Virulent Phages Isolated from a Smear-Ripened Cheese Are Also Detected in Reservoirs of the Cheese Factory
Abstract
:1. Introduction
2. Material and Methods
2.1. Sampling Procedure
2.2. Microbiological Analysis
2.2.1. Enumeration and Isolation of Microorganisms
2.2.2. Identification of Bacterial Isolates
2.3. Isolation of Bacteriophages, Purification and Concentration
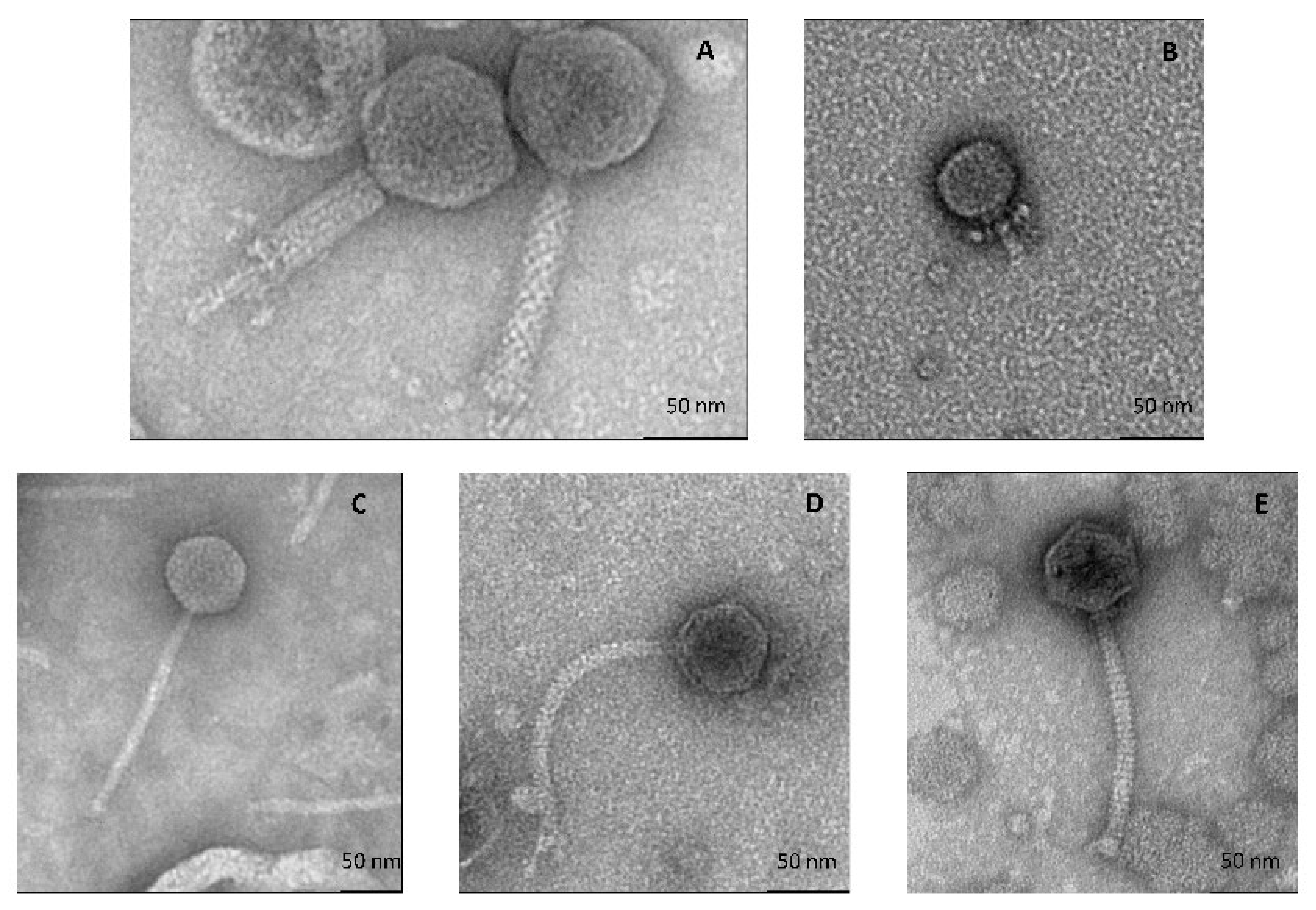
2.4. Transmission Electronic Microscopy (TEM)
2.5. Viral Genomic DNA Extraction and Sequencing
2.6. Phage Genome Assembly
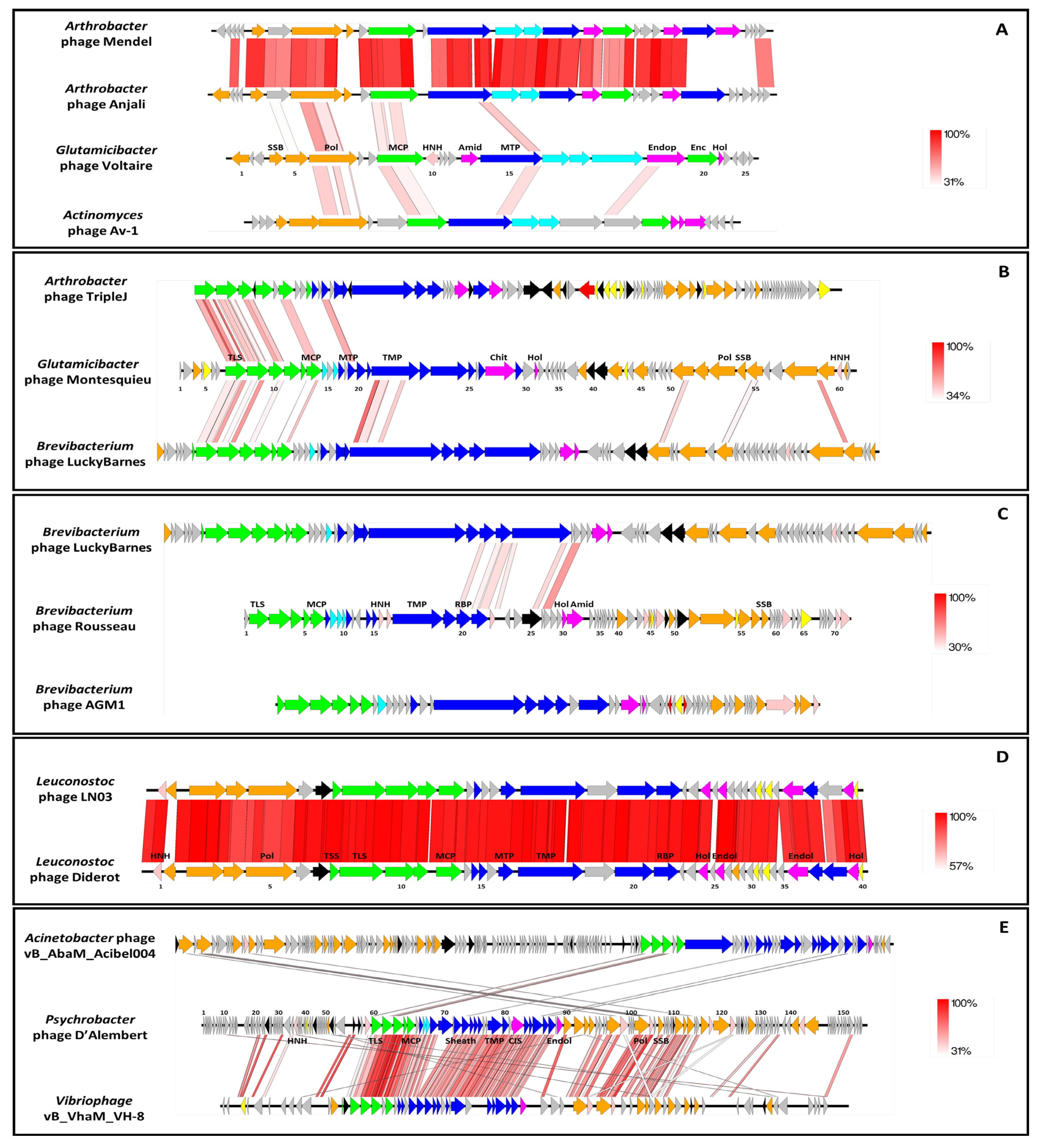
2.7. Structural and Functional Annotation
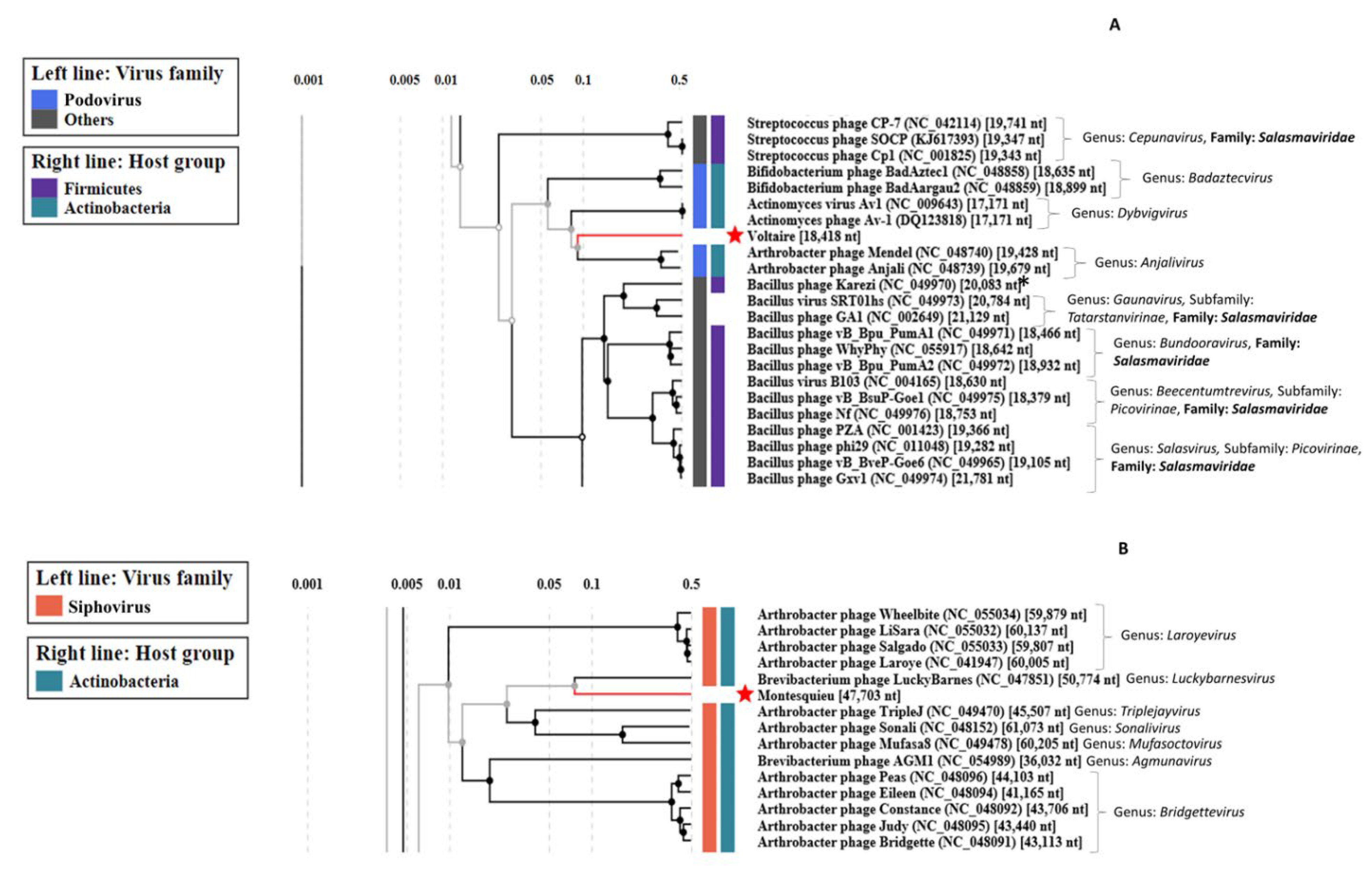
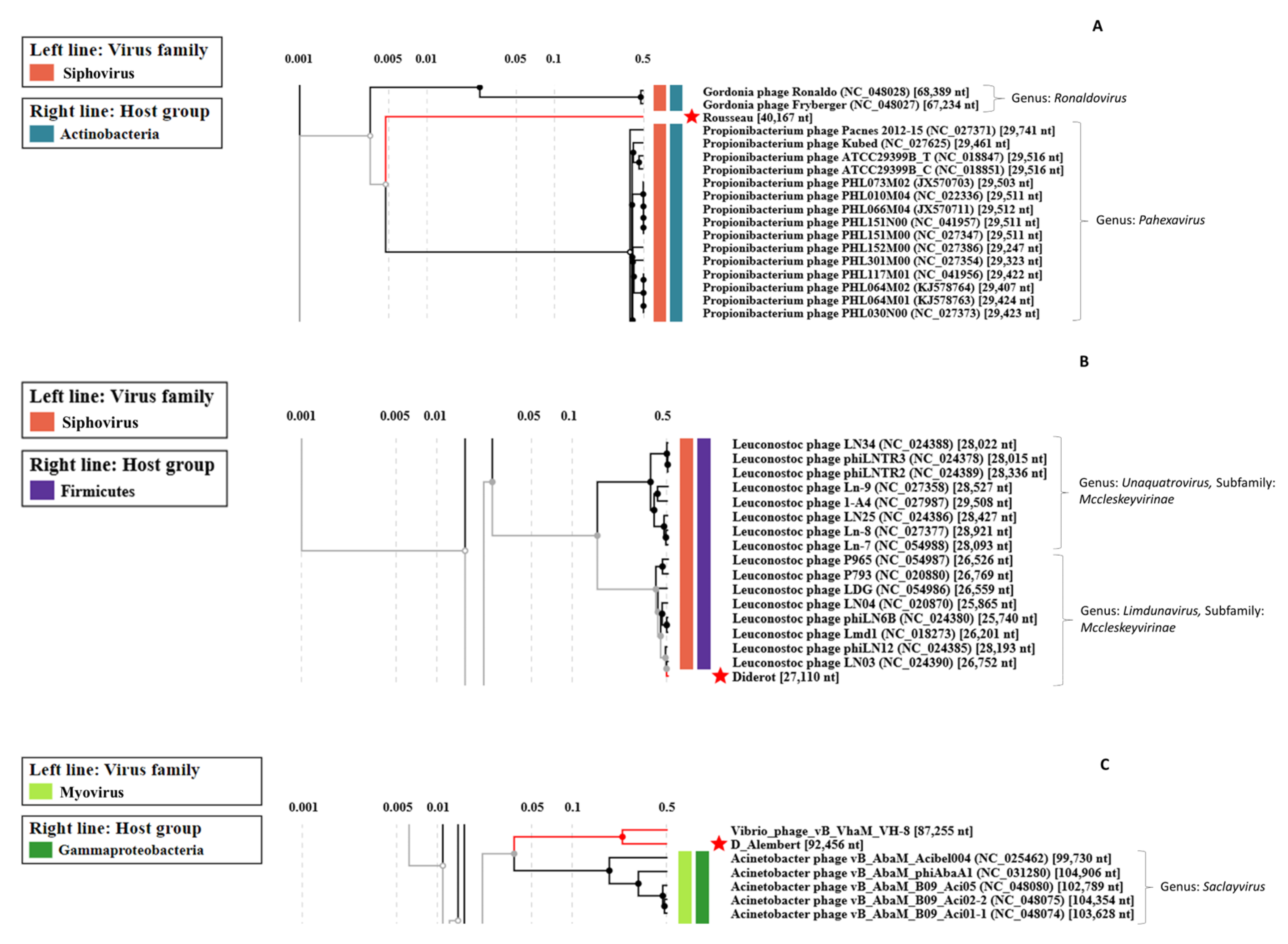
2.8. Genome-Based Classification of Phages
2.9. Host Range Determination
2.10. Effect of Temperature on Phage Infection
2.11. Identification of Phage Reservoirs in a Dairy Plant
3. Results
3.1. Construction of a Bacterial Collection from the Cheese Surface
3.2. Five Bacteriophages Isolated from the Cheese Surface
3.2.1. All Cultivable Phages Are Tailed Phages
3.2.2. All Five Phages Have Narrow Host Ranges
3.2.3. Effect of Temperature on Phage Infection
3.2.4. Uncovering Three Completely New Phage Genomes
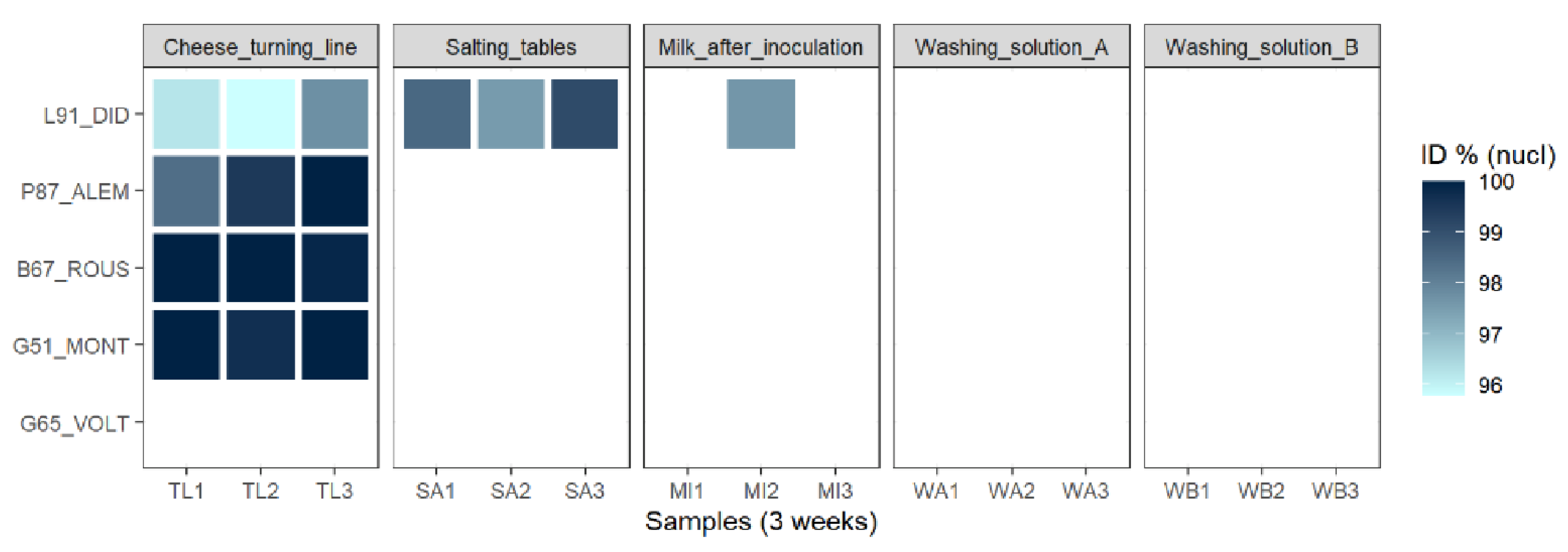
3.2.5. Most of the Identified Phages Are Also Present in the Dairy Plant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irlinger, F.; Layec, S.; Hélinck, S.; Dugat-Bony, E. Cheese Rind Microbial Communities: Diversity, Composition and Origin. FEMS Microbiol. Lett. 2015, 362, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokulich, N.A.; Mills, D.A. Facility-Specific “House” Microbiome Drives Microbial Landscapes of Artisan Cheesemaking Plants. Appl. Environ. Microbiol. 2013, 79, 5214–5223. [Google Scholar] [CrossRef] [Green Version]
- Montel, M.-C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional Cheeses: Rich and Diverse Microbiota with Associated Benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef]
- Penland, M.; Falentin, H.; Parayre, S.; Pawtowski, A.; Maillard, M.-B.; Thierry, A.; Mounier, J.; Coton, M.; Deutsch, S.-M. Linking Pélardon Artisanal Goat Cheese Microbial Communities to Aroma Compounds during Cheese-Making and Ripening. Int. J. Food Microbiol. 2021, 345, 109130. [Google Scholar] [CrossRef]
- Panthi, R.R.; Jordan, K.N.; Kelly, A.L.; Sheehan, J.J. Selection and Treatment of Milk for Cheesemaking. In Cheese, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, CA, USA, 2017; Chapter 2; pp. 23–50. ISBN 978-0-12-417012-4. [Google Scholar]
- Parente, E.; Cogan, T.M.; Powell, I.B. Starter Cultures: General Aspects. In Cheese, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, CA, USA, 2017; Chapter 8; pp. 201–226. ISBN 978-0-12-417012-4. [Google Scholar]
- Helinck, S.; Le Bars, D.; Moreau, D.; Yvon, M. Ability of Thermophilic Lactic Acid Bacteria to Produce Aroma Compounds from Amino Acids. Appl. Environ. Microbiol. 2004, 70, 3855–3861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leistner, L.; Gorris, L.G.M. Food Preservation by Hurdle Technology. Trends Food Sci. Technol. 1995, 6, 41–46. [Google Scholar] [CrossRef]
- Brum, J.R.; Ignacio-Espinoza, J.C.; Roux, S.; Doulcier, G.; Acinas, S.G.; Alberti, A.; Chaffron, S.; Cruaud, C.; de Vargas, C.; Gasol, J.M.; et al. Patterns and Ecological Drivers of Ocean Viral Communities. Science 2015, 348, 1261498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, O.H.; Kushmaro, A.; Brenner, A. Bacteriophage Predation Regulates Microbial Abundance and Diversity in a Full-Scale Bioreactor Treating Industrial Wastewater. ISME J. 2010, 4, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, T.D.S.; Hill, C. Gut Bacteriophage: Current Understanding and Challenges. Front. Endocrinol. 2019, 10, 784. [Google Scholar] [CrossRef] [PubMed]
- Paillet, T.; Dugat-Bony, E. Bacteriophage Ecology of Fermented Foods: Anything New under the Sun? Curr. Opin. Food Sci. 2021, 40, 102–111. [Google Scholar] [CrossRef]
- Brüssow, H. Phages of Dairy Bacteria. Annu. Rev. Microbiol. 2001, 55, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H.; Kutter, E. Phage Ecology. In Bacteriophages; Kutter, E., Sulakvelidze, A., Eds.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0-8493-1336-3. [Google Scholar]
- Garneau, J.E.; Moineau, S. Bacteriophages of Lactic Acid Bacteria and Their Impact on Milk Fermentations. Microb. Cell Fact 2011, 10, S20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcó, M.B.; Moineau, S.; Quiberoni, A. Bacteriophages and Dairy Fermentations. Bacteriophage 2012, 2, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüssow, H.; Fremont, M.; Bruttin, A.; Sidoti, J.; Constable, A.; Fryder, V. Detection and Classification of Streptococcus Thermophilus Bacteriophages Isolated from Industrial Milk Fermentation. Appl. Environ. Microbiol. 1994, 60, 4537–4543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautier, M.; Rouault, A.; Sommer, P.; Briandet, R. Occurrence of Propionibacterium Freudenreichii Bacteriophages in Swiss Cheese. Appl. Environ. Microbiol. 1995, 61, 2572–2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madera, C.; Monjardín, C.; Suárez, J.E. Milk Contamination and Resistance to Processing Conditions Determine the Fate of Lactococcus Lactis Bacteriophages in Dairies. Appl. Environ. Microbiol. 2004, 70, 7365–7371. [Google Scholar] [CrossRef] [Green Version]
- Wagner, N.; Brinks, E.; Samtlebe, M.; Hinrichs, J.; Atamer, Z.; Kot, W.; Franz, C.M.A.P.; Neve, H.; Heller, K.J. Whey Powders Are a Rich Source and Excellent Storage Matrix for Dairy Bacteriophages. Int. J. Food Microbiol. 2017, 241, 308–317. [Google Scholar] [CrossRef]
- Bockelmann, W. Cheese | Smear-Ripened Cheeses. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 753–766. ISBN 978-0-12-374407-4. [Google Scholar]
- Mounier, J.; Coton, M.; Irlinger, F.; Landaud, S.; Bonnarme, P. Smear-Ripened Cheeses. In Cheese, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, CA, USA, 2017; Chapter 38; pp. 955–996. ISBN 978-0-12-417012-4. [Google Scholar]
- Dugat-Bony, E.; GARNIER, L.; Denonfoux, J.; Ferreira, S.; Sarthou, A.-S.; Bonnarme, P.; Irlinger, F. Highlighting the Microbial Diversity of 12 French Cheese Varieties. Int. J. Food Microbiol. 2016, 238, 265–273. [Google Scholar] [CrossRef]
- Irlinger, F.; Monnet, C. Temporal Differences in Microbial Composition of Époisses Cheese Rinds during Ripening and Storage. J. Dairy Sci. 2021, 104, 7500–7508. [Google Scholar] [CrossRef]
- Mounier, J.; Gelsomino, R.; Goerges, S.; Vancanneyt, M.; Vandemeulebroecke, K.; Hoste, B.; Scherer, S.; Swings, J.; Fitzgerald, G.F.; Cogan, T.M. Surface Microflora of Four Smear-Ripened Cheeses. Appl. Environ. Microbiol. 2005, 71, 6489–6500. [Google Scholar] [CrossRef] [Green Version]
- Dugat-Bony, E.; Lossouarn, J.; De Paepe, M.; Sarthou, A.-S.; Fedala, Y.; Petit, M.-A.; Chaillou, S. Viral Metagenomic Analysis of the Cheese Surface: A Comparative Study of Rapid Procedures for Extracting Viral Particles. Food Microbiol. 2020, 85, 103278. [Google Scholar] [CrossRef] [PubMed]
- De Melo, A.G.; Rousseau, G.M.; Tremblay, D.M.; Labrie, S.J.; Moineau, S. DNA Tandem Repeats Contribute to the Genetic Diversity of Brevibacterium Aurantiacum Phages. Environ. Microbiol. 2020, 22, 3413–3428. [Google Scholar] [CrossRef] [PubMed]
- Edwards, U.; Rogall, T.; Blöcker, H.; Emde, M.; Böttger, E.C. Isolation and Direct Complete Nucleotide Determination of Entire Genes. Characterization of a Gene Coding for 16S Ribosomal RNA. Nucleic Acids Res. 1989, 17, 7843–7853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S RRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Jurczak-Kurek, A.; Gąsior, T.; Nejman-Faleńczyk, B.; Bloch, S.; Dydecka, A.; Topka, G.; Necel, A.; Jakubowska-Deredas, M.; Narajczyk, M.; Richert, M.; et al. Biodiversity of Bacteriophages: Morphological and Biological Properties of a Large Group of Phages Isolated from Urban Sewage. Sci. Rep. 2016, 6, 34338. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Jakočiūnė, D.; Moodley, A. A Rapid Bacteriophage DNA Extraction Method. Methods Protoc. 2018, 1, 27. [Google Scholar] [CrossRef] [Green Version]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanfear, R.; Schalamun, M.; Kainer, D.; Wang, W.; Schwessinger, B. MinIONQC: Fast and Simple Quality Control for MinION Sequencing Data. Bioinformatics 2019, 35, 523–525. [Google Scholar] [CrossRef] [Green Version]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Completing Bacterial Genome Assemblies with Multiplex MinION Sequencing. Microb. Genom. 2017, 3, e000132. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Cerdeira, L.T.; Hawkey, J.; Méric, G.; Vezina, B.; Wyres, K.L.; Holt, K.E. Trycycler: Consensus Long-Read Assemblies for Bacterial Genomes. Genome Biol. 2021, 22, 266. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of Long, Error-Prone Reads Using Repeat Graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Vaser, R.; Šikić, M. Time- and Memory-Efficient Genome Assembly with Raven. Nat. Comput. Sci. 2021, 1, 332–336. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikheenko, A.; Prjibelski, A.; Saveliev, V.; Antipov, D.; Gurevich, A. Versatile Genome Assembly Evaluation with QUAST-LG. Bioinformatics 2018, 34, i142–i150. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Garneau, J.R.; Depardieu, F.; Fortier, L.-C.; Bikard, D.; Monot, M. PhageTerm: A Tool for Fast and Accurate Determination of Phage Termini and Packaging Mechanism Using next-Generation Sequencing Data. Sci Rep 2017, 7, 8292. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, L.; Stephens, A.; Nam, S.-Z.; Rau, D.; Kübler, J.; Lozajic, M.; Gabler, F.; Söding, J.; Lupas, A.N.; Alva, V. A Completely Reimplemented MPI Bioinformatics Toolkit with a New HHpred Server at Its Core. J. Mol. Biol. 2018, 430, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The Conserved Domain Database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchler-Bauer, A.; Bryant, S.H. CD-Search: Protein Domain Annotations on the Fly. Nucleic Acids Res. 2004, 32, W327–W331. [Google Scholar] [CrossRef]
- Terzian, P.; Olo Ndela, E.; Galiez, C.; Lossouarn, J.; Pérez Bucio, R.E.; Mom, R.; Toussaint, A.; Petit, M.-A.; Enault, F. PHROG: Families of Prokaryotic Virus Proteins Clustered Using Remote Homology. NAR Genom. Bioinform. 2021, 3, lqab067. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Tavares, P.; Petit, M.-A.; Guérois, R.; Zinn-Justin, S. Automated Classification of Tailed Bacteriophages According to Their Neck Organization. BMC Genom. 2014, 15, 1027. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A Reference Database for Bacterial Virulence Factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef] [Green Version]
- Florensa, A.F.; Kaas, R.S.; Clausen, P.T.L.C.; Aytan-Aktug, D.; Aarestrup, F.M.Y. ResFinder—An Open Online Resource for Identification of Antimicrobial Resistance Genes in next-Generation Sequencing Data and Prediction of Phenotypes from Genotypes. Microb. Genom. 2022, 8, 000748. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Kropinski, A.M.; Adriaenssens, E.M. A Roadmap for Genome-Based Phage Taxonomy. Viruses 2021, 13, 506. [Google Scholar] [CrossRef]
- Nishimura, Y.; Yoshida, T.; Kuronishi, M.; Uehara, H.; Ogata, H.; Goto, S. ViPTree: The Viral Proteomic Tree Server. Bioinformatics 2017, 33, 2379–2380. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.B.; Weitz, J.S.; Wilhelm, S. Viral Ecology Comes of Age. Environ. Microbiol. Rep. 2017, 9, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wahab, L.; Gill, J.J. Development and Validation of a Microtiter Plate-Based Assay for Determination of Bacteriophage Host Range and Virulence. Viruses 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogan, T.M.; Chamba, J.-F.; Hohenegger, M.; Guéguen, M.; Ward, A.C.; Gelsomino, R.; Jamet, E.; Swings, J.; Irlinger, F.; Larpin, S.; et al. Biodiversity of the Surface Microbial Consortia from Limburger, Reblochon, Livarot, Tilsit, and Gubbeen Cheeses. In Cheese and Microbes; Donnelly, C.W., Ed.; American Society of Microbiology: Washington, DC, USA, 2014; pp. 219–250. ISBN 978-1-55581-586-8. [Google Scholar]
- Larpin-Laborde, S.; Imran, M.; Bonaïti, C.; Bora, N.; Gelsomino, R.; Goerges, S.; Irlinger, F.; Goodfellow, M.; Ward, A.C.; Vancanneyt, M.; et al. Surface Microbial Consortia from Livarot, a French Smear-Ripened Cheese. Can. J. Microbiol. 2011, 57, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Quigley, L.; O’Sullivan, O.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. High-Throughput Sequencing for Detection of Subpopulations of Bacteria Not Previously Associated with Artisanal Cheeses. Appl. Environ. Microbiol. 2012, 78, 5717–5723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irlinger, F.; Helinck, S.; Jany, J.L. Secondary and Adjunct Cultures. In Cheese, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, CA, USA, 2017; Chapter 11; pp. 273–300. ISBN 978-0-12-417012-4. [Google Scholar]
- Feurer, C.; Vallaeys, T.; Corrieu, G.; Irlinger, F. Does Smearing Inoculum Reflect the Bacterial Composition of the Smear at the End of the Ripening of a French Soft, Red-Smear Cheese? J. Dairy Sci. 2004, 87, 3189–3197. [Google Scholar] [CrossRef]
- Goerges, S.; Mounier, J.; Rea, M.C.; Gelsomino, R.; Heise, V.; Beduhn, R.; Cogan, T.M.; Vancanneyt, M.; Scherer, S. Commercial Ripening Starter Microorganisms Inoculated into Cheese Milk Do Not Successfully Establish Themselves in the Resident Microbial Ripening Consortia of a South German Red Smear Cheese. Appl. Env. Microbiol. 2008, 74, 2210–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertuzzi, A.S.; Walsh, A.M.; Sheehan, J.J.; Cotter, P.D.; Crispie, F.; McSweeney, P.L.H.; Kilcawley, K.N.; Rea, M.C. Omics-Based Insights into Flavor Development and Microbial Succession within Surface-Ripened Cheese. mSystems 2018, 3, e00211-17. [Google Scholar] [CrossRef] [Green Version]
- Pham, N.-P.; Layec, S.; Dugat-Bony, E.; Vidal, M.; Irlinger, F.; Monnet, C. Comparative Genomic Analysis of Brevibacterium Strains: Insights into Key Genetic Determinants Involved in Adaptation to the Cheese Habitat. BMC Genom. 2017, 18, 955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irlinger, F.; Bimet, F.; Delettre, J.; Lefèvre, M.; Grimont, P.A.D.Y. Arthrobacter Bergerei Sp. Nov. and Arthrobacter Arilaitensis Sp. Nov., Novel Coryneform Species Isolated from the Surfaces of Cheeses. Int. J. Syst. Evol. Microbiol. 2005, 55, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Monnet, C.; Loux, V.; Gibrat, J.-F.; Spinnler, E.; Barbe, V.; Vacherie, B.; Gavory, F.; Gourbeyre, E.; Siguier, P.; Chandler, M.; et al. The Arthrobacter Arilaitensis Re117 Genome Sequence Reveals Its Genetic Adaptation to the Surface of Cheese. PLoS ONE 2010, 5, e15489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erkus, O.; de Jager, V.C.; Spus, M.; van Alen-Boerrigter, I.J.; van Rijswijck, I.M.; Hazelwood, L.; Janssen, P.W.; van Hijum, S.A.; Kleerebezem, M.; Smid, E.J. Multifactorial Diversity Sustains Microbial Community Stability. ISME J. 2013, 7, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Spus, M.; Li, M.; Alexeeva, S.; Wolkers-Rooijackers, J.C.M.; Zwietering, M.H.; Abee, T.; Smid, E.J. Strain Diversity and Phage Resistance in Complex Dairy Starter Cultures. J. Dairy Sci. 2015, 98, 5173–5182. [Google Scholar] [CrossRef] [Green Version]
- Somerville, V.; Berthoud, H.; Schmidt, R.S.; Bachmann, H.-P.; Meng, Y.H.; Fuchsmann, P.; von Ah, U.; Engel, P. Functional Strain Redundancy and Persistent Phage Infection in Swiss Hard Cheese Starter Cultures. ISME J. 2022, 16, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.R.; Zeng, Q.; Kelly, L.; Huang, K.H.; Singer, A.U.; Stubbe, J.; Chisholm, S.W. Phage Auxiliary Metabolic Genes and the Redirection of Cyanobacterial Host Carbon Metabolism. Proc. Natl. Acad. Sci. USA 2011, 108, E757–E764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, R.E.; Lovell, S.C.; Misquitta, S.A.; Bateman, R.C. Human Glutaminyl Cyclase and Bacterial Zinc Aminopeptidase Share a Common Fold and Active Site. BMC Biol. 2004, 2, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taudte, N.; Linnert, M.; Rahfeld, J.-U.; Piechotta, A.; Ramsbeck, D.; Buchholz, M.; Kolenko, P.; Parthier, C.; Houston, J.A.; Veillard, F.; et al. Mammalian-like Type II Glutaminyl Cyclases in Porphyromonas Gingivalis and Other Oral Pathogenic Bacteria as Targets for Treatment of Periodontitis. J. Biol. Chem. 2021, 296, 100263. [Google Scholar] [CrossRef] [PubMed]
- Lamers, S.; Feng, Q.; Cheng, Y.; Yu, S.; Sun, B.; Lukman, M.; Jiang, J.; Ruiz-Carrillo, D. Structural and Kinetic Characterization of Porphyromonas Gingivalis Glutaminyl Cyclase. Biol Chem 2021, 402, 759–768. [Google Scholar] [CrossRef]
- Blower, T.R.; Evans, T.J.; Przybilski, R.; Fineran, P.C.; Salmond, G.P.C. Viral Evasion of a Bacterial Suicide System by RNA–Based Molecular Mimicry Enables Infectious Altruism. PLoS Genet. 2012, 8, e1003023. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, Y.; Yonesaki, T. Dmd of Bacteriophage T4 Functions as an Antitoxin against Escherichia Coli LsoA and RnlA Toxins. Mol. Microbiol. 2012, 83, 669–681. [Google Scholar] [CrossRef]
- Weinbauer, M.G. Ecology of Prokaryotic Viruses. FEMS Microbiol. Rev. 2004, 28, 127–181. [Google Scholar] [CrossRef] [Green Version]
- Abedon, S.T. Prophages Preventing Phage Superinfection. In Bacteriophages as Drivers of Evolution: An Evolutionary Ecological Perspective; Abedon, S.T., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 179–191. ISBN 978-3-030-94309-7. [Google Scholar]
- Chopin, A.; Bolotin, A.; Sorokin, A.; Ehrlich, S.D.; Chopin, M.-C. Analysis of Six Prophages in Lactococcus Lactis IL1403: Different Genetic Structure of Temperate and Virulent Phage Populations. Nucleic Acids Res. 2001, 29, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Mahony, J.; Fitzgerald, G.F.; van Sinderen, D. Bacteriophages Infecting Lactic Acid Bacteria. In Cheese, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: San Diego, CA, USA, 2017; Chapter 10; pp. 249–272. ISBN 978-0-12-417012-4. [Google Scholar]
- Neve, H.; Kemper, U.; Geis, A.; Heller, K.J. Monitoring and characterization of lactococcal bacteriophages in a dairy plant. Kiel. Milchwirtsch. Forsch. 1994, 46, 167–178. [Google Scholar]
- Verreault, D.; Gendron, L.; Rousseau, G.M.; Veillette, M.; Massé, D.; Lindsley, W.G.; Moineau, S.; Duchaine, C. Detection of Airborne Lactococcal Bacteriophages in Cheese Manufacturing Plants. Appl. Environ. Microbiol. 2011, 77, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mounier, J.; Goerges, S.; Gelsomino, R.; Vancanneyt, M.; Vandemeulebroecke, K.; Hoste, B.; Brennan, N.M.; Scherer, S.; Swings, J.; Fitzgerald, G.F.; et al. Sources of the Adventitious Microflora of a Smear-Ripened Cheese. J. Appl. Microbiol. 2006, 101, 668–681. [Google Scholar] [CrossRef] [PubMed]
| Strain or Isolate | Isolation Source | Tested Phages |
|---|---|---|
| Glutamicibacter arilaitensis G16 | Studied cheese | Voltaire and Montesquieu |
| Glutamicibacter arilaitensis G26 | Studied cheese | |
| Glutamicibacter arilaitensis G33 | Studied cheese | |
| Glutamicibacter arilaitensis G43 | Studied cheese | |
| Glutamicibacter arilaitensis G51 | Studied cheese | |
| Glutamicibacter arilaitensis G52 | Studied cheese | |
| Glutamicibacter arilaitensis G53 | Studied cheese | |
| Glutamicibacter arilaitensis G65 | Studied cheese | |
| Glutamicibacter arilaitensis G119 | Studied cheese | |
| Glutamicibacter arilaitensis G135 | Studied cheese | |
| Glutamicibacter arilaitensis G183 | Studied cheese | |
| Glutamicibacter arilaitensis G186 | Studied cheese | |
| Glutamicibacter arilaitensis G201 | Studied cheese | |
| Glutamicibacter arilaitensis DSM 16368 | Reblochon cheese | |
| Glutamicibacter bergerei DSM 16367 | Camembert cheese | |
| Glutamicibacter nicotianae DSM 20123 | Air of tobacco warehouses | |
| Glutamicibacter uratoxydans DSM 20647 | Humus soil | |
| Brevibacterium aurantiacum B20 | Studied cheese | Rousseau |
| Brevibacterium aurantiacum B67 | Studied cheese | |
| Brevibacterium aurantiacum 2M23 | Cheese | |
| Brevibacterium aurantiacum FME9 | Cheese | |
| Brevibacterium aurantiacum FME34 | Cheese | |
| Brevibacterium aurantiacum FME43 | Cheese | |
| Brevibacterium aurantiacum FME45 | Cheese | |
| Brevibacterium aurantiacum FME48 | Cheese | |
| Brevibacterium aurantiacum FME49 | Cheese | |
| Brevibacterium aurantiacum ATCC 9174 | Cheese | |
| Brevibacterium aurantiacum ATCC 9175 (DSM 20426) | Camembert cheese | |
| Brevibacterium aurantiacum 25 | Camembert cheese | |
| Brevibacterium aurantiacum 299 | Camembert cheese | |
| Brevibacterium aurantiacum B3 | Langres cheese | |
| Brevibacterium aurantiacum CAM-4 | Camembert cheese | |
| Brevibacterium aurantiacum CAM 12C | Camembert cheese | |
| Brevibacterium casei CIP 102111 (DSM 20657) | Cheese | |
| Brevibacterium epidermidis NCDO 2286T (DSM 20660) | Skin | |
| Brevibacterium iodinum ATCC 49514T (DSM 20626) | Skin | |
| Brevibacterium linens ATCC 9172 (DSM 20425) | Cheese | |
| Brevibacterium sandarakinum DSM 22082 | Wall surface | |
| Leuconostoc falkenbergense 90 | Studied cheese | Diderot |
| Leuconostoc falkenbergense 91 | Studied cheese | |
| Leuconostoc falkenbergense 92 | Studied cheese | |
| Leuconostoc falkenbergense 93 | Studied cheese | |
| Leuconostoc falkenbergense 96 | Studied cheese | |
| Leuconostoc falkenbergense 98 | Studied cheese | |
| Leuconostoc falkenbergense 99 | Studied cheese | |
| Leuconostoc falkenbergense 114 | Studied cheese | |
| Leuconostoc falkenbergense 116 | Studied cheese | |
| Leuconostoc mesenteroides 88 | Studied cheese | |
| Leuconostoc mesenteroides 89 | Studied cheese | |
| Leuconostoc mesenteroides 95 | Studied cheese | |
| Leuconostoc mesenteroides 97 | Studied cheese | |
| Leuconostoc mesenteroides 101 | Studied cheese | |
| Leuconostoc mesenteroides 102 | Studied cheese | |
| Leuconostoc mesenteroides 107 | Studied cheese | |
| Leuconostoc mesenteroides 108 | Studied cheese | |
| Leuconostoc mesenteroides 113 | Studied cheese | |
| Leuconostoc mesenteroides 115 | Studied cheese | |
| Leuconostoc citreum MSE2 | Milk | |
| Leuconostoc lactis NCW1 | Cheese | |
| Leuconostoc mesenteroides ssp. cremoris DSM 20346 | Cheese | |
| Leuconostoc pseudomesenteroides MSE7 | Cheese | |
| Psychrobacter aquimaris 15 | Studied cheese | D’Alembert |
| Psychrobacter aquimaris 54 | Studied cheese | |
| Psychrobacter aquimaris 59 | Studied cheese | |
| Psychrobacter aquimaris 60 | Studied cheese | |
| Psychrobacter aquimaris 69 | Studied cheese | |
| Psychrobacter aquimaris 87 | Studied cheese | |
| Psychrobacter aquimaris 124 | Studied cheese | |
| Psychrobacter aquimaris 129 | Studied cheese | |
| Psychrobacter aquimaris 184 | Studied cheese | |
| Psychrobacter aquimaris 200 | Studied cheese | |
| Psychrobacter cibarius 132 | Studied cheese | |
| Psychrobacter cibarius 139 | Studied cheese | |
| Psychrobacter cibarius 140 | Studied cheese | |
| Psychrobacter cibarius 157 | Studied cheese | |
| Psychrobacter cibarius 158 | Studied cheese | |
| Psychrobacter cibarius 160 | Studied cheese | |
| Psychrobacter cibarius 165 | Studied cheese | |
| Psychrobacter cibarius 171 | Studied cheese | |
| Psychrobacter cibarius 181 | Studied cheese | |
| Psychrobacter cibarius 198 | Studied cheese | |
| Psychrobacter aquimaris ER15 174 BHI7 | Saint-Nectaire cheese | |
| Psychrobacter celer DSM 23510 | Munster cheese | |
| Psychrobacter cibarius DSM 16327 | Epoisses cheese | |
| Psychrobacter faecalis | Livarot cheese | |
| Psychrobacter namhaensis 1439 | Camembert cheese |
| Phage | Primer | Targeted CDS | Product | Sequence (5′-3′) | Annealing Temperature (°C) | Amplicon Size (bp) |
|---|---|---|---|---|---|---|
| Voltaire | VOLT_F | VOLT_18 | Pre-neck protein | actacctaccctgcccctaa | 57 | 705 |
| VOLT_R | ttcgttgaccagcacacaag | |||||
| Rousseau | ROUS_F | ROUS_20 | Receptor-binding protein | ggcggttcggagggtattag | 57 | 877 |
| ROUS_R | gaaccaaaccttcatcgcca | |||||
| Diderot | DID_F | DID_20 | Tail tape measure protein | aaaactgctgtgactcgtgg | 57 | 931 |
| DID_R | caccaaacacgccagagaaa | |||||
| D’Alembert | ALEM_F | DAL_18 | RNA ligase | tggtactaatgcaggtatcggt | 57 | 714 |
| ALEM_R | tcaacctcaaagcccatctct | |||||
| Montesquieu | MONT_F | MONT_53 | DNA polymerase I | tgacggcaagttcaatcagc | 57 | 683 |
| MONT_R | gctggttcggagtagtgtct |
| Phage | Capsid Size (nm ± SD 1) | Tail Size (nm ± SD) | Morphotype | Plaque Morphology |
|---|---|---|---|---|
| D’Alembert | 88 ± 2 | 113 ± 2.6 | myophage | Clear, small |
| Voltaire | 47 ± 1.1 | 30 ± 3.8 | podophage | Clear, small |
| Montesquieu | 64 ± 1.8 | 184 ± 5.5 | siphophage | Clear, large |
| Rousseau | 62 ± 5.5 | 177 ± 15.6 | siphophage | Clear, large |
| Diderot | 57 ± 4.3 | 141 ± 0.9 | siphophage | Clear, large |
| Phage | Sensitive Isolates/Tested Isolates (Same Species as the Host) | Sensitive Species/Tested Species (Same Genus but Different Species as the Host) | ||
|---|---|---|---|---|
| Propagation Strain | Isolated from the Studied Cheese | From Other Sources | ||
| D’Alembert | Psychrobacter aquimaris 87 | 3/10 | 0/5 | 0/4 |
| Voltaire | Glutamicibacter arilaitensis G65 | 2/13 | 0/1 | 0/3 |
| Montesquieu | Glutamicibacter arilaitensis G51 | 7/13 | 0/1 | 0/3 |
| Rousseau | Brevibacterium aurantiacum B67 | 1/2 | 0/16 | 0/5 |
| Diderot | Leuconostoc falkenbergense 91 | 7/9 | 0/1 | 0/4 |
| Phage | Raw Reads Count | Average Size of Reads (bp) | Number of Contigs | Genome Size (kb) | Number of ORFs | Terminal Repeat Size (bp) | Best Blast Hit 2 | ||
|---|---|---|---|---|---|---|---|---|---|
| Illumina | Nanopore | Illumina | Nanopore | ||||||
| Voltaire | 8.9 × 106 | 63,494 | 2 × 150 | 4320 | 1 | 18.4 | 26 | 176 | Brevibacterium phage Cantare (83.33% id 1% cov) |
| Montesquieu 1 | 5.6 × 106 | - | 2 × 150 | - | 1 | 47.7 | 62 | - | Arthrobacter phage TripleJ (75.25% id 2% cov) |
| Rousseau | 5.5 × 106 | 15,649 | 2 × 150 | 3576 | 1 | 40.2 | 71 | - | Siphoviridae sp. Isolate ctmmc7 (75.54% id 0% cov) |
| Diderot | 6.9 × 106 | 1,736,125 | 2 × 150 | 3074 | 1 | 27.1 | 40 | - | Leuconostoc phage LN03 (98.20% id 98% cov) |
| D’Alembert | 7.1 × 106 | 115,763 | 2 × 150 | 9872 | 1 | 92.5 | 158 | 5719 | Vibrio phage vB_VhaM_VH-8 (83.95% id 34% cov) |
| Illumina Only Assembly | Final assembly | ||||
|---|---|---|---|---|---|
| Size in bp | Terminal Repeat | PhageTerm Prediction: Boundaries and Encapsidation Strategy | Size in bp after Polishing | Terminal Repeat | |
| Voltaire | 18,300 | / | Redundant, permuted and unknown 1 | 18,418 | 176 bps ITR |
| Montesquieu | 47,703 | 127 bp DTR, assembly artefact removed | Headful (pac) | 47,576 | / |
| Rousseau | 40,294 | 127 bp DTR, assembly artefact removed | Cos (3′) | 40,167 | / |
| Diderot | / | / | Cos (3′) | 27,116 | / |
| D’Alembert | 86,864 | 127 bp DTR, assembly artefact removed | 5719 bps DTR (long) | 92,456 | 5719 bps DTR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paillet, T.; Lossouarn, J.; Figueroa, C.; Midoux, C.; Rué, O.; Petit, M.-A.; Dugat-Bony, E. Virulent Phages Isolated from a Smear-Ripened Cheese Are Also Detected in Reservoirs of the Cheese Factory. Viruses 2022, 14, 1620. https://doi.org/10.3390/v14081620
Paillet T, Lossouarn J, Figueroa C, Midoux C, Rué O, Petit M-A, Dugat-Bony E. Virulent Phages Isolated from a Smear-Ripened Cheese Are Also Detected in Reservoirs of the Cheese Factory. Viruses. 2022; 14(8):1620. https://doi.org/10.3390/v14081620
Chicago/Turabian StylePaillet, Thomas, Julien Lossouarn, Clarisse Figueroa, Cédric Midoux, Olivier Rué, Marie-Agnès Petit, and Eric Dugat-Bony. 2022. "Virulent Phages Isolated from a Smear-Ripened Cheese Are Also Detected in Reservoirs of the Cheese Factory" Viruses 14, no. 8: 1620. https://doi.org/10.3390/v14081620
APA StylePaillet, T., Lossouarn, J., Figueroa, C., Midoux, C., Rué, O., Petit, M.-A., & Dugat-Bony, E. (2022). Virulent Phages Isolated from a Smear-Ripened Cheese Are Also Detected in Reservoirs of the Cheese Factory. Viruses, 14(8), 1620. https://doi.org/10.3390/v14081620






