sperm Capacitation Potential. Biochem. Biophys. Res. Commun. 1998, 246, 148–154. [Google Scholar] [CrossRef]
- Dumas, F.; Preira, P.; Salomé, L. Membrane organization of virus and target cell plays a role in HIV entry. Biochimie 2014, 107, 22–27. [Google Scholar] [CrossRef]
- Raulin, J. Lipids and retroviruses. Lipids 2000, 35, 123–130. [Google Scholar] [CrossRef]
- Kočar, E.; Režen, T.; Rozman, D. Cholesterol, lipoproteins, and COVID-19: Basic concepts and clinical applications. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2021, 1866, 158849. [Google Scholar] [CrossRef]
- Molotkovsky, R.; Alexandrova, V.; Galimzyanov, T.; Jiménez-Munguía, I.; Pavlov, K.; Batishchev, O.; Akimov, S. Lateral Membrane Heterogeneity Regulates Viral-Induced Membrane Fusion during HIV Entry. Int. J. Mol. Sci. 2018, 19, 1483. [Google Scholar] [CrossRef] [Green Version]
- Fantini, J.; Epand, R.M.; Barrantes, F.J. Cholesterol-Recognition Motifs in Membrane Proteins. In Direct Mechanisms in Cholesterol Modulation of Protein Function; Rosenhouse-Dantsker, A., Bukiya, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 3–25. [Google Scholar]
- Campbell, S.M.; Crowe, S.M.; Mak, J. Lipid rafts and HIV-1: From viral entry to assembly of progeny virions. J. Clin. Virol. 2001, 22, 217–227. [Google Scholar] [CrossRef]
- Ivankin, A.; Kuzmenko, I.; Gidalevitz, D. Cholesterol Mediates Membrane Curvature during Fusion Events. Phys. Rev. Lett. 2012, 108, 238103. [Google Scholar] [CrossRef]
- Razinkov, V.I.; Melikyan, G.B.; Epand, R.M.; Epand, R.F.; Cohen, F.S. Effects of Spontaneous Bilayer Curvature on Influenza Virus–mediated Fusion Pores. J. Gen. Physiol. 1998, 112, 409–422. [Google Scholar] [CrossRef]
- Stiasny, K.; Heinz, F.X. Effect of Membrane Curvature-Modifying Lipids on Membrane Fusion by Tick-Borne Encephalitis Virus. J. Virol. 2004, 78, 8536–8542. [Google Scholar] [CrossRef]
- Inamdar, K.; Tsai, F.C.; Dibsy, R.; de Poret, A.; Manzi, J.; Merida, P.; Muller, R.; Lappalainen, P.; Roingeard, P.; Mak, J.; et al. Full assembly of HIV-1 particles requires assistance of the membrane curvature factor IRSp53. Elife 2021, 10, e67321. [Google Scholar] [CrossRef]
- Grover, J.R.; Llewellyn, G.N.; Soheilian, F.; Nagashima, K.; Veatch, S.L.; Ono, A. Roles played by capsid-dependent induction of membrane curvature and Gag-ESCRT interactions in tetherin recruitment to HIV-1 assembly sites. J. Virol. 2013, 87, 4650–4664. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperber, H.S.; Sutter, K.; Müller, K.; Müller, P.; Schwarzer, R. The Bovine Seminal Plasma Protein PDC-109 Possesses Pan-Antiviral Activity. Viruses 2022, 14, 2031. https://doi.org/10.3390/v14092031
Sperber HS, Sutter K, Müller K, Müller P, Schwarzer R. The Bovine Seminal Plasma Protein PDC-109 Possesses Pan-Antiviral Activity. Viruses. 2022; 14(9):2031. https://doi.org/10.3390/v14092031
Chicago/Turabian StyleSperber, Hannah Sabeth, Kathrin Sutter, Karin Müller, Peter Müller, and Roland Schwarzer. 2022. "The Bovine Seminal Plasma Protein PDC-109 Possesses Pan-Antiviral Activity" Viruses 14, no. 9: 2031. https://doi.org/10.3390/v14092031
APA StyleSperber, H. S., Sutter, K., Müller, K., Müller, P., & Schwarzer, R. (2022). The Bovine Seminal Plasma Protein PDC-109 Possesses Pan-Antiviral Activity. Viruses, 14(9), 2031. https://doi.org/10.3390/v14092031






