HepG2-NTCP Subclones Exhibiting High Susceptibility to Hepatitis B Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Generation of HepG2-NTCP Subclones
2.2. NTCP Expression
2.3. Peptide Labelling and Staining of NTCP Expressing Cells
2.4. HBV Infection and Quantification of HBV Genomes
2.5. Immunofluorescence for HBV Core Protein
2.6. Statistical Analysis
3. Results
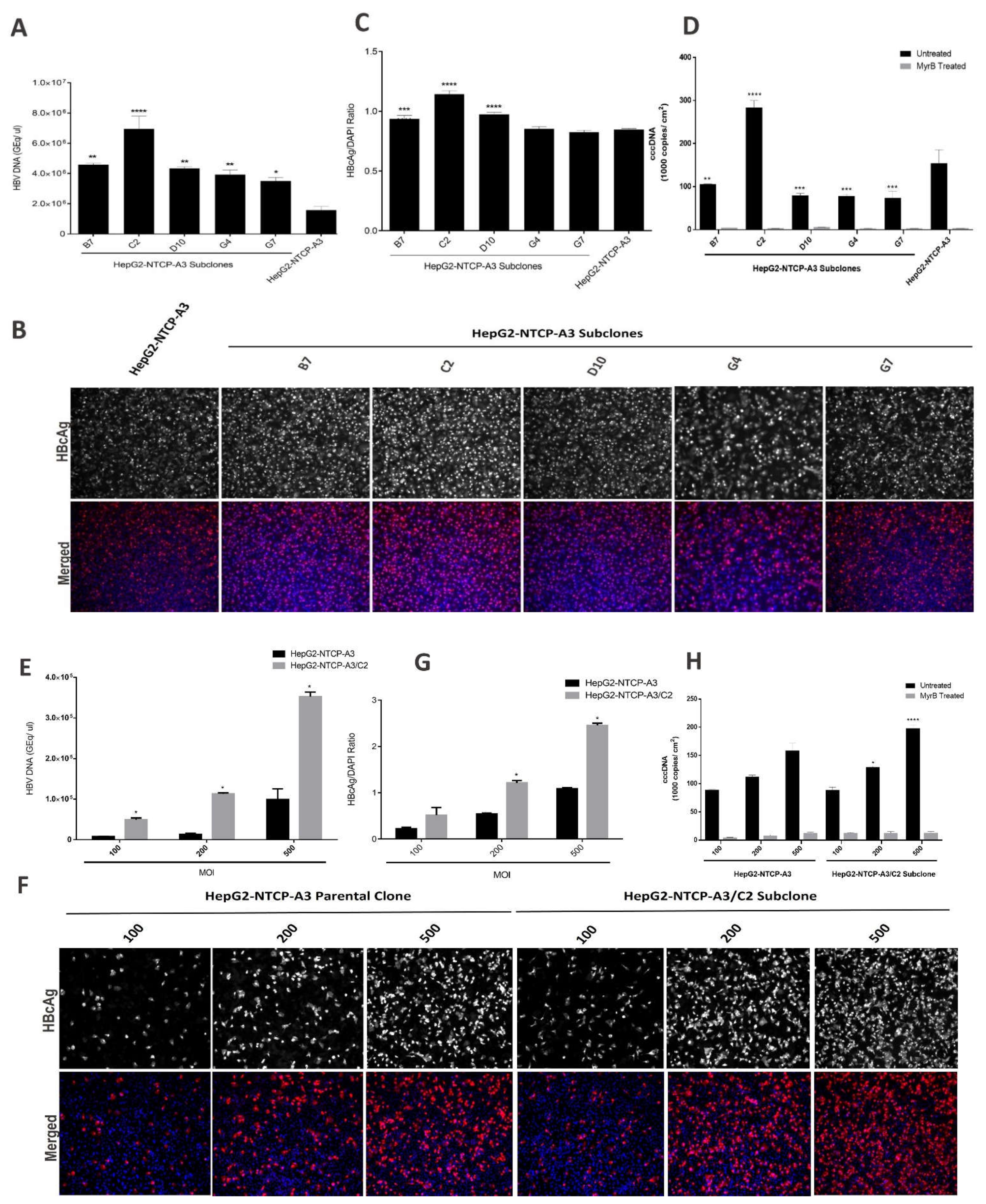
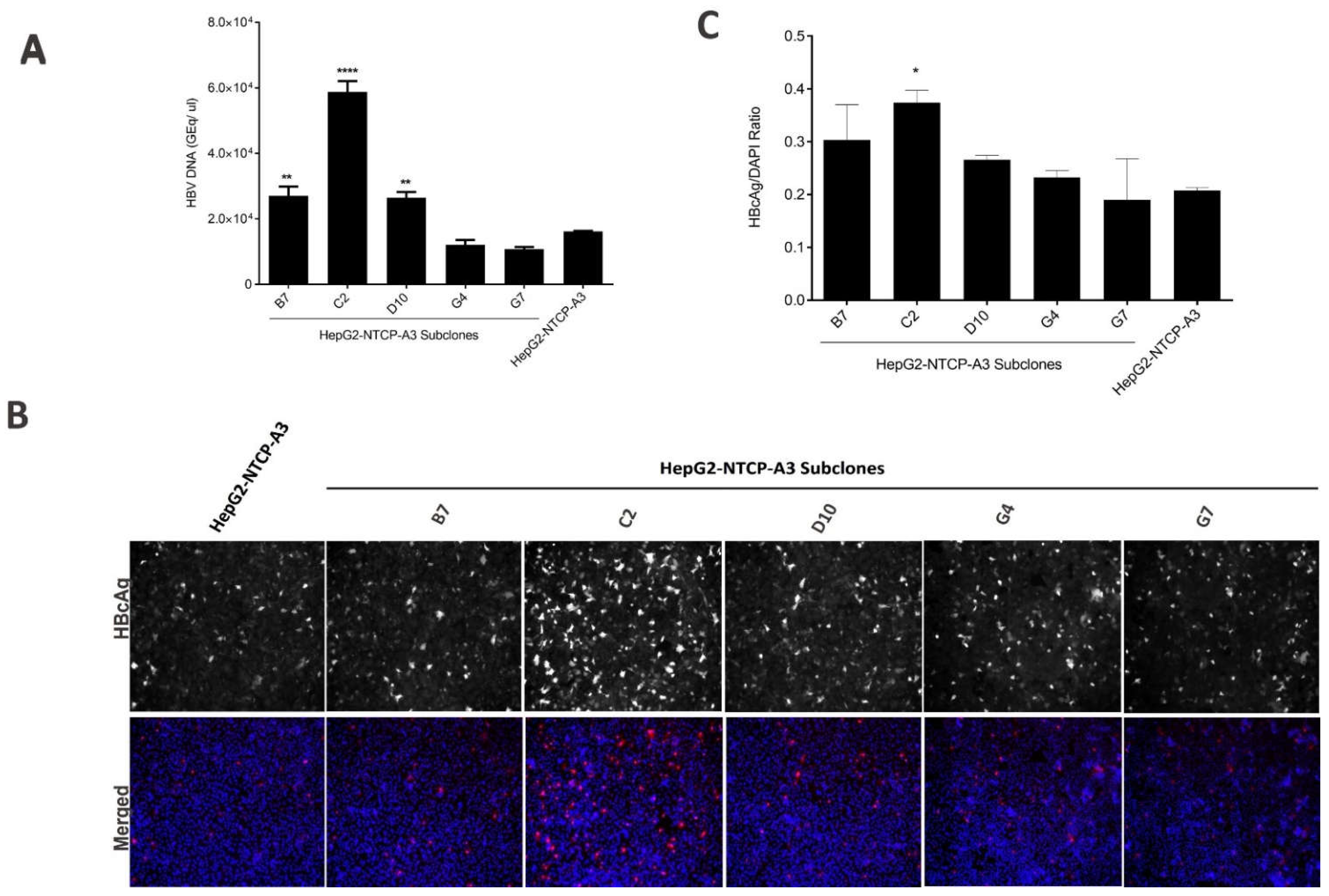
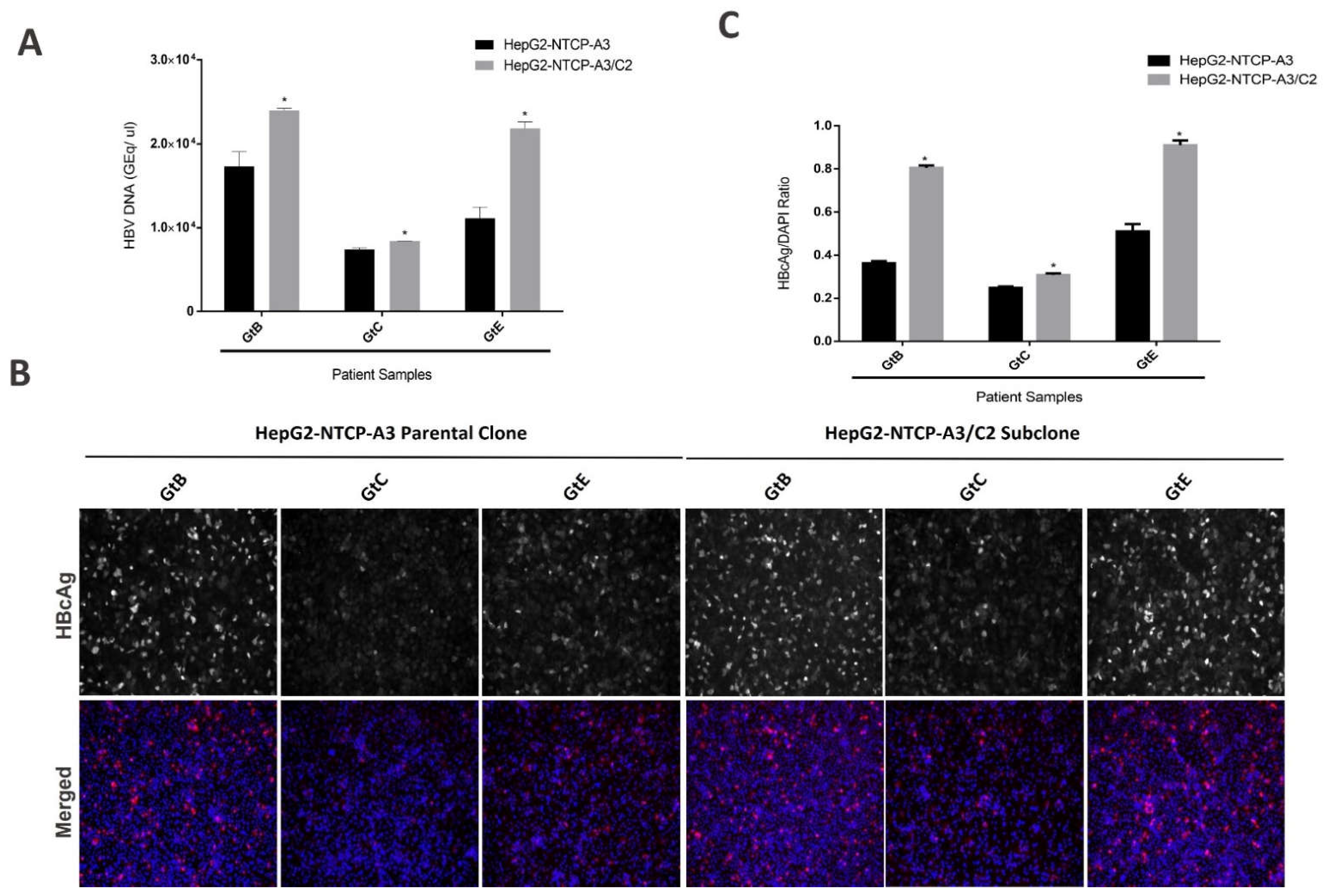
3.1. Enhanced HBV Infection in Subclones of HepG2-NTCP Cells
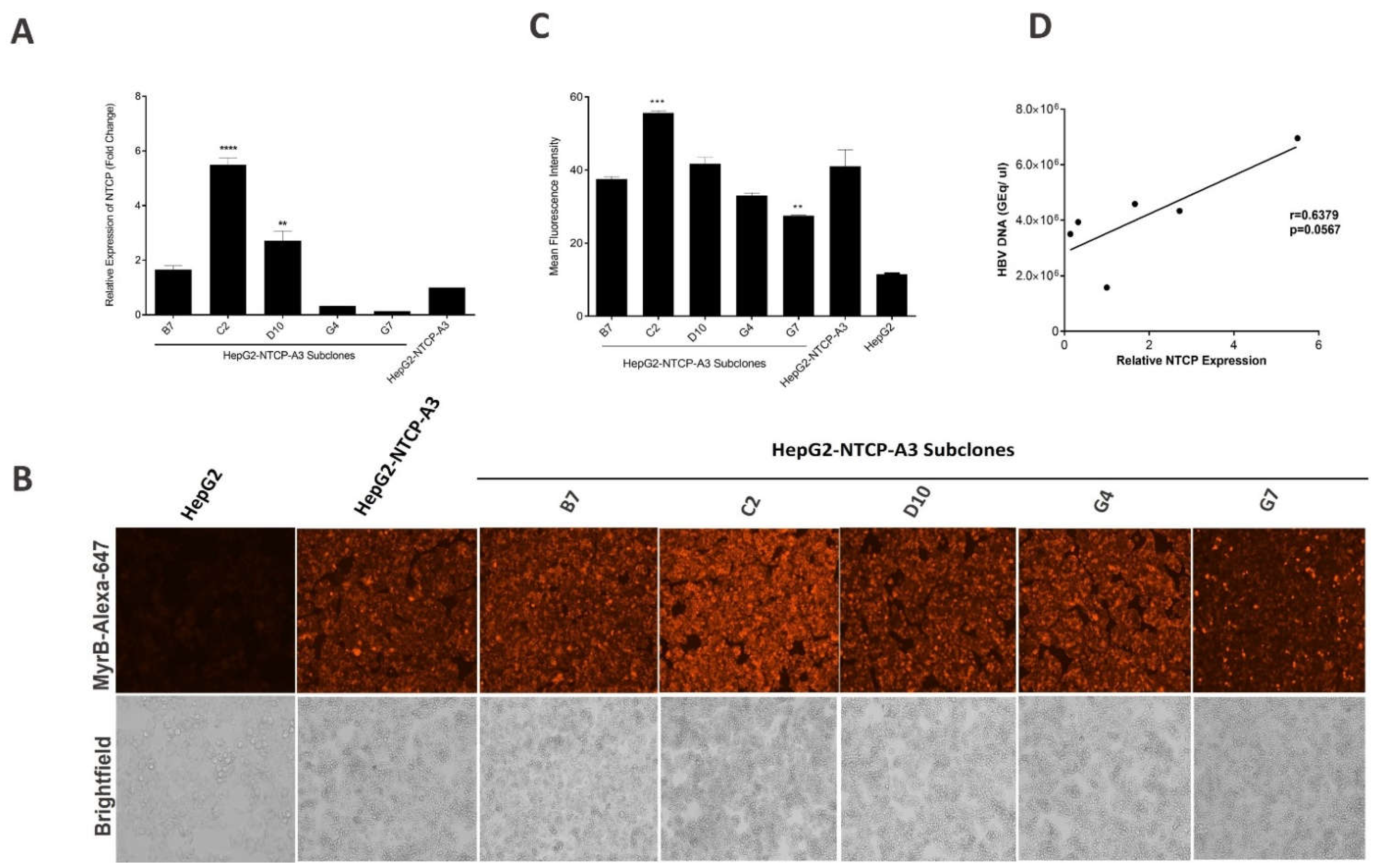
3.2. NTCP Expression in Subclones of HepG2-NTCP Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Razavi-Shearer, D.; Gamkrelidze, I.; Nguyen, M.H.; Chen, D.-S.; van Damme, P.; Abbas, Z.; Abdulla, M.; Abou Rached, A.; Adda, D.; Aho, I.; et al. Global Prevalence, Treatment, and Prevention of Hepatitis B Virus Infection in 2016: A Modelling Study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef]
- Schweitzer, A.; Horn, J.; Mikolajczyk, R.T.; Krause, G.; Ott, J.J. Estimations of Worldwide Prevalence of Chronic Hepatitis B Virus Infection: A Systematic Review of Data Published between 1965 and 2013. Lancet 2015, 386, 1546–1555. [Google Scholar] [CrossRef]
- Trépo, C.; Chan, H.L.Y.; Lok, A. Hepatitis B Virus Infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef]
- El-Serag, H.B. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology 2012, 142, 1264–1273. [Google Scholar] [CrossRef]
- Yan, H.; Liu, Y.; Sui, J.; Li, W. NTCP Opens the Door for Hepatitis B Virus Infection. Antivir. Res. 2015, 121, 24–30. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, Y.; Peng, B.; Li, W. NTCP-Reconstituted In Vitro HBV Infection System. In Hepatitis B Virus; Humana Press: New York, NY, USA, 2017; pp. 1–14. [Google Scholar]
- Kuipery, A.; Sanchez Vasquez, J.D.; Mehrotra, A.; Feld, J.J.; Janssen, H.L.A.; Gehring, A.J. Immunomodulation and RNA Interference Alter Hepatitis B Virus-specific CD8 T Cell Recognition of Infected HepG2-NTCP. Hepatology 2021, 75, 1539–1550. [Google Scholar] [CrossRef]
- Zahoor, M.A.; Philip, S.; Zhi, H.; Giam, C.-Z. NF-κB Inhibition Facilitates the Establishment of Cell Lines That Chronically Produce Human T-Lymphotropic Virus Type 1 Viral Particles. J. Virol. 2014, 88, 3496–3504. [Google Scholar] [CrossRef]
- Zahoor, M.A.; Woods, M.W.; Dizzell, S.; Nazli, A.; Mueller, K.M.; Nguyen, P.V.; Verschoor, C.P.; Kaushic, C. Transcriptional Profiling of Primary Endometrial Epithelial Cells Following Acute HIV-1 Exposure Reveals Gene Signatures Related to Innate Immunity. Am. J. Reprod. Immunol. 2018, 79, e12822. [Google Scholar] [CrossRef]
- Okuyama-Dobashi, K.; Kasai, H.; Tanaka, T.; Yamashita, A.; Yasumoto, J.; Chen, W.; Okamoto, T.; Maekawa, S.; Watashi, K.; Wakita, T.; et al. Hepatitis B Virus Efficiently Infects Non-Adherent Hepatoma Cells via Human Sodium Taurocholate Cotransporting Polypeptide. Sci. Rep. 2015, 5, 17047. [Google Scholar] [CrossRef]
- Gripon, P.; Cannie, I.; Urban, S. Efficient Inhibition of Hepatitis B Virus Infection by Acylated Peptides Derived from the Large Viral Surface Protein. J. Virol. 2005, 79, 1613–1622. [Google Scholar] [CrossRef]
- Ni, Y.; Lempp, F.A.; Mehrle, S.; Nkongolo, S.; Kaufman, C.; Fälth, M.; Stindt, J.; Königer, C.; Nassal, M.; Kubitz, R.; et al. Hepatitis B and D Viruses Exploit Sodium Taurocholate Co-Transporting Polypeptide for Species-Specific Entry into Hepatocytes. Gastroenterology 2014, 146, 1070–1083.e6. [Google Scholar] [CrossRef]
- Chakraborty, A.; Ko, C.; Henning, C.; Lucko, A.; Harris, J.M.; Chen, F.; Zhuang, X.; Wettengel, J.M.; Roessler, S.; Protzer, U.; et al. Synchronised Infection Identifies Early Rate-limiting Steps in the Hepatitis B Virus Life Cycle. Cell. Microbiol. 2020, 22, e13250. [Google Scholar] [CrossRef]
- Le, C.; Sirajee, R.; Steenbergen, R.; Joyce, M.A.; Addison, W.R.; Tyrrell, D.L. In Vitro Infection with Hepatitis B Virus Using Differentiated Human Serum Culture of Huh7.5-NTCP Cells without Requiring Dimethyl Sulfoxide. Viruses 2021, 13, 97. [Google Scholar] [CrossRef]
- Ladner, S.K.; Otto, M.J.; Barker, C.S.; Zaifert, K.; Wang, G.H.; Guo, J.T.; Seeger, C.; King, R.W. Inducible Expression of Human Hepatitis B Virus (HBV) in Stably Transfected Hepatoblastoma Cells: A Novel System for Screening Potential Inhibitors of HBV Replication. Antimicrob. Agents Chemother. 1997, 41, 1715–1720. [Google Scholar] [CrossRef]
- König, A.; Yang, J.; Jo, E.; Park, K.H.P.; Kim, H.; Than, T.T.; Song, X.; Qi, X.; Dai, X.; Park, S.; et al. Efficient Long-Term Amplification of Hepatitis B Virus Isolates after Infection of Slow Proliferating HepG2-NTCP Cells. J. Hepatol. 2019, 71, 289–300. [Google Scholar] [CrossRef]
- Ko, C.; Chakraborty, A.; Chou, W.-M.; Hasreiter, J.; Wettengel, J.M.; Stadler, D.; Bester, R.; Asen, T.; Zhang, K.; Wisskirchen, K.; et al. Hepatitis B Virus Genome Recycling and de Novo Secondary Infection Events Maintain Stable CccDNA Levels. J. Hepatol. 2018, 69, 1231–1241. [Google Scholar] [CrossRef]
- Chua, C.G.; Mehrotra, A.; Mazzulli, T.; Wong, D.K.; Feld, J.J.; Janssen, H.L.A.; Gehring, A.J. Optimized Ex Vivo Stimulation Identifies Multi-Functional HBV-Specific T Cells in a Majority of Chronic Hepatitis B Patients. Sci. Rep. 2020, 10, 11344. [Google Scholar] [CrossRef]
- Michailidis, E.; Pabon, J.; Xiang, K.; Park, P.; Ramanan, V.; Hoffmann, H.-H.; Schneider, W.M.; Bhatia, S.N.; de Jong, Y.P.; Shlomai, A.; et al. A Robust Cell Culture System Supporting the Complete Life Cycle of Hepatitis B Virus. Sci. Rep. 2017, 7, 16616. [Google Scholar] [CrossRef]
- Choijilsuren, G.; Jhou, R.-S.; Chou, S.-F.; Chang, C.-J.; Yang, H.-I.; Chen, Y.-Y.; Chuang, W.-L.; Yu, M.-L.; Shih, C. Heparin at Physiological Concentration Can Enhance PEG-Free in Vitro Infection with Human Hepatitis B Virus. Sci. Rep. 2017, 7, 14461. [Google Scholar] [CrossRef]
- Qi, Y.; Gao, Z.; Xu, G.; Peng, B.; Liu, C.; Yan, H.; Yao, Q.; Sun, G.; Liu, Y.; Tang, D.; et al. DNA Polymerase κ Is a Key Cellular Factor for the Formation of Covalently Closed Circular DNA of Hepatitis B Virus. PLoS Pathog. 2016, 12, e1005893. [Google Scholar] [CrossRef]
- Qu, B.; Urban, S. Quantification of Hepatitis B Virus Covalently Closed Circular DNA in Infected Cell Culture Models by Quantitative PCR. Bio-Protocol 2019, 9, 553–559. [Google Scholar] [CrossRef]
- Nair, S.; Zlotnick, A. HBV Core Protein Is in Flux between Cytoplasmic, Nuclear, and Nucleolar Compartments. mBio 2021, 12, e03514-20. [Google Scholar] [CrossRef]
- Yan, Y.; Allweiss, L.; Yang, D.; Kang, J.; Wang, J.; Qian, X.; Zhang, T.; Liu, H.; Wang, L.; Liu, S.; et al. Down-Regulation of Cell Membrane Localized NTCP Expression in Proliferating Hepatocytes Prevents Hepatitis B Virus Infection. Emerg. Microbes Infect. 2019, 8, 879–894. [Google Scholar] [CrossRef]
- Iwamoto, M.; Saso, W.; Sugiyama, R.; Ishii, K.; Ohki, M.; Nagamori, S.; Suzuki, R.; Aizaki, H.; Ryo, A.; Yun, J.-H.; et al. Epidermal Growth Factor Receptor Is a Host-Entry Cofactor Triggering Hepatitis B Virus Internalization. Proc. Natl. Acad. Sci. USA 2019, 116, 8487–8492. [Google Scholar] [CrossRef]
- Bouezzedine, F.; Fardel, O.; Gripon, P. Interleukin 6 Inhibits HBV Entry through NTCP down Regulation. Virology 2015, 481, 34–42. [Google Scholar] [CrossRef]
- Eller, C.; Heydmann, L.; Colpitts, C.C.; el Saghire, H.; Piccioni, F.; Jühling, F.; Majzoub, K.; Pons, C.; Bach, C.; Lucifora, J.; et al. A Genome-Wide Gain-of-Function Screen Identifies CDKN2C as a HBV Host Factor. Nat. Commun. 2020, 11, 2707. [Google Scholar] [CrossRef]
- Li, J.; Zong, L.; Sureau, C.; Barker, L.; Wands, J.R.; Tong, S. Unusual Features of Sodium Taurocholate Cotransporting Polypeptide as a Hepatitis B Virus Receptor. J. Virol. 2016, 90, 8302–8313. [Google Scholar] [CrossRef]
- Gripon, P.; Diot, C.; Guguen-Guillouzo, C. Reproducible High Level Infection of Cultured Adult Human Hepatocytes by Hepatitis B Virus: Effect of Polyethylene Glycol on Adsorption and Penetration. Virology 1993, 192, 534–540. [Google Scholar] [CrossRef]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium Taurocholate Cotransporting Polypeptide Is a Functional Receptor for Human Hepatitis B and D Virus. eLife 2012, 1, e00049. [Google Scholar] [CrossRef]
- Ou, G.; He, L.; Wang, L.; Song, J.; Lai, X.; Tian, X.; Wang, L.; Zhang, K.; Zhang, X.; Deng, J.; et al. The Genotype (A to H) Dependent N-Terminal Sequence of HBV Large Surface Protein Affects Viral Replication, Secretion and Infectivity. Front. Microbiol. 2021, 12, 687785. [Google Scholar] [CrossRef]
- Sainz, B.; Barretto, N.; Uprichard, S.L. Hepatitis C Virus Infection in Phenotypically Distinct Huh7 Cell Lines. PLoS ONE 2009, 4, e6561. [Google Scholar] [CrossRef] [PubMed]
| 4% PEG | No Peg | |||||||
|---|---|---|---|---|---|---|---|---|
| Extracellular HBV DNA | Intracellular HBV DNA | Extracellular HBV DNA | Intracellular HBV DNA | |||||
| HBV DNA log10Copies/mL (SD) | Fold Change vs. Parental | HBcAg MFI | Fold Change vs. Parental | HBV DNA log10Copies/mL (SD) | Fold Change vs. Parental | HBV DNA log10Copies/mL (SD) | Fold Change vs. Parental | |
| HepG2-NTCP-A3 | 6.20 ± 5.52 | 1.0 | 69.1 ± 1.3 | 1.0 | 4.21 ± 2.15 | 1.0 | 16.1 ± 0.4 | 1.0 |
| B7 | 6.66 ± 4.94 a | 2.98 | 84.7 ± 2.6 | 1.22 | 4.43 ± 3.45 b | 1.67 | 16.7 ± 0.4 | 1.03 |
| C2 | 6.84 ± 5.93 a | 4.45 | 93.0 ± 2.3 a | 1.35 | 4.77 ± 2.51 b | 3.63 | 24.7 ±1.5 b | 1.48 |
| D10 | 6.65 ± 4.06 a | 2.80 | 79.6 ± 1.3 | 1.15 | 4.42 ± 2.43 b | 1.63 | 20.4 ± 0.6 | 1.22 |
| G4 | 6.59 ± 4.07 a | 2.57 | 77.3 ± 1.5 | 1.12 | 4.08 ± 2.17 | 0.74 | 16.6 ± 0.9 | 0.99 |
| G7 | 6.54 ± 5.37 a | 2.25 | 74.6 ± 1.5 | 1.08 | 4.03 ± 2.79 | 0.67 | 10.1 ± 4.1 | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahoor, M.A.; Kuipery, A.; Mosa, A.I.; Gehring, A.J.; Feld, J.J. HepG2-NTCP Subclones Exhibiting High Susceptibility to Hepatitis B Virus Infection. Viruses 2022, 14, 1800. https://doi.org/10.3390/v14081800
Zahoor MA, Kuipery A, Mosa AI, Gehring AJ, Feld JJ. HepG2-NTCP Subclones Exhibiting High Susceptibility to Hepatitis B Virus Infection. Viruses. 2022; 14(8):1800. https://doi.org/10.3390/v14081800
Chicago/Turabian StyleZahoor, Muhammad Atif, Adrian Kuipery, Alexander I. Mosa, Adam J. Gehring, and Jordan J. Feld. 2022. "HepG2-NTCP Subclones Exhibiting High Susceptibility to Hepatitis B Virus Infection" Viruses 14, no. 8: 1800. https://doi.org/10.3390/v14081800
APA StyleZahoor, M. A., Kuipery, A., Mosa, A. I., Gehring, A. J., & Feld, J. J. (2022). HepG2-NTCP Subclones Exhibiting High Susceptibility to Hepatitis B Virus Infection. Viruses, 14(8), 1800. https://doi.org/10.3390/v14081800







