SARS-CoV-2 Omicron Variant Neutralization after Third Dose Vaccination in PLWH
Abstract
:1. Introduction
2. Materials and Methods
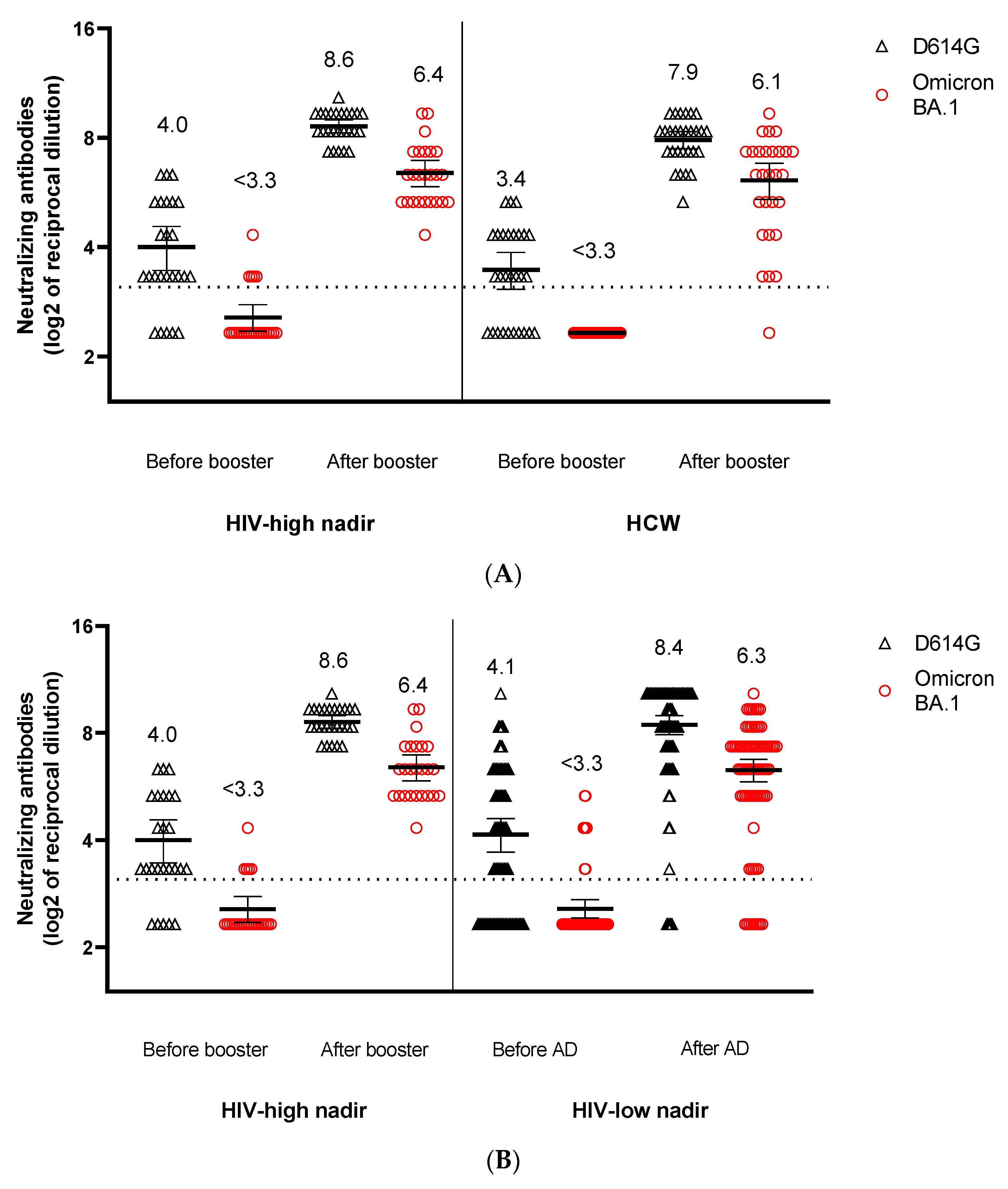
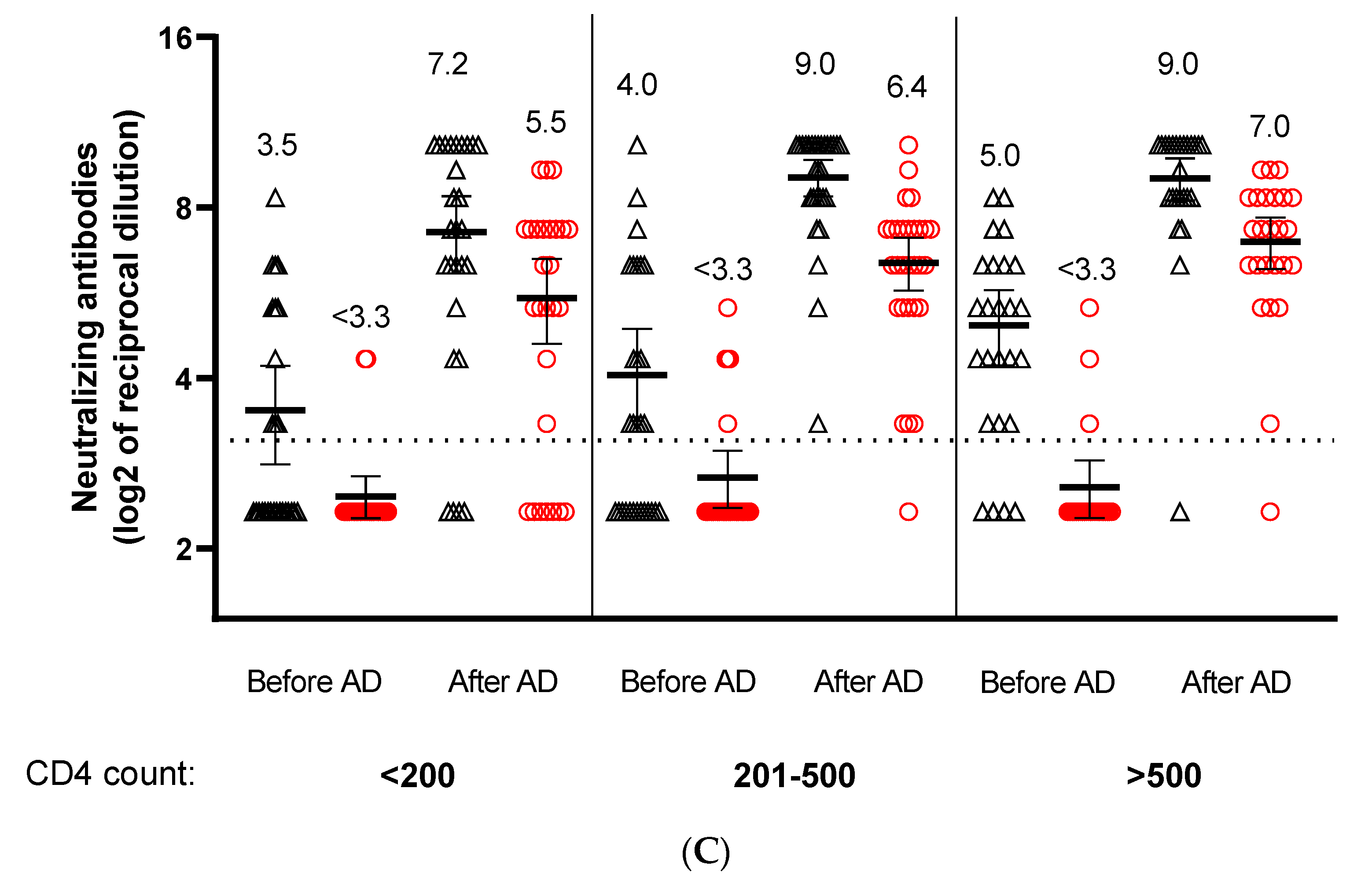
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danwang, C.; Noubiap, J.J.; Robert, A.; Yombi, J.C. Outcomes of patients with HIV and COVID-19 co-infection: A systematic review and meta-analysis. AIDS Res Ther. 2022, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Vergori, A.; Cicalini, S.; Cozzi Lepri, A.; Matusali, G.; Bordoni, V.; Lanini, S.; Colavita, F.; Cimini, E.; Iannazzo, R.; De Pascale, L.; et al. Immunogenicity and Reactogenicity to COVID-19 mRNA Vaccine Additional Dose in PLWH. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Virtual, 12–16 February 2022. [Google Scholar]
- Antinori, A.; Cicalini, S.; Meschi, S.; Bordoni, V.; Lorenzini, P.; Vergori, A.; Lanini, S.; De Pascale LMatusali, G.; Mariotti, D.; Cozzi Lepri, A.; et al. HIV-VAC Study Group. Humoral and cellular immune response elicited by mRNA vaccination against SARS-CoV-2 in people living with HIV (PLWH) receiving antiretroviral therapy (ART) according with current CD4 T-lymphocyte count. Clin. Infect. Dis. 2022, ciac238. [Google Scholar] [CrossRef] [PubMed]
- Tuekprakhon, A.; Huo, J.; Nutalai, R.; Dijokaite-Guraliuc, A.; Zhou, D.; Ginn, H.M.; Sekvaraj, M.; Liu, C.; Mentzer, A.J.; Supasa, P.; et al. Further antibody escape by Omicron BA.4 and BA.5 from vaccine and BA.1 serum. bioRxiv 2002. [Google Scholar] [CrossRef]
- Meschi, S.; Matusali, G.; Colavita, F.; Lapa, D.; Bordi, L.; Puro, V.; Leoni, B.D.; Galli, C.; Capobianchi, M.R.; Castilletti, C.; et al. Predicting the protective humoral response to a SARS-CoV-2 mRNA vaccine. Clin. Chem. Lab. Med. 2021, 59, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergori, A.; Cozzi-Lepri, A.; Matusali, G.; Colavita, F.; Cicalini, S.; Gallì, P.; Garbuglia, A.R.; Fusto, M.; Puro, V.; Maggi, F.; et al. SARS-CoV-2 Omicron Variant Neutralization after Third Dose Vaccination in PLWH. Viruses 2022, 14, 1710. https://doi.org/10.3390/v14081710
Vergori A, Cozzi-Lepri A, Matusali G, Colavita F, Cicalini S, Gallì P, Garbuglia AR, Fusto M, Puro V, Maggi F, et al. SARS-CoV-2 Omicron Variant Neutralization after Third Dose Vaccination in PLWH. Viruses. 2022; 14(8):1710. https://doi.org/10.3390/v14081710
Chicago/Turabian StyleVergori, Alessandra, Alessandro Cozzi-Lepri, Giulia Matusali, Francesca Colavita, Stefania Cicalini, Paola Gallì, Anna Rosa Garbuglia, Marisa Fusto, Vincenzo Puro, Fabrizio Maggi, and et al. 2022. "SARS-CoV-2 Omicron Variant Neutralization after Third Dose Vaccination in PLWH" Viruses 14, no. 8: 1710. https://doi.org/10.3390/v14081710
APA StyleVergori, A., Cozzi-Lepri, A., Matusali, G., Colavita, F., Cicalini, S., Gallì, P., Garbuglia, A. R., Fusto, M., Puro, V., Maggi, F., Girardi, E., Vaia, F., & Antinori, A., on behalf of the HIV-VAC Study Group. (2022). SARS-CoV-2 Omicron Variant Neutralization after Third Dose Vaccination in PLWH. Viruses, 14(8), 1710. https://doi.org/10.3390/v14081710






