Epidemiology and Ecology of Influenza A Viruses among Wildlife in the Arctic
Abstract
1. Introduction
2. Methods
2.1. Literature Search and Data Collection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Search Results and Study Selection
3.2. Characteristics of the Included Studies
3.3. Natural History and Epidemiology of IAVs in Arctic Regions
3.4. A Broad Avian Host Range for Numerous Subtypes of IAVs in Arctic Regions
| Family Taxa | Host Species | Sampling Year(s) | Location | Prevalence | Seroprevalence | Author, Year | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive Samples | Total Samples | Prevalence % | Virus Subtypes Identified (LP: Low Path, HP: High Path) | n/N (%) | Subtype-Specific Antibodies Identified, n/N (%) (LP: Low Path, HP: High Path) | |||||
| Accipitridae | 0 | 0 | 0% | |||||||
| Northern Goshawk (Accipiter gentilis) | 2016 | Troms, Norway | 0 | - | 0% | - | 0/29 (0) | - | Lee, 2019 [41] | |
| White-tailed Eagle (Haliaeetus albicilla) | 2016 | Steigen, Norway | 0 | - | 0% | - | 0/43 (0) | - | Lee, 2019 [41] | |
| Alcidae | 150 | 3685 | 4% | |||||||
| Dovekie (Alle alle) | 2008–2011 | Newfoundland and Labrador, Canada | 0 | 52 | 0% | - | - | - | Wille, 2014 [57] | |
| Crested Auklet (Aethia cristatetlla) | 2006–2007 | Alaska | 0 | 207 | 0% | - | - | - | Ip, 2008 [58] | |
| Parakeet Auklet (Aethia psittacula) | 2006–2007 | Alaska | 0 | 8 | 0% | - | - | - | Ip, 2008 [58] | |
| Least Auklet (Aethia pusilla) | 2006–2007 | Alaska | 0 | 30 | 0% | - | - | - | Ip, 2008 [58] | |
| Razorbill (Alca torda) | 2008–2011 | Newfoundland and Labrador, Canada | 0 | 196 | 0% | - | - | - | Wille, 2014 [57] | |
| Black Guillemot (Cepphus grille) | 2008–2011 | Newfoundland and Labrador, Canada | 0 | 1 | 0% | - | - | - | Wille, 2014 [57] | |
| 2010–2011 | Iceland | 0 | 10 | 0% | - | - | - | Dusek, 2014 [4] | ||
| Atlantic Puffin (Fratercula arctica) | 2008–2011 | Newfoundland and Labrador, Canada | 0 | 365 | 0% | - | - | - | Wille, 2014 [57] | |
| Tufted Puffin (Fratercula cirrhata) | 2006–2007 | Alaska | 0 | 4 | 0% | - | - | - | Ip, 2008 [58] | |
| Horned Puffin (Fratercula corniculata) | 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | |
| Common Murre (Uria aalge) | 2006–2007 | Alaska | 1 | 76 | 1% | - | - | - | Ip, 2008 [58] | |
| 2008–2011 | Newfoundland and Labrador, Canada | 70 | 1273 | 6% | - | - | - | Wille, 2014 [57] | ||
| 2010–2011 | Iceland | 0 | 1 | 0% | - | - | - | Dusek, 2014 [4] | ||
| 2011 | Newfoundland and Labrador, Canada | 68 | 452 | 15% | - | - | - | Huang, 2014 [59] | ||
| Thick-billed Murre (Uria lomvia) | 2006–2007 | Alaska | 7 | 235 | 3% | - | - | - | Ip, 2008 [58] | |
| 2007–2011 | Greenland | 0 | 64 | 0% | - | - | - | Hjulsager, 2012 [42] | ||
| 2008–2011 | Newfoundland and Labrador, Canada | 1 | 621 | 0% | - | - | - | Wille, 2014 [57] | ||
| 2014 | Greenland | 2 | 44 | 5% | H11N2: 2 birds | - | - | Hartby, 2016 [60] | ||
| 2014 | Greenland | 1 | 44 | 2% | H5N1 (LP): 1 bird | - | - | Hartby, 2016 [60] | ||
| Aluadidae | 0 | 1 | 0% | |||||||
| Horned Lark (Eremophila alpestris) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Anatidae Anatini | 1142 | 10,022 | 11% | |||||||
| Northern Pintail (Anas acuta) | 2005 | Minto Flats State Game Refuge, Alaska | 1 | 19 | 5% | - | - | - | Runstadler, 2007 [61] | |
| 2005 | Minto Flats State Game Refuge, Alaska | 178 | 738 | 24% | H3N6: 3/38 (7.9%); H4N6: 9/38 (23.7%); H8N4: 1/38 (2.6%); H12N5: 7/38 (18.4%) | - | - | Runstadler, 2007 [61] | ||
| 2005 | Pt. Barrow, Alaska | 3 | 133 | 2% | - | - | - | Wahlgren, 2008 [62] | ||
| 2005 | Chuchki Peninsula, Russia | 0 | 2 | 0% | - | - | - | Wahlgren, 2008 [62] | ||
| 2006–2007 | Alaska | 51 | 539 | 10% | - | - | - | Ip, 2008 [58] | ||
| 2006–2007 | Alaska | 87 | 1426 | 6% | - | - | - | Ip, 2008 [58] | ||
| 2006–2008 | Izembek Lagoon, Alaska | 29 | 258 | 11% | - | - | - | Ramey, 2011 [63] | ||
| 2007–2008 | Minto Flats State Game Refuge, Alaska | 101 | 1291 | 8% | - | - | - | Hill, 2012 [3] | ||
| 2007–2008 | Minto Flats State Game Refuge, Alaska | 19 | 215 | 9% | - | - | - | Hill, 2012 [64] | ||
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 104 | 709 | 15% | - | - | - | Ramey, 2015 [65]; Reeves, 2018 [66] | ||
| 2014 | Izembek National Wildlife Refuge, Alaska | 17 | 69 | 25% | - | - | - | Ramey, 2016 [67]; Reeves, 2018 [66] | ||
| 2015 | Alaska | - | - | - | - | 28/209 (13.4%) | H5 *: 0/45 (0%) | Stallknecht, 2020 [49] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 1 | 15 | 7% | - | - | - | Ramey, 2016 [67] | ||
| American Wigeon (Anas americana) | 2005 | Minto Flats State Game Refuge, Alaska | 1 | 2 | 50% | - | - | - | Runstadler, 2007 [61] | |
| 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | ||
| 2007–2008 | Minto Flats State Game Refuge, Alaska | 7 | 241 | 3% | - | - | - | Hill, 2012 [64] | ||
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 34 | 0% | - | - | - | Ramey, 2015 [65]; Reeves, 2018 [66] | ||
| 2014 | Izembek National Wildlife Refuge, Alaska | 1 | 11 | 9% | - | - | - | Ramey, 2016 [67]; Reeves, 2018 [66] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 1 | 0% | - | - | - | Ramey, 2016 [67] | ||
| Northern Shoveler (Anas clypeata) | 2006–2007 | Alaska | 1 | 22 | 5% | - | - | - | Ip, 2008 [58] | |
| 2007–2008 | Minto Flats State Game Refuge, Alaska | 38 | 118 | 32% | - | - | - | Hill, 2012 [64] | ||
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 1 | 5 | 20% | - | - | - | Ramey, 2015 [65]; Reeves, 2018 [66] | ||
| 2014 | Izembek National Wildlife Refuge, Alaska | 1 | 5 | 20% | - | - | - | Ramey, 2016 [68]; Reeves, 2018 [66] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 1 | 19 | 5% | - | - | - | Ramey, 2016 [67] | ||
| American Green-winged Teal (Anas crecca) | 2011–2013 | Izembek National Wildlife Refuge, Alaska | 26 | 111 | 23% | - | - | - | Ramey, 2015 [69]; Reeves, 2018 [66] | |
| 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 4 | 0% | - | - | - | Ramey, 2016 [67] | ||
| Blue-winged Teal (Anas discors) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Eurasian Wigeon (Anas Penelope) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 18 | 0% | - | - | - | Ramey, 2015 [69] | ||
| 2014 | Belaya Gora, Sakha Republic, Russia | 1 | H5N8 (HP) (tracheal specimen) | - | - | Marchenko, 2015 [70] | ||||
| 2014 | Izembek National Wildlife Refuge, Alaska | 0 | 1 | 0% | - | - | - | Ramey, 2016 [68] | ||
| Mallard (Anas platyrhynchos) | 2005 | Minto Flats State Game Refuge, Alaska | 45 | 121 | 37% | H3N8: 2/38 (5.2%); H4N6: 2/38 (5.2%); H8N4: 2/38 (5.2%); H12N5: 2/38 (5.2%) | - | - | Runstadler, 2007 [61] | |
| 2006–2007 | Alaska | 25 | 273 | 9% | - | - | - | Ip, 2008 [58] | ||
| 2007–2011 | Greenland | 2 | 623 | 0% | - | - | - | Hjulsager, 2012 [42] | ||
| 2007–2008 | Minto Flats State Game Refuge, Alaska | 124 | 1004 | 12% | - | - | - | Hill, 2012 [3] | ||
| 2010–2011 | Iceland | 1 | 14 | 7% | - | - | - | Dusek, 2014 [4] | ||
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 7 | 72 | 10% | - | - | - | Ramey, 2015 [69]; Reeves, 2018 [66] | ||
| 2012–2015 | Fairbanks and Anchorage, Alaska | 181 | 1062 | 17% | - | 457/984 (46.4%) | - | Spivey, 2017 [71] | ||
| 2013–2014 | Subarctic Ontario, Canada | 12 | 304 | 4% | H3: 3/12 (25%) H4: 1/12 (8.3%) | - | - | Liberda, 2017 [48] | ||
| 2014 | Izembek National Wildlife Refuge, Alaska | 18 | 49 | 37% | - | - | - | Ramey, 2016 [68]; Reeves, 2018 [66] | ||
| 2014–2015 | Anchorage, Alaska | 63 | 700 | - | - | - | Hill, 2017 [10] | |||
| 2015 | Alaska | - | - | 98/210 (46.7%) | H5 *: 24/91 (26.4%) | Stallknecht, 2020 [49] | ||||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 12 | 0% | - | - | - | Ramey, 2016 [67] | ||
| Gadwall (Anas strepera) | 2006–2007 | Alaska | 0 | 16 | 0% | - | - | - | Ip, 2008 [58] | |
| 2007–2008 | Minto Flats State Game Refuge, Alaska | 0 | 1 | 0% | - | - | - | Hill, 2012 [3] | ||
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 14 | 0% | - | - | - | Ramey, 2015 [69] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 1 | 0% | - | - | - | Ramey, 2016 [67] | ||
| Miscellaneous duck species *** | 2005 | Pt. Barrow, Alaska | 0 | 1 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2005 | Newfoundland and Labrador, Canada | 8 | 73 | 11% | - | - | - | Parmley, 2008 [72] | ||
| 2013–2014 | St. Paul Island and Prince William Sound, Alaska | 2 | 185 | 1% | - | - | - | Ramey, 2015 [65] | ||
| 2016 | Fairbanks, Alaska | 48 | 188 | 26% | H5N2 (HP): 1/188 (0.5%) | - | - | Lee, 2017 [73] | ||
| Anatidae Anserini | 322 | 11,636 | 3% | |||||||
| Greater White-fronted Goose (Anser albifrons) | 2005 | Pt. Barrow, Alaska | 0 | 11 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2006–2007 | Alaska | 40 | 1081 | 4% | - | - | - | Ip, 2008 [58] | ||
| 2007–2008 | Minto Flats State Game Refuge, Alaska | 0 | 3 | 0% | - | - | - | Hill, 2012 [3] | ||
| 2007–2011 | Greenland | 0 | 179 | 0% | - | - | - | Hjulsager, 2012 [42] | ||
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 2 | 0% | - | - | - | Ramey, 2015 [69] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 39 | 298 | 13% | - | - | - | Ramey, 2016 [67] | ||
| 2012–2016 | Alaska | - | - | 35/76 (46%) | H1N2: 4/15 (26.6%) | Van Hemert, 2019 [40] | ||||
| H6N2: 5/15 (33.3%) | ||||||||||
| H9N2: 6/15 (40%) | ||||||||||
| Greylag Goose (Anser anser) | 2010–2011 | Iceland | 11 | 223 | 5% | - | - | - | Dusek, 2014 [4] | |
| Pink-footed Goose (Anser brachyrhynchus) | 2010–2011 | Iceland | 1 | 13 | 8% | - | - | - | Dusek, 2014 [4] | |
| 2016 | Greenland | 0 | 150 | 0% | - | - | - | Gaidet, 2018 [74] | ||
| Brent Goose (Branta bernicula) | 2005 | Chukchi Peninsula, Russia | 0 | 12 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2005 | Pt. Barrow, Alaska | 0 | 1 | 0% | - | - | - | Wahlgren, 2008 [62] | ||
| Black Brant (Branta bernicla nigricans) | 2006–2007 | Alaska | 8 | 2075 | 0% | - | - | - | Ip, 2008 [58] | |
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 192 | 0% | - | - | - | Ramey, 2015 [69]; Reeves, 2018 [66] | ||
| 2012–2016 | Alaska | - | - | 56/110 (50.9%) | - | Van Hemert, 2019 [40] | ||||
| 2014 | Izembek National Wildlife Refuge, Alaska | 0 | 21 | 0% | - | - | - | Ramey, 2016 [68]; Reeves, 2018 [66] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 3 | 41 | 7% | - | - | - | Ramey, 2016 [67] | ||
| Canada Goose (Branta canadensis) | 2006–2007 | Alaska | 9 | 260 | 4% | - | - | - | Ip, 2008 [58] | |
| 2007–2011 | Greenland | 0 | 221 | 0% | - | - | - | Hjulsager, 2012 [42] | ||
| Cackling Goose (Branta hutchinsii) | 2006–2007 | Alaska | 2 | 373 | 1% | - | - | - | Ip, 2008 [58] | |
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 5 | 221 | 2% | - | - | - | Ramey, 2015 [69]; Reeves, 2018 [66] | ||
| 2015 | Alaska | - | - | 33/105 (31.4%) | H5 *: 1/23 (4.3%) | Stallknecht, 2020 [49] | ||||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 9 | 315 | 3% | - | - | - | Ramey, 2016 [67] | ||
| Barnacle Goose (Branta leucopsis) | 2016 | Greenland | 0 | 150 | 0% | - | - | - | Gaidet, 2018 [74] | |
| Emperor Goose (Chen canagica) | 2005 | Chukchi Peninsula, Russia | 0 | 19 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2006–2007 | Alaska | 11 | 685 | 2% | - | - | - | Ip, 2008 [58] | ||
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 26 | 663 | 4% | - | - | - | Ramey, 2015 [69] | ||
| 2014 | Izembek National Wildlife Refuge, Alaska | 14 | 294 | 5% | - | - | H1–H12, H14, H15 ** | Ramey, 2016 [68]; Reeves, 2018 [66] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 4 | 19 | 21% | - | - | - | Ramey, 2016 [67] | ||
| 2015–2016 | Izembek National Wildlife Refuge, Alaska | 59 | 390 | 15% | - | - | H1–H12, H14, H15 ** | Ramey, 2019 [56] | ||
| 2015–2017 | Kodiak Island, Alaska | 41 | 800 | 5% | - | - | H1–H12, H14, H15 ** | Ramey, 2019 [56] | ||
| 2016 | Shemya Island, Alaska | 2 | 400 | 1% | - | - | H1–H12, H14, H15 ** | Ramey, 2019 [56] | ||
| 2016 | Nelson Lagoon, Alaska | 1 | 194 | 1% | - | - | H1–H12, H14, H15 ** | Ramey, 2019 [56] | ||
| 2016 | Cinder Lagoon, Alaska | 3 | 225 | 1% | - | - | H1–H12, H14, H15 ** | Ramey, 2019 [56] | ||
| 2016–2017 | Adak Island, Alaska | 9 | 800 | 1% | - | - | H1–H12, H14, H15 ** | Ramey, 2019 [56] | ||
| Snow Goose (Chen caerulescens) | 1993–1996 | Wrangel Island, Russia | - | - | - | - | 513/983 (52.2%) | - | Samuel, 2015 [75] | |
| 1993–1996 | Banks Island, Canada | - | - | - | - | 855/1996 (42.8%) | - | Samuel, 2015 [75] | ||
| 2005 | Chukchi Peninsula, Russia | 0 | 222 | 0% | - | - | - | Wahlgren, 2008 [62] | ||
| 2006–2007 | Alaska | 17 | 720 | 2% | - | - | - | Ip, 2008 [58] | ||
| 2007–2011 | Greenland | 0 | 283 | 0% | - | - | - | Hjulsager, 2012 [42] | ||
| 2007–2008 | Minto Flats State Game Refuge, Alaska | 0 | 2 | 0% | - | - | - | Hill, 2012 [3] | ||
| 2013–2014 | Subarctic Ontario, Canada | 3 | 16 | 19% | - | - | - | Liberda, 2017 [48] | ||
| 2015 | Alaska | - | - | 92/200 (46%) | H5 *: 8/75 (10.7%) | Stallknecht, 2020 [49] | ||||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 5 | 62 | 8% | - | - | - | Ramey, 2016 [67] | ||
| 2012–2016 | Alaska | - | - | 57/108 (52.8%) | - | Van Hemert, 2019 [40] | ||||
| Anatidae Aythyini | 10 | 221 | 5% | |||||||
| Redhead (Aythya americana) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Lesser Scaup (Aythya affinis) | 2006–2007 | Alaska | 0 | 19 | 0% | - | - | - | Ip, 2008 [58] | |
| 2007–2008 | Minto Flats State Game Refuge, Alaska | 0 | 19 | 0% | - | - | - | Hill, 2012 [3] | ||
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 2 | 0% | - | - | - | Ramey, 2015 [69] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 7 | 62 | 11% | - | - | - | Ramey, 2016 [67] | ||
| Ring-necked Duck (Aythya collaris) | 2007–2008 | Minto Flats State Game Refuge, Alaska | 0 | 16 | 0% | - | - | - | Hill, 2012 [3] | |
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 1 | 0% | - | - | - | Ramey, 2015 [69] | ||
| Canvasback (Aythya valisineria) | 2006–2007 | Alaska | 0 | 8 | 0% | - | - | - | Ip, 2008 [58] | |
| 2007–2008 | Minto Flats State Game Refuge, Alaska | 0 | 23 | 0% | - | - | - | Hill, 2012 [3] | ||
| 2014 | Izembek National Wildlife Refuge, Alaska | 0 | 1 | 0% | - | - | - | Ramey, 2016 [68] | ||
| Greater Scaup (Aythya marila) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 1 | 36 | 3% | - | - | - | Ramey, 2015 [69]; Reeves, 2018 [66] | ||
| 2014 | Izembek National Wildlife Refuge, Alaska | 0 | 21 | 0% | - | - | - | Ramey, 2016 [68]; Reeves, 2018 [66] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 2 | 11 | 18% | - | - | - | Ramey, 2016 [67] | ||
| Anatidae Cygnini | 10 | 637 | 2% | |||||||
| Trumpeter Swan (Cyngus buccinator) | 2006–2007 | Alaska | 0 | 10 | 0% | - | - | - | Ip, 2008 [58] | |
| Tundra Swan (Cyngus columbianus) | 2005 | Chukchi Peninsula, Russia | 0 | 3 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2006–2007 | Alaska | 7 | 583 | 1% | - | - | - | Ip, 2008 [58] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 3 | 41 | 7% | - | - | - | Ramey, 2016 [67] | ||
| Anatidae Mergini | 62 | 3956 | 2% | |||||||
| Bufflehead (Bucephala albeola) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| 2007–2008 | Minto Flats State Game Refuge, Alaska | 0 | 29 | 0% | - | - | - | Hill, 2012 [3] | ||
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 3 | 15 | 20% | - | - | - | Ramey, 2015 [69]; Reeves, 2018 [66] | ||
| 2014 | Izembek National Wildlife Refuge, Alaska | 2 | 10 | 20% | - | - | - | Ramey, 2016 [68]; Reeves, 2018 [66] | ||
| Common Goldeneye (Bucephala clangula) | 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 2 | 0% | - | - | - | Ramey, 2015 [69] | |
| 2014 | Izembek National Wildlife Refuge, Alaska | 0 | 2 | 0% | - | - | - | Ramey, 2016 [68] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 2 | 0% | - | - | - | Ramey, 2016 [67] | ||
| Long-tailed Duck (Clangula hyemalis) | 2006–2007 | Alaska | 1 | 52 | 2% | - | - | - | Ip, 2008 [58] | |
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 1 | 16 | 6% | - | - | - | Ramey, 2015 [69]; Reeves, 2018 [66] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 1 | 6 | 17% | - | - | - | Ramey, 2016 [67] | ||
| Harlequin Duck (Histronicus histronicus) | 2006–2007 | Alaska | 0 | 65 | 0% | - | - | - | Ip, 2008 [58] | |
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 48 | 0% | - | - | - | Ramey, 2015 [69] | ||
| 2014 | Izembek National Wildlife Refuge, Alaska | 0 | 2 | 0% | - | - | - | Ramey, 2016 [68] | ||
| White-winged Scoter (Malanitta fusca) | 2006–2007 | Alaska | 0 | 41 | 0% | - | - | - | Ip, 2008 [58] | |
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 15 | 0% | - | - | - | Ramey, 2015 [69] | ||
| 2014 | Izembek National Wildlife Refuge, Alaska | 0 | 23 | 0% | - | - | - | Ramey, 2016 [68] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 1 | 16 | 6% | - | - | - | Ramey, 2016 [67] | ||
| Black Scoter (Melanitta nigra) | 2006–2007 | Alaska | 0 | 10 | 0% | - | - | - | Ip, 2008 [58] | |
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 9 | 0% | - | - | - | Ramey, 2015 [69] | ||
| 2014 | Izembek National Wildlife Refuge, Alaska | 0 | 1 | 0% | - | - | - | Ramey, 2016 [68] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 4 | 83 | 5% | - | - | - | Ramey, 2016 [67] | ||
| Surf Scoter (Melanitta perspicillata) | 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | |
| 2015 | Yukon-Kuskokwim Delta, Alaska | 1 | 5 | 20% | - | - | - | Ramey, 2016 [67] | ||
| Common Merganser (Mergus merganser) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 1 | 0% | - | - | - | Ramey, 2016 [67] | ||
| Red-breasted Merganser (Mergus serrator) | 2007–2011 | Greenland | 0 | 5 | 0% | - | - | - | Hjulsager, 2012 [42] | |
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 4 | 0% | - | - | - | Ramey, 2015 [69] | ||
| 2014 | Izembek National Wildlife Refuge, Alaska | 0 | 6 | 0% | - | - | - | Ramey, 2016 [68] | ||
| Steller’s Eider (Polysticta stelleri) | 2006–2007 | Alaska | 6 | 737 | 1% | - | - | - | Ip, 2008 [58] | |
| 2006–2008 | Izembek Lagoon, Alaska | 1 | 457 | 0% | - | - | - | Ramey, 2011 [63] | ||
| 2006–2008 | Nelson Lagoon, Alaska | 30 | 779 | 4% | - | - | - | Ramey, 2011 [63] | ||
| Spectacled Eider (Somateria fischeri) | 2006–2007 | Alaska | 2 | 348 | 1% | - | - | - | Ip, 2008 [58] | |
| Common Eider (Somateria mollissima) | 2005 | Chukchi Peninsula, Russia | 0 | 7 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2006–2007 | Alaska | 1 | 395 | 0% | - | - | - | Ip, 2008 [58] | ||
| 2007–2011 | Greenland | 0 | 20 | 0% | - | - | - | Hjulsager, 2012 [42] | ||
| 2007–2011 | Nunavut, Canada | - | - | 304/552 (55.1%) | - | Hall, 2015 [76] | ||||
| 2010–2011 | Iceland | 0 | 35 | 0% | - | - | - | Dusek, 2014 [4] | ||
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 0 | 20 | 0% | - | - | - | Ramey, 2015 [69] | ||
| 2012 | Iceland | - | - | 31/38 (81.6%) | - | Hall, 2015 [76] | ||||
| 2014 | Izembek National Wildlife Refuge, Alaska | 0 | 1 | 0% | - | - | - | Ramey, 2016 [68] | ||
| King Eider (Somateria spectabilis) | 2006–2007 | Alaska | 6 | 680 | 1% | - | - | - | Ip, 2008 [58] | |
| 2011–2013 | Izembek National Wildlife Refuge, Alaska | 2 | 5 | 40% | - | - | - | Ramey, 2015 [69]; Reeves, 2018 [66] | ||
| Certhiidae | 0 | 1 | 0% | |||||||
| Brown Creeper (Certhia americana) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Charadriidae | 0 | 177 | 0% | |||||||
| Common Ringed Plover (Charadrius hiaticula) | 2005 | Chukchi Peninsula, Russia | 0 | 10 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2010–2011 | Iceland | 0 | 4 | 0% | - | - | - | Dusek, 2014 [4] | ||
| 2012 | Iceland | 0 | 10 | 0% | - | 0/10 (0%) | - | Hall, 2014 [77] | ||
| 2013 | Iceland | 0 | 13 | 0% | - | - | - | Hall, 2014 [77] | ||
| Semipalmated Plover (Charadrius semipalmatus) | 2006–2007 | Alaska | 0 | 4 | 0% | - | - | - | Ip, 2008 [58] | |
| American Golden Plover (Pluvialis dominica) | 2006–2007 | Alaska | 0 | 15 | 0% | - | - | - | Ip, 2008 [58] | |
| Pacific Golden Plover (Pluvialis fulva) | 2006–2007 | Alaska | 0 | 42 | 0% | - | - | - | Ip, 2008 [58] | |
| Black-bellied Plover (Pluvialis squatarola) | 2006–2007 | Alaska | 0 | 73 | 0% | - | - | - | Ip, 2008 [58] | |
| Golden Plover spp. (Pluvialis spp.) | 2006–2007 | Alaska | 0 | 6 | 0% | - | - | - | Ip, 2008 [58] | |
| Corvidae | 0 | 201 | 0% | |||||||
| Common Raven (Corus corax) | 2007–2011 | Greenland | 0 | 199 | 0% | - | - | - | Hjulsager, 2012 [42] | |
| Gray Jay (Perisoreus canadensis) | 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | |
| Emberizidae | 0 | 463 | 0% | |||||||
| Lapland Longspur (Calcarius lapponicus) | 2006–2007 | Alaska | 0 | 8 | 0% | - | - | - | Ip, 2008 [58] | |
| 2007–2011 | Greenland | 0 | 51 | 0% | - | - | - | Hjulsager, 2012 [42] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 1 | 0% | - | - | - | Ramey, 2016 [67] | ||
| 2005 | Chukchi Peninsula, Russia | 0 | 2 | 0% | - | - | - | Wahlgren, 2008 [62] | ||
| Dark-eyed Junco (Junco hyemalis) | 2006–2007 | Alaska | 0 | 16 | 0% | - | - | - | Ip, 2008 [58] | |
| Lincoln’s Sparrow (Melospiza lincolnii) | 2006–2007 | Alaska | 0 | 20 | 0% | - | - | - | Ip, 2008 [58] | |
| Fox Sparrow (Passerella iliaca) | 2006–2007 | Alaska | 0 | 26 | 0% | - | - | - | Ip, 2008 [58] | |
| Savannah Sparrow (Passerculus sandwichensis) | 2006–2007 | Alaska | 0 | 92 | 0% | - | - | - | Ip, 2008 [58] | |
| Snow Bunting (Plectrophenax nivalis) | 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | |
| 2007–2011 | Greenland | 0 | 80 | 0% | - | - | - | Hjulsager, 2012 [42] | ||
| American Tree Sparrow (Spizella arborea) | 2006–2007 | Alaska | 0 | 96 | 0% | - | - | - | Ip, 2008 [58] | |
| Golden-crowned Sparrow (Zonotrichia atricapilla) | 2006–2007 | Alaska | 0 | 20 | 0% | - | - | - | Ip, 2008 [58] | |
| White-crowned Sparrow (Zonotrichia leucophrys) | 2006–2007 | Alaska | 0 | 49 | 0% | - | - | - | Ip, 2008 [58] | |
| Falconidae | 0 | 17 | 0% | |||||||
| Peregrine Falcon (Falco peregrinus) | 2007–2011 | Greenland | 0 | 16 | 0% | - | - | - | Hjulsager, 2012 [42] | |
| Greyfalcon (Falco rusticolus) | 2014 | Izembek National Wildlife Refuge, Alaska | 0 | 1 | 0% | - | - | - | Ramey, 2016 [68] | |
| Fringillidae | 0 | 149 | 0% | |||||||
| Common Redpoll (Acanthis flammea) | 2007–2011 | Greenland | 0 | 82 | 0% | - | - | - | Hjulsager, 2012 [42] | |
| Hoary Redpoll (Carduelis hornemanni) | 2006–2007 | Alaska | 0 | 10 | 0% | - | - | - | Ip, 2008 [58] | |
| Common Redpoll (Carduelis flammea) | 2006–2007 | Alaska | 0 | 52 | 0% | - | - | - | Ip, 2008 [58] | |
| Brambling (Fringilla montifringilla) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| White-winged Crossbill (Loxia leucoptera) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Pine Grosbeak (Pinicola enucleator) | 2006–2007 | Alaska | 0 | 3 | 0% | - | - | - | Ip, 2008 [58] | |
| Gaviidae | 1 | 10 | 10% | |||||||
| Yellow-billed Loon (Gavia adamsii) | 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | |
| 2012–2014 | Beaufort Sea coast, Alaska | - | - | 1/29 (3.4%) | - | Uher-Koch, 2019 [54] | ||||
| 2012–2016 | Alaska | - | - | 1/29 (3.4%) | H5N2: 1/20 (5%) | Van Hemert, 2019 [40] | ||||
| 2017 | Chukchi Sea coast, Alaska | - | - | 1/7 (14.2%) | - | Uher-Koch, 2019 [54] | ||||
| Arctic Loon (Gavia arctica) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Common Loon (Gavia immer) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Pacific Loon (Gavia pacifica) | 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | |
| 2008–2010 | Chukchi Sea coast, Alaska | - | - | 15/28 (53.6%) | - | Uher-Koch, 2019 [54] | ||||
| 2012–2016 | Alaska | - | - | 5/48 (10.4%) | - | Van Hemert, 2019 [40] | ||||
| 2012–2016 | Beaufort Sea coast, Alaska | - | - | 5/48 (10.4 %) | - | Uher-Koch, 2019 [54] | ||||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 1 | 1 | 100% | - | - | - | Ramey, 2016 [67] | ||
| 2016 | Yukon-Kuskokwim Delta, Alaska | - | - | 2/14 (14.2%) | - | Uher-Koch, 2019 [54] | ||||
| Red-throated Loon (Gavia stellata) | 2006–2007 | Alaska | 0 | 3 | 0% | - | - | - | Ip, 2008 [58] | |
| 2008–2010 | Chukchi Sea coast, Alaska | - | - | 15/33 (45.5%) | - | Uher-Koch, 2019 [54] | ||||
| 2010, 2012–2014 | Beaufort Sea coast, Alaska | - | - | 3/13 (23%) | - | Uher-Koch, 2019 [54] | ||||
| 2012–2016 | Alaska | - | - | 2/10 (20%) | H4N2: 1/20 (5%) | Van Hemert, 2019 [40] | ||||
| Gruidae | 2 | 192 | 1% | |||||||
| Sandhill Crane (Grus canadensis) | 2006–2007 | Alaska | 0 | 161 | 0% | - | - | - | Ip, 2008 [58] | |
| 2015 | Yukon-Kuskokwim Delta, Alaska | 2 | 31 | 7% | - | - | - | Ramey, 2016 [67] | ||
| Haematopodidae | 0 | 16 | 0% | |||||||
| Eurasian Oystercatcher (Haematopus ostralegus) | 2010–2011 | Iceland | 0 | 16 | 0% | - | - | - | Dusek, 2014 [4] | |
| Hydrobatidae | 0 | 377 | 0% | |||||||
| Leach’s Storm Petrel (Oceandroma leucorhoa) | 2008–2011 | Newfoundland and Labrador, Canada | 0 | 377 | 0% | - | - | - | Wille, 2014 [57] | |
| Hirundinidae | 0 | 2 | 0% | |||||||
| Cliff Swallow (Petrochelidon pyrrhonota) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Tree Swallow (Tachycineta bicolor) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Laniida | 0 | 2 | 0% | |||||||
| Northern Shrike (Lanius excubitor) | 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | |
| Laridae | 118 | 5568 | 2% | |||||||
| Black-headed Gull (Chroicocephalus ridibundus) | 2008–2011 | Newfoundland and Labrador, Canada | 0 | 1 | 0% | - | - | - | Huang, 2014 [39] | |
| 2010–2011 | Iceland | 2 | 168 | 1% | - | - | - | Dusek, 2014 [4] | ||
| Herring Gull (Larus argenatus smithsonianus) | 2005 | Chukchi Peninsula, Russia | 0 | 63 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2006–2007 | Alaska | 0 | 4 | 0% | - | - | - | Ip, 2008 [58] | ||
| 2008–2011 | Newfoundland and Labrador, Canada | 13 | 1083 | 1% | H13N6 (n = 5), H16N3 (n = 3), N6 (n = 1) | - | - | Huang, 2014 [39] | ||
| 2010–2011 | Iceland | 5 | 121 | 4% | - | - | - | Dusek, 2014 [4] | ||
| Common Gull (Larus canus) | 2006–2007 | Alaska | 0 | 5 | 0% | - | - | - | Ip, 2008 [58] | |
| 2008–2011 | Newfoundland and Labrador, Canada | 0 | 2 | 0% | - | - | - | Huang, 2014 [39] | ||
| 2010–2011 | Iceland | 0 | 1 | 0% | - | - | - | Dusek, 2014 [4] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 2 | 0% | - | - | - | Ramey, 2016 [67] | ||
| Ring-billed Gull (Larus delawarensis) | 2008–2011 | Newfoundland and Labrador, Canada | 1 | 21 | 5% | H13N6 | - | - | Huang, 2014 [39] | |
| Lesser Black-backed Gull (Larus fuscus) | 2010–2011 | Iceland | 1 | 96 | 1% | - | - | - | Dusek, 2014 [4] | |
| Glaucous-winged Gull (Larus glaucescens) | 2011–2013 | Izembek National Wildlife Refuge, Alaska | 34 | 710 | 5% | - | - | - | Ramey, 2015 [69] | |
| 2014 | Izembek National Wildlife Refuge, Alaska | 6 | 348 | 2% | - | - | - | Ramey, 2016 [68]; Reeves, 2018 [66] | ||
| Iceland Gull (Larus glaucoides) | 2007–2011 | Greenland | 1 | 398 | 0% | - | - | - | Hjulsager, 2012 [42] | |
| 2008–2011 | Newfoundland and Labrador, Canada | 0 | 19 | 0% | - | - | - | Huang, 2014 [39] | ||
| 2010–2011 | Iceland | 1 | 19 | 5% | - | - | - | Dusek, 2014 [4] | ||
| Glaucous Gull (Larus hyperboreus) | 2005 | Chukchi Peninsula, Russia | 0 | 3 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2005 | Pt. Barrow, Alaska | 0 | 33 | 0% | - | - | - | Wahlgren, 2008 [62] | ||
| 2006–2007 | Alaska | 5 | 138 | 4% | - | - | - | Ip, 2008 [58] | ||
| 2007–2011 | Greenland | 2 | 110 | 2% | - | - | - | Hjulsager, 2012 [42] | ||
| 2008–2011 | Newfoundland and Labrador, Canada | 0 | 24 | 0% | - | - | - | Huang, 2014 [39] | ||
| 2010–2011 | Iceland | 2 | 98 | 2% | - | - | - | Dusek, 2014 [4] | ||
| 2017 | Adentfjorden/Sassendalen, Norway | 5 | 15 | 33% | Lee, 2020 [11] | |||||
| Glaucous Gull x Herring Gull hybrid (Larus hyperboreus x L. argenatus) | 2007–2011 | Greenland | 0 | 47 | 0% | - | - | - | Hjulsager, 2012 [42] | |
| Great Black-backed Gull (Larus marinus) | 2008–2011 | Newfoundland and Labrador, Canada | 6 | 200 | 3% | H1, H13N6, H9N9 | - | - | Huang, 2014 [39] | |
| 2010–2011 | Iceland | 1 | 12 | 8% | - | - | - | Dusek, 2014 [4] | ||
| 2010–2011 | Iceland | 4 | 38 | 11% | - | - | - | Dusek, 2014 [4] | ||
| 2021 | St. John’s, Newfoundland and Labrador, Canada | 1 | - | - | H5N1 (HP; n = 1) (tissue sample) | - | - | Caliendo, 2022 [78] | ||
| Miscellaneous Gulls (Larus spp.) | 2007–2011 | Greenland | 0 | 747 | 0% | - | - | - | Hjulsager, 2012 [42] | |
| Aleutian Tern (Onychoprion aleuticus) | 2006–2007 | Alaska | 1 | 302 | 0% | - | - | - | Ip, 2008 [58] | |
| Common Tern (Sterna hirundo) | 2008–2011 | Newfoundland and Labrador, Canada | 0 | 21 | 0% | - | - | - | Wille, 2014 [57] | |
| Arctic Tern (Sterna paradisaea) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| 2007–2011 | Greenland | 0 | 165 | 0% | - | - | - | Hjulsager, 2012 [42] | ||
| 2008–2011 | Newfoundland and Labrador, Canada | 0 | 9 | 0% | - | - | - | Wille, 2014 [57] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 1 | 1 | 100% | - | - | - | Ramey, 2016 [67] | ||
| Black-legged Kittiwake (Rissa tridactyla) | 2005 | Chukchi Peninsula, Russia | 0 | 1 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | ||
| 2007–2011 | Greenland | 0 | 207 | 0% | - | - | - | Hjulsager, 2012 [42] | ||
| 2008–2009 | Hornøya, Norway | 25 | 200 | 13% | H4: 1/200 (0.5%) | 57/98 (58.2%) | H13: 18/48 (37.5%); H16: 39/48 (81.3%) | Tønnessen, 2011 [53] | ||
| 2008–2011 | Newfoundland and Labrador, Canada | 0 | 109 | 0% | - | - | - | Wille, 2014 [57] | ||
| 2014–2017 | Kongsfjorden, Norway | - | - | 9/53 (17.0%) | - | Lee, 2020 [11] | ||||
| Sabine’s Gull (Xema sabini) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| 2007–2011 | Greenland | 0 | 22 | 0% | - | - | - | Hjulsager, 2012 [42] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 1 | 1 | 100% | - | - | - | Ramey, 2016 [67] | ||
| Motacillidae | 0 | 308 | 0% | |||||||
| Yellow Wagtail (Motacilla flava) | 2005 | Chukchi Peninsula, Russia | 0 | 2 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| American Pipit (Anthus rubescens) | 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | |
| Eastern Yellow Wagtail (Motacilla tschutschensis) | 2006–2007 | Alaska | 0 | 304 | 0% | - | - | - | Ip, 2008 [58] | |
| Paridae | 0 | 1 | 0% | |||||||
| Black-capped Chickadee (Poecile atricapillus) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Parulidae | 0 | 135 | 0% | |||||||
| Bay-breasted Warbler (Dendroica castanea) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Yellow-rumped Warbler (Dendroica coronata) | 2006–2007 | Alaska | 0 | 11 | 0% | - | - | - | Ip, 2008 [58] | |
| Yellow Warbler (Dendroica petechia) | 2006–2007 | Alaska | 0 | 7 | 0% | - | - | - | Ip, 2008 [58] | |
| Blackpoll Warbler (Dendroica striata) | 2006–2007 | Alaska | 0 | 3 | 0% | - | - | - | Ip, 2008 [58] | |
| Northern Waterthrush (Seiurus noveboracensis) | 2006–2007 | Alaska | 0 | 27 | 0% | - | - | - | Ip, 2008 [58] | |
| Wilson’s Warbler (Wilsonia pusilla) | 2006–2007 | Alaska | 0 | 42 | 0% | - | - | - | Ip, 2008 [58] | |
| Orange-crowned Warbler (Vermivora celata) | 2006–2007 | Alaska | 0 | 44 | 0% | - | - | - | Ip, 2008 [58] | |
| Phalacrocoracidae | 0 | 30 | 0% | |||||||
| European Shag (Phalacrocorax aristotelis) | 2010–2011 | Iceland | 0 | 6 | 0% | - | - | - | Dusek, 2014 [4] | |
| Great Cormorant (Phalacrocorax carbo) | 2007–2011 | Greenland | 0 | 6 | 0% | - | - | - | Hjulsager, 2012 [42] | |
| 2010–2011 | Iceland | 0 | 2 | 0% | - | - | - | Dusek, 2014 [4] | ||
| Pelagic Cormorant (Phalacrocorax pelagicus) | 2005 | Chukchi Peninsula, Russia | 0 | 11 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2006–2007 | Alaska | 0 | 3 | 0% | - | - | - | Ip, 2008 [58] | ||
| Red-faced Cormorant (Phalacrocorax urile) | 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | |
| Phasianidae | 1 | 15 | 7% | |||||||
| Willow Ptarmigan (Lagopus lagopus) | 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 3 | 0% | - | - | - | Ramey, 2016 [67] | |
| Rock Ptarmigan (Lagopus muta) | 2007–2011 | Greenland | 0 | 1 | 0% | - | - | - | Hjulsager, 2012 [42] | |
| Partridge/Sharp-tailed Grouse (Tympanuchus phasianellus) | 2013–2014 | Subarctic Ontario, Canada | 1 | 11 | 9% | - | - | - | Liberda, 2017 [48] | |
| Picidae | 0 | 1 | 0% | |||||||
| Northern Flicker (Colaptes auratus) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Podicipedidae | 0 | 1 | 0% | |||||||
| Red-necked Grebe (Podiceps grisegena) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Procellariidae | 0 | 17 | 0% | |||||||
| Northern Fulmar (Fulmarus glacialis) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| 2008–2011 | Newfoundland and Labrador, Canada | 0 | 4 | 0% | - | - | - | Wille, 2014 [57] | ||
| Manx Shearwater (Puffinus puffinus) | 2008–2011 | Newfoundland and Labrador, Canada | 0 | 12 | 0% | - | - | - | Wille, 2014 [57] | |
| Regulidae | 0 | 2 | 0% | |||||||
| Ruby-crowned Kinglet (Regulus calendula) | 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | |
| Scolopacidae | 17 | 4333 | 0.4% | |||||||
| Ruddy Turnstone (Arenaria interpres) | 2005 | Chukchi Peninsula, Russia | 0 | 1 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2006–2007 | Alaska | 0 | 30 | 0% | - | - | - | Ip, 2008 [58] | ||
| 2010–2011 | Iceland | 0 | 64 | 0% | - | - | - | Dusek, 2014 [4] | ||
| 2012 | Iceland | 1 | 68 | 2% | - | 51/60 (85%) | - | Hall, 2014 [77] | ||
| 2013 | Iceland | 1 | 88 | 1% | - | 46/70 (66%) | - | Hall, 2014 [77] | ||
| Black Turnstone (Arenaria melanocephala) | 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | |
| 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 3 | 0% | - | - | - | Ramey, 2016 [67] | ||
| Sharp-tailed Sandpiper (Calidris acuminate) | 2006–2007 | Alaska | 0 | 225 | 0% | - | - | - | Ip, 2008 [58] | |
| 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 3 | 0% | - | - | - | Ramey, 2016 [67] | ||
| Baird’s Sandpiper (Calidris bairdii) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Sanderling (Calidris alba) | 2010–2011 | Iceland | 0 | 129 | 0% | - | - | - | Dusek, 2014 [4] | |
| 2012 | Iceland | 1 | 297 | 0% | - | 1/48 (2.1%) | - | Hall, 2014 [77] | ||
| 2013 | Iceland | 1 | 388 | 0% | - | 0/1 (0%) | - | Hall, 2014 [77] | ||
| Dunlin (Calidris alpina) | 2005 | Chukchi Peninsula, Russia | 0 | 22 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2005 | Pt. Barrow, Alaska | 1 | 1 | 100% | - | - | - | Wahlgren, 2008 [62] | ||
| 2006–2007 | Alaska | 2 | 897 | 0% | - | - | - | Ip, 2008 [58] | ||
| 2010–2011 | Iceland | 0 | 2 | 0% | - | - | - | Dusek, 2014 [4] | ||
| 2012 | Iceland | 0 | 3 | 0% | - | 0/3 (0%) | - | Hall, 2014 [77] | ||
| 2013 | Iceland | 0 | 12 | 0% | - | - | - | Hall, 2014 [77] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 1 | 9 | 11% | - | - | - | Ramey, 2016 [67] | ||
| Red Knot (Calidris canutus) | 2006–2007 | Alaska | 0 | 79 | 0% | - | - | - | Ip, 2008 [58] | |
| 2010–2011 | Iceland | 0 | 1 | 0% | - | - | - | Dusek, 2014 [4] | ||
| 2013 | Iceland | 0 | 21 | 0% | - | 52/114 (45.6%) | - | Hall, 2014 [77] | ||
| Stilt Sandpiper (Calidris himantopus) | 2006–2007 | Alaska | 0 | 3 | 0% | - | - | - | Ip, 2008 [58] | |
| Purple Sandpiper (Calidris maritima) | 2010–2011 | Iceland | 0 | 2 | 0% | - | - | - | Dusek, 2014 [4] | |
| 2013 | Iceland | 0 | 6 | 0% | - | 0/1 (0%) | - | Hall, 2014 [77] | ||
| Western Sandpiper (Calidris mauri) | 2005 | Chukchi Peninsula, Russia | 0 | 75 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2005 | Pt. Barrow, Alaska | 0 | 6 | 0% | - | - | - | Wahlgren, 2008 [62] | ||
| 2006–2007 | Alaska | 0 | 128 | 0% | - | - | - | Ip, 2008 [58] | ||
| Pectoral Sandpiper (Calidris melanotos) | 2005 | Chukchi Peninsula, Russia | 0 | 1 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2006–2007 | Alaska | 0 | 580 | 0% | - | - | - | Ip, 2008 [58] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 1 | 16 | 6% | - | - | - | Ramey, 2016 [67] | ||
| Rock Sandpiper (Calidris ptilocnemis) | 2005 | Chukchi Peninsula, Russia | 0 | 1 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2006–2007 | Alaska | 0 | 173 | 0% | - | - | - | Ip, 2008 [58] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 1 | 0% | - | - | - | Ramey, 2016 [67] | ||
| Semipalmated Sandpiper (Calidris pusilla) | 2005 | Chukchi Peninsula, Russia | 0 | 1 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2005 | Pt. Barrow, Alaska | 0 | 5 | 0% | - | - | - | Wahlgren, 2008 [62] | ||
| 2006–2007 | Alaska | 0 | 212 | 0% | - | - | - | Ip, 2008 [58] | ||
| Temminck’s Stint (Calidris temminckii) | 2005 | Chukchi Peninsula, Russia | 0 | 2 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| Spoon-billed Sandpiper (Eurynorhynchus pygmeus) | 2005 | Chukchi Peninsula, Russia | 0 | 1 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| Common Snipe (Gallinago gallinago) | 2010–2011 | Iceland | 0 | 1 | 0% | - | - | - | Dusek, 2014 [4] | |
| Wilson’s Snipe (Gallinago delicata) | 2006–2007 | Alaska | 0 | 9 | 0% | - | - | - | Ip, 2008 [58] | |
| 2015 | Yukon-Kuskokwim Delta, Alaska | 0 | 1 | 0% | - | - | - | Ramey, 2016 [67] | ||
| Long-billed Dowitcher (Limnodromus scolopaceus) | 2006–2007 | Alaska | 0 | 165 | 0% | - | - | - | Ip, 2008 [58] | |
| 2015 | Yukon-Kuskokwim Delta, Alaska | 1 | 3 | 33% | - | - | - | Ramey, 2016 [67] | ||
| Bar-tailed Godwit (Limosa lapponica) | 2006–2007 | Alaska | 3 | 210 | 1% | - | - | - | Ip, 2008 [58] | |
| 2015 | Yukon-Kuskokwim Delta, Alaska | 1 | 14 | 7% | - | - | - | Ramey, 2016 [67] | ||
| Whimbrel (Neumenius phaeopus) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Bristle-thighed Curlew (Neumenius tahitiensis) | 2006–2007 | Alaska | 0 | 9 | 0% | - | - | - | Ip, 2008 [58] | |
| Grey Phalarope (Phalaropus fulicaria) | 2005 | Chukchi Peninsula, Russia | 0 | 8 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2005 | Pt. Barrow, Alaska | 0 | 5 | 0% | - | - | - | Wahlgren, 2008 [62] | ||
| Red Phalarope (Phaloropus fulicarius) | 2006–2007 | Alaska | 0 | 179 | 0% | - | - | - | Ip, 2008 [58] | |
| Red-necked Phalarope (Phalaropus lobatus) | 2005 | Chukchi Peninsula, Russia | 0 | 3 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2005 | Pt. Barrow, Alaska | 0 | 5 | 0% | - | - | - | Wahlgren, 2008 [62] | ||
| 2006–2007 | Alaska | 0 | 38 | 0% | - | - | - | Ip, 2008 [58] | ||
| 2015 | Yukon-Kuskokwim Delta, Alaska | 3 | 23 | 13% | - | - | - | Ramey, 2016 [67] | ||
| Ruff (Philomachus pugnax) | 2006–2007 | Alaska | 0 | 6 | 0% | - | - | - | Ip, 2008 [58] | |
| Greater Yellowlegs (Tringa melanoleuca) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Common Redshank (Tringa tetanus) | 2010–2011 | Iceland | 0 | 1 | 0% | - | - | - | Dusek, 2014 [4] | |
| Buff-breasted Sandpiper (Tryngites subruficollis) | 2006–2007 | Alaska | 0 | 92 | 0% | - | - | - | Ip, 2008 [58] | |
| Slyvioidea | 0 | 769 | 0% | |||||||
| Arctic Warbler (Phylloscopus borealis) | 2006–2007 | Alaska | 0 | 769 | 0% | - | - | - | Ip, 2008 [58] | |
| Strigidae | 0 | 2 | 0% | |||||||
| Short-eared Owl (Asio flammeus) | 2006–2007 | Alaska | 0 | 1 | 0% | - | - | - | Ip, 2008 [58] | |
| Snowy Owl (Nyctea scandiaca) | 2005 | Pt. Barrow, Alaska | 0 | 1 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| Stercorariidae | 0 | 6 | 0% | |||||||
| Long-tailed Jaeger (Stercorarius longicaudus) | 2006–2007 | Alaska | 0 | 2 | 0% | - | - | - | Ip, 2008 [58] | |
| Pomarine Jaeger (Stercorarius pomarinus) | 2006–2007 | Alaska | 0 | 4 | 0% | - | - | - | Ip, 2008 [58] | |
| Sulidae | 0 | 77 | 0% | |||||||
| Northern Gannet (Morus bassanus) | 2008–2011 | Newfoundland and Labrador, Canada | 0 | 76 | 0% | - | - | - | Wille, 2014 [57] | |
| 2010–2011 | Iceland | 0 | 1 | 0% | - | - | - | Dusek, 2014 [4] | ||
| Turdidae | 0 | 333 | 0% | |||||||
| Hermit Thrush (Catharus guttatus) | 2006–2007 | Alaska | 0 | 10 | 0% | - | - | - | Ip, 2008 [58] | |
| Gray-checked Thrush (Catharus minimus) | 2006–2007 | Alaska | 0 | 229 | 0% | - | - | - | Ip, 2008 [58] | |
| Swainson’s Thrush (Catharus ustulatus) | 2006–2007 | Alaska | 0 | 20 | 0% | - | - | - | Ip, 2008 [58] | |
| Varied Thrush (Ixoreus naevius) | 2006–2007 | Alaska | 0 | 10 | 0% | - | - | - | Ip, 2008 [58] | |
| Bluethroat (Luscina svecica) | 2005 | Chukchi Peninsula, Russia | 0 | 2 | 0% | - | - | - | Wahlgren, 2008 [62] | |
| 2006–2007 | Alaska | 0 | 12 | 0% | - | - | - | Ip, 2008 [58] | ||
| Northern Wheatear (Oenanthe Oenanthe) | 2006–2007 | Alaska | 0 | 10 | 0% | - | - | - | Ip, 2008 [58] | |
| 2007–2011 | Greenland | 0 | 29 | 0% | - | - | - | Hjulsager, 2012 [42] | ||
| American Robin (Turdus migratorius) | 2006–2007 | Alaska | 0 | 11 | 0% | - | - | - | Ip, 2008 [58] | |
| Tyrannidae | 0 | 5 | 0% | |||||||
| Alder Flycatcher (Empidonax alnorum) | 2006–2007 | Alaska | 0 | 5 | 0% | - | - | - | Ip, 2008 [58] | |
3.5. Marine and Terrestrial Mammals in the Arctic Are Exposed to IAVs, despite Major Gaps in Surveillance
| Family Taxa | Host Species | Sampling Year(s) | Location | Prevalence | Seroprevalence | Author, Year | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive Samples | Total Samples | Prevalence % | Virus Subtypes Identified | n/N (%) | Subtype-Specific Antibodies Identified, n/N (%) | |||||
| Balaenidae | 0 | 4 | 0% | |||||||
| Bowhead whale (Balaena mysticetus) | 1984–1998 | Canadian Arctic | 0 | 4 | 0% | - | - | - | Nielsen, 2001 [45] | |
| Canidae | 1 | 231 | 0.43% | |||||||
| Arctic fox (Vulpes lagopus) | 2012–2016 | Alaska | 1 | 231 | 0.43% | - | - | - | Van Hemert, 2019 [40] | |
| Cervidae | 0 | 46 | 0.00% | |||||||
| Caribou (Rangifer tarandus) | 2012–2016 | Alaska | 0 | 46 | 0% | - | - | - | Van Hemert, 2019 [40] | |
| Monodontidae | 5 | 494 | 1.01% | |||||||
| Beluga whale (Delphinapterus leucas) | 1984–1998 | Canadian Arctic | 5 | 418 | 1.20% | - | - | - | Nielsen, 2001 [45] | |
| Narwhal (Monodon monoceros) | 1984–1998 | Canadian Arctic | 0 | 76 | 0% | - | - | - | Nielsen, 2001 [45] | |
| Odobenidae | 8 | 248 | 3.23% | |||||||
| Pacific walrus (Odobenus rosmarus divergens) | 1994–1996 | Aleutian Islands, Alaska | 8 | 38 | 21% | - | - | H10: 8/8 (100%) | Calle, 2002 [85] | |
| Atlantic walrus (Odobenus rosmarus rosmarus) | 1984–1998 | Canadian Arctic | 0 | 210 | 0% | - | - | - | Nielsen, 2001 [45] | |
| Phocidae | 207 | 1621 | 12.77% | |||||||
| Hooded Seal (Cystophora cristata) | 1991–1992 | Jan Mayen Island, Norway | 8 | 92 | 8.70% | - | - | - | Stuen, 1994 [86] | |
| 1994–2005 | Eastern Canadian Subarctic | 4 | 36 | 11% | - | - | H3: 0 animals * | Measures, 2021 [87] | ||
| H4: 0 animals * | ||||||||||
| H5: 0 animals * | ||||||||||
| Bearded seal (Erignathus barbatus) | 2000–2017 | Alaska | 0 | 3 | 0% | - | - | - | Goertz, 2019 [88] | |
| Grey seal (Halichoerus grypus) | 1994–2005 | Eastern Canadian Subarctic | 18 | 80 | 23% | - | - | H3: 13 animals * | Measures, 2021 [87] | |
| H4: 0 animals * | ||||||||||
| H10: 5 animals * | ||||||||||
| Harp seal (Phoca groenlandica) | 1991–1992 | Barents Sea and Jan Mayen Island, Norway | 42 | 183 | 22.90% | - | - | - | Stuen, 1994 [86] | |
| 1994–2005 | Eastern Canadian Subarctic | 86 | 206 | 41.70% | - | - | H3: 72 animals * | Measures, 2021 [87] | ||
| H4: 4 animals * | ||||||||||
| H10: 11 animals * | ||||||||||
| Ringed seal (Phoca hispida) | 1978–1995 | Alaska | 1 | 32 | 3.10% | - | - | H3N2, H3N3, H7N7: 1 animal tested | Danner, 1998 [84] | |
| 1984–1998 | Canadian Arctic | 23 | 903 | 2.50% | - | - | - | Nielsen, 2001 [45] | ||
| 2000–2017 | Alaska | 0 | 11 | 0% | - | - | - | Goertz, 2019 [88] | ||
| 2004 | Central Russian Arctic | 5 | 6 | 83.30% | - | - | H3N2: 4/6 (66.6%) | Ohishi, 2004 [44] | ||
| H7N7: 1/6 (16.6%) | ||||||||||
| Spotted seal (Phoca largha) | 2000–2017 | Alaska | 0 | 8 | 0% | - | - | - | Goertz, 2019 [88] | |
| Baikal seal (Phoca sibrica) | 2004 | Central Russian Arctic | 2 | 7 | 28.50% | - | - | H3N2: 1/7 (14.2%) | Ohishi, 2004 [44] | |
| Harbor seal (Phoca vitulina) | 1994–2005 | Eastern Canadian Subarctic | 18 | 54 | 33% | - | - | H3: 0 animals * | Measures, 2021 [87] | |
| H4: 0 animals * | ||||||||||
| H5: 0 animals * | ||||||||||
| Ursidae | 8 | 255 | 3.14% | |||||||
| Brown bear (Ursus Arctos) | 2013–2016 | Alaska | 8 | 155 | 5.20% | - | - | - | Ramey, 2019 [89] | |
| Polar Bear (Ursus maritimus) | 2012–2016 | Alaska | 0 | 100 | 0% | - | - | - | Van Hemert, 2019 [40] | |
3.6. Arctic Environment Serves as an Important Source of IAV Infection for Wildlife
| Environmental Matrix | Sampling Year(s) | Location | Prevalence | Sero-Prevalence | Author, Year | ||||
|---|---|---|---|---|---|---|---|---|---|
| Positive Samples | Total Samples | Prevalence % | Virus Subtypes Identified | n/N (%) | Subtype-Specific Antibodies Identified, n/N (%) | ||||
| Lake/pond water samples | 57 | 194 | 29.38% | ||||||
| 1992–2005 | Lake Hood, Alaska | 1 | 6 | 16.67% | H4N6: 1/6 (16.6%) | - | - | Ito, 1995 [93] | |
| 1992–2005 | Lake Cheney, Alaska | 0 | 6 | 0% | - | - | - | Ito, 1995 [93] | |
| 1992–2005 | Potter Marsh, Alaska | 0 | 4 | 0% | - | - | - | Ito, 1995 [93] | |
| 1992–2005 | Westchester Lagoon, Alaska | 0 | 1 | 0% | - | - | - | Ito, 1995 [93] | |
| 1992–2005 | Lake Hanger, Alaska | 0 | 4 | 0% | - | - | - | Ito, 1995 [93] | |
| 1992–2005 | Fairbanks, Alaska | 0 | 5 | 0% | - | - | - | Ito, 1995 [93] | |
| 1992–2005 | Big Minto Lake, Alaska | 10 | 44 | 22.73% | - | - | - | Ito, 1995 [93] | |
| 1992–2005 | Mallard Lake, Alaska | 1 | 24 | 4.17% | - | - | - | Ito, 1995 [93] | |
| 1992–2005 | Heart Lake, Alaska | 0 | 5 | 0% | - | - | - | Ito, 1995 [93] | |
| 1992–2005 | Canvasback Lake, Alaska | 0 | 3 | 0% | - | - | - | Ito, 1995 [93] | |
| 1992–2005 | Corville Delta, Alaska | 0 | 1 | 0% | - | - | - | Ito, 1995 [93] | |
| 2001–2002 | Lake Edoma, Russia | 1 IAV sequence characterized | - | - | - | Zhang, 2006 [94] | |||
| 2001–2002 | Lake Park, Russia | 83 IAV sequences characterized | - | - | - | Zhang, 2006 [94] | |||
| 2001–2002 | Lake Shchychie, Russia | 0 IAV sequences characterized | - | - | - | Zhang, 2006 [94] | |||
| 2005–2006 | Fairbanks, Alaska (pond 1) | 17 | 38 | 44.74% | H3, H12 | - | - | Lang, 2008 [98] | |
| 2005–2006 | Fairbanks, Alaska (pond 2) | 20 | 37 | 54.05% | H3, H8, H11, H12 | - | - | Lang, 2008 [98] | |
| 2005–2006 | Fairbanks, Alaska (pond 3) | 8 | 16 | 50.00% | H3, H11, H12 | - | - | Lang, 2008 [98] | |
| Common Murre excreta samples | 1 | 44 | 2.27% | ||||||
| 2008–2011 | Newfoundland and Labrador, Canada | 1 | 44 | 2.27% | - | - | - | Wille, 2014 [57] | |
| Duck excreta samples, species unspecified | 142 | 5105 | 2.78% | ||||||
| 1991–1994 | Lake Hood, Alaska | 2 | 660 | 0.30% | H4N6: 1/317 (0.31%); H10N7: 1/317 (0.31%) | - | - | Ito, 1995 [93] | |
| 1991–1994 | Lake Cheney, Alaska | 0 | 460 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Potter March, Alaska | 0 | 19 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Westchester Lagoon, Alaska | 0 | 134 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Lake Hanger, Alaska | 0 | 44 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Fairbanks, Alaska | 0 | 326 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Nenana, Alaska | 16 | 108 | 14.81% | H3N8: 14/108 (13%) | - | - | Ito, 1995 [93] | |
| 1991–1994 | Delta Junction, Alaska | 0 | 27 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Denali National Park, Alaska | 0 | 6 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Big Minto Lake, Alaska | 27 | 382 | 7.07% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Mallard Lake, Alaska | 53 | 180 | 29.44% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Heart Lake, Alaska | 6 | 38 | 15.79% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Canvasback Lake, Alaska | 0 | 6 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Corville Delta, Alaska | 0 | 14 | 0% | - | - | - | Ito, 1995 [93] | |
| 1995–1998 | Petropavlovsk-Kamchatsky, Russia | 0 | 26 | 0% | - | - | - | Okazaki, 2000 [43] | |
| 1995–1998 | Lake Kanycheva, Russia | 0 | 95 | 0% | - | - | - | Okazaki, 2000 [43] | |
| 1995–1998 | 40 Islands, Russia | 0 | 1185 | 0% | - | - | - | Okazaki, 2000 [43] | |
| 1995–1998 | Koybyaysky, Russia | 32 | 943 | 3.39% | H4N6: 19/819 (2.3%); H4N9: 1/819 (0.12%); H11N1: 1/819 (0.12%); H11N6: 2/819 (0.24%); H11N9: 8/819 (0.97%); H4N6: 1/124 (0.80%) | - | - | Okazaki, 2000 [43] | |
| 1995–1998 | Kemkeme National Park, Russia | 0 | 23 | 0% | - | - | - | Okazaki, 2000 [43] | |
| 1995–1998 | Kharyyalah, Russia | 0 | 146 | 0% | - | - | - | Okazaki, 2000 [43] | |
| 1995–1998 | Buotama, Russia | 0 | 51 | 0% | - | - | - | Okazaki, 2000 [43] | |
| 1995–1998 | Yakutsk, Russia | 6 | 232 | 2.59% | H3N8: 5/62 (8.1%); H13N6: 1/170 (0.58%); H1N1: 1/170 (0.58%); H5N3: 1/170 (0.58%); H5N4 (LP): 1/170 (0.58%); H6N7: 1/170 (0.58%); H8N1: 1/170 (0.58%); H8N3: 1/170 (0.58%) | - | - | Okazaki, 2000 [43] | |
| Gull excreta samples | 12 | 317 | 3.79% | ||||||
| 1991–1994 | Lake Hood, Alaska | 0 | 3 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Potter Marsh, Alaska | 0 | 4 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Fairbanks, Alaska | 0 | 3 | 0% | - | - | - | Ito, 1995 [93] | |
| 1995–1998 | 40 Islands, Russia | 0 | 10 | 0% | - | - | - | Okazaki, 2000 [43] | |
| 2008–2011 | Newfoundland and Labrador, Canada | 10 | 295 | 3.39% | - | - | - | Huang, 2014 [39] | |
| 2010 | Nuuk, Greenland | 1 sample * | - | - | - | Hjulsager, 2012 [42] | |||
| 2016 | Kamchatka, Russia | 1 sample * | - | - | - | Marchenko, 2018 [99] | |||
| Goose excreta samples | 4 | 794 | 0.50% | ||||||
| 1991–1994 | Lake Hood, Alaska | 4 | 238 | 1.68% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Lake Cheney, Alaska | 0 | 196 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Potter Marsh, Alaska | 0 | 130 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Westchester Lagoon, Alaska | 0 | 2 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Big Minto Lake, Alaska | 0 | 9 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Mallard Lake, Alaska | 0 | 3 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Corville Delta, Alaska | 0 | 16 | 0% | - | - | - | Ito, 1995 [93] | |
| 1991–1994 | Prudhoe Bay, Alaska | 0 | 69 | 0% | - | - | - | Ito, 1995 [93] | |
| 1995–1998 | 40 Islands, Russia | 0 | 126 | 0% | - | - | - | Okazaki, 2000 [43] | |
| 1995–1998 | Kemkeme National Park, Russia | 0 | 5 | 0% | - | - | - | Okazaki, 2000 [43] | |
| Shorebird excreta samples | 0 | 31 | 0% | ||||||
| 1991–1994 | Fairbanks, Alaska | 0 | 7 | 0% | - | - | - | Ito, 1995 [93] | |
| 1995–1998 | 40 Islands, Russia | 0 | 24 | 0% | - | - | - | Okazaki, 2000 [43] | |
| Swan excreta samples | 0 | 4 | 0% | ||||||
| 1995–1998 | Kemkeme National Park, Russia | 0 | 4 | 0% | - | - | - | Okazaki, 2000 [43] | |
| 1991–1994 | Corville Delta, Alaska | 0 | 6 | 0% | - | - | - | Ito, 1995 [93] | |
| Mallard excreta samples | 2 | 2 | 100% | ||||||
| 2007 | Nuuk and Sisimiut, Greenland | 2 samples * | - | - | - | Hjulsager, 2012 [42] | |||
4. Host–Pathogen–Environmental Ecology of IAVs in Arctic Regions
4.1. Trans-Continental Movement of IAV via the Arctic during Migration and Breeding
4.2. Detection of HPAI Viruses in the Arctic Is Infrequent, but Serological Data Suggest Circulation
4.3. Inter-Continental Reassortment of IAVs in Arctic Regions Is Common
4.4. Climate Change Is Altering the Ecology of IAVs and Hosts of IAVs
5. Discussion
5.1. Gaps in Knowledge
- What role do mammals serve as potential sources and sinks for inter-species transmission in Arctic regions? Are marine and/or terrestrial mammals sources of onward IAV transmission to other animals? Do Arctic mammals serve as reservoir or incidental hosts for IAVs in the region?
- How do wetlands throughout Arctic regions contribute to the dispersal of IAVs via waterfowl migration at regional and global scales? Are wetlands in Arctic regions associated with environmental or inter-species transmission from waterfowl to sympatric avian species and/or terrestrial mammals?
- What is the impact of climate change (dynamics of air temperature, sea-surface temperature, ice and permafrost melt, etc.) on IAV infection, transmission dynamics, and migratory ecology of avian and mammalian species in the Arctic?
- What are spatiotemporal trends (i.e., seasonal changes or geographic differences) in IAV prevalence and seroprevalence within the Arctic and how do overlaps in migratory flyways influence the global dispersal of IAVs?
- To what degree do environmentally derived IAV samples in the Arctic reflect the spatiotemporal dynamics of IAVs in resident and migratory birds in Arctic regions?
5.2. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.; Munster, V.J.; Wallensten, A.; Waldenström, J.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Global Patterns of Influenza A Virus in Wild Birds. Science 2006, 312, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.J.; Takekawa, J.Y.; Cardona, C.J.; Meixell, B.W.; Ackerman, J.T.; Runstadler, J.A.; Boyce, W.M. Cross-seasonal patterns of avian influenza virus in breeding and wintering migratory birds: A flyway perspective. Vector Borne Zoonotic Dis. 2012, 12, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Dusek, R.J.; Hallgrimsson, G.T.; Ip, H.S.; Jónsson, J.E.; Sreevatsan, S.; Nashold, S.W.; TeSlaa, J.L.; Enomoto, S.; Halpin, R.A.; Lin, X.; et al. North Atlantic Migratory Bird Flyways Provide Routes for Intercontinental Movement of Avian Influenza Viruses. PLoS ONE 2014, 9, e92075. [Google Scholar] [CrossRef]
- Gass, J.D.; Dusek, R.J.; Hall, J.S.; Hallgrimsson, G.T.; Halldorsson, H.P.; Vignisson, S.R.; Ragnarsdottir, S.B.; Jonsson, J.E.; Krauss, S.; Wong, S.; et al. Global dissemination of Influenza A virus is driven by wild bird migration through arctic and subarctic zones. Authorea 2022, 1–22. [Google Scholar] [CrossRef]
- Lee, D.H.; Torchetti, M.K.; Winker, K.; Ip, H.S.; Song, C.S.; Swayne, D.E. Intercontinental Spread of Asian-Origin H5N8 to North America through Beringia by Migratory Birds. J. Virol. 2015, 89, 6521–6524. [Google Scholar] [CrossRef]
- Conservation of Arctic Flora and Fauna (CAFF) and GRiD Arendal. Definitions of the Arctic. 2005. Available online: https://www.grida.no/resources/7010 (accessed on 16 May 2022).
- Herrick, K.A.; Huettmann, F.; Lindgren, M.A. A global model of avian influenza prediction in wild birds: The importance of northern regions. Vet. Res. 2013, 44, 42. [Google Scholar] [CrossRef]
- The Global Consortium for H5N8 and Related Influenza Viruses. Role for migratory wild birds in the global spread of avian influenza H5N8. Science 2016, 354, 213–217. [Google Scholar] [CrossRef]
- Hill, N.; Hussein, I.; Davis, K.; Ma, E.; Spivey, T.; Ramey, A.; Puryear, W.; Das, S.; Halpin, R.; Lin, X.; et al. Reassortment of Influenza A Viruses in Wild Birds in Alaska before H5 Clade 2.3.4.4 Outbreaks. Emerg. Infect. Dis. 2017, 23, 654–657. [Google Scholar] [CrossRef]
- Lee, M.M.; Jaspers, V.L.B.; Gabrielsen, G.W.; Jenssen, B.M.; Ciesielski, T.M.; Mortensen, Å.-K.; Lundgren, S.S.; Waugh, C.A. Evidence of avian influenza virus in seabirds breeding on a Norwegian high-Arctic archipelago. BMC Vet. Res. 2020, 16, 48. [Google Scholar] [CrossRef]
- Marra, P.P.; Hobson, K.A.; Holmes, R.T. Linking Winter and Summer Events in a Migratory Bird by Using Stable-Carbon Isotopes. Science 1998, 282, 1884–1886. [Google Scholar] [CrossRef]
- Olson, S.H.; Parmley, J.; Soos, C.; Gilbert, M.; Latorre-Margalef, N.; Hall, J.S.; Hansbro, P.M.; Leighton, F.; Munster, V.; Joly, D. Sampling strategies and biodiversity of influenza A subtypes in wild birds. PLoS ONE 2014, 9, e90826. [Google Scholar] [CrossRef]
- Martin, G.; Becker, D.J.; Plowright, R.K. Environmental Persistence of Influenza H5N1 Is Driven by Temperature and Salinity: Insights From a Bayesian Meta-Analysis. Front. Ecol. Evol. 2018, 6, 131. [Google Scholar] [CrossRef]
- Webby, R.J.; Webster, R.G.; Richt, J.A. Influenza Viruses in Animal Wildlife Populations. In Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission; Childs, J.E., Mackenzie, J.S., Richt, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 67–83. [Google Scholar]
- Hall, J.; Dusek, R.; Nashold, S.; TeSlaa, J.; Allen, R.; Grear, D. Avian influenza virus prevalence in marine birds is dependent on ocean temperatures. Ecol. Appl. 2020, 30, e02040. [Google Scholar] [CrossRef]
- Gamblin, S.J.; Skehel, J.J. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J. Biol. Chem. 2010, 285, 28403–28409. [Google Scholar] [CrossRef]
- Suarez, D.L. Evolution of avian influenza viruses. Vet. Microbiol. 2000, 74, 15–27. [Google Scholar] [CrossRef]
- Chen, R.; Holmes, E.C. Avian Influenza Virus Exhibits Rapid Evolutionary Dynamics. Mol. Biol. Evol. 2006, 23, 2336–2341. [Google Scholar] [CrossRef]
- Chen, J.; Deng, Y.-M. Influenza virus antigenic variation, host antibody production and new approach to control epidemics. Virol. J. 2009, 6, 30. [Google Scholar] [CrossRef]
- Nao, N.; Yamagishi, J.; Miyamoto, H.; Igarashi, M.; Manzoor, R.; Ohnuma, A.; Tsuda, Y.; Furuyama, W.; Shigeno, A.; Kajihara, M.; et al. Genetic Predisposition To Acquire a Polybasic Cleavage Site for Highly Pathogenic Avian Influenza Virus Hemagglutinin. mBio 2017, 8, e02298-16. [Google Scholar] [CrossRef]
- Scheibner, D.; Ulrich, R.; Fatola, O.I.; Graaf, A.; Gischke, M.; Salaheldin, A.H.; Harder, T.C.; Veits, J.; Mettenleiter, T.C.; Abdelwhab, E.M. Variable impact of the hemagglutinin polybasic cleavage site on virulence and pathogenesis of avian influenza H7N7 virus in chickens, turkeys and ducks. Sci. Rep. 2019, 9, 11556. [Google Scholar] [CrossRef]
- Kosik, I.; Yewdell, J.W. Influenza Hemagglutinin and Neuraminidase: Yin–Yang Proteins Coevolving to Thwart Immunity. Viruses 2019, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Afanador-Villamizar, A.; Gomez-Romero, C.; Diaz, A.; Ruiz-Saenz, J. Avian influenza in Latin America: A systematic review of serological and molecular studies from 2000–2015. PLoS ONE 2017, 12, e0179573. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.J. An overview of the epidemiology of avian influenza. Vaccine 2007, 25, 5637–5644. [Google Scholar] [CrossRef] [PubMed]
- Worobey, M.; Han, G.-Z.; Rambaut, A. A synchronized global sweep of the internal genes of modern avian influenza virus. Nature 2014, 508, 254. [Google Scholar] [CrossRef]
- Runstadler, J.; Hill, N.; Hussein, I.T.M.; Puryear, W.; Keogh, M. Connecting the study of wild influenza with the potential for pandemic disease. Infect. Genet. Evol. 2013, 17, 162–187. [Google Scholar] [CrossRef]
- Verhagen, J.H.; Fouchier, R.A.M.; Lewis, N. Highly Pathogenic Avian Influenza Viruses at the Wild-Domestic Bird Interface in Europe: Future Directions for Research and Surveillance. Viruses 2021, 13, 212. [Google Scholar] [CrossRef]
- Hill, A.; Gillings, S.; Berriman, A.; Brouwer, A.; Breed, A.C.; Snow, L.; Ashton, A.; Byrne, C.; Irvine, R.M. Quantifying the spatial risk of Avian Influenza introduction into British poultry by wild birds. Sci. Rep. 2019, 9, 19973. [Google Scholar] [CrossRef]
- Ramey, A.M.; Hill, N.J.; DeLiberto, T.J.; Gibbs, S.E.J.; Camille Hopkins, M.; Lang, A.S.; Poulson, R.L.; Prosser, D.J.; Sleeman, J.M.; Stallknecht, D.E.; et al. Highly pathogenic avian influenza is an emerging disease threat to wild birds in North America. J. Wildl. Manag. 2022, 86, e22171. [Google Scholar] [CrossRef]
- Cappelle, J.; Gaidet, N.; Iverson, S.A.; Takekawa, J.Y.; Newman, S.H.; Fofana, B.; Gilbert, M. Characterizing the interface between wild ducks and poultry to evaluate the potential of transmission of avian pathogens. Int. J. Health Geogr. 2011, 10, 60. [Google Scholar] [CrossRef]
- Capua, I.; Marangon, S. Control of avian influenza in poultry. Emerg. Infect. Dis. 2006, 12, 1319–1324. [Google Scholar] [CrossRef]
- Arctic Monitoring and Assessment Programme (AMAP). AMAP Climate Change Update 2019: An Update to Key Findings of Snow, Water, Ice and Permafrost in the Arctic; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 2019. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Heather, J.M.; Chain, B. The sequence of sequencers: The history of sequencing DNA. Genomics 2016, 107, 1–8. [Google Scholar] [CrossRef]
- Law, M.; Stewart, D.; Pollock, N.; Letts, L.; Bosch, J.; Westorland, M. Guidelines for Critical Review Form—Quantitative Studies. McMaster University Occupational Therapy Evidence-Based Practice Research Group. 1998. Available online: https://canchild.ca/system/tenon/assets/attachments/000/000/366/original/quantguide.pdf (accessed on 16 May 2022).
- JMP, version 14.0.0; SAS Institute Inc.: Cary, NC, USA, 1989–2019.
- SAS, v. 2022; SAS Institute Inc.: Cary, NC, USA, 1989–2019.
- Huang, Y.; Wille, M.; Benkaroun, J.; Munro, H.; Bond, A.L.; Fifield, D.A.; Robertson, G.J.; Ojkic, D.; Whitney, H.; Lang, A.S. Perpetuation and reassortment of gull influenza A viruses in Atlantic North America. Virology 2014, 456–457, 353–363. [Google Scholar] [CrossRef]
- Van Hemert, C.; Spivey, T.J.; Uher-Koch, B.D.; Atwood, T.C.; Sinnett, D.R.; Meixell, B.W.; Hupp, J.W.; Jiang, K.; Adams, L.G.; Gustine, D.D.; et al. Survey of arctic alaskan wildlife for influenza a antibodies: Limited evidence for exposure of mammals. J. Wildl. Dis. 2019, 55, 387–398. [Google Scholar] [CrossRef]
- Lee, M.M.; Jaspers, V.L.B.; Løseth, M.E.; Briels, N.; Nygård, T.; Bustnes, J.O.; Waugh, C.A. No evidence of avian influenza antibodies in two species of raptor nestlings inhabiting Norway. BMC Vet. Res. 2019, 15, 375. [Google Scholar] [CrossRef]
- Hjulsager, C.K. Surveillance for Avian Influenza Viruses in Wild Birds in Denmark and Greenland, 2007–2010. Avian Dis. 2012, 56, 992–998. [Google Scholar] [CrossRef]
- Okazaki, K.; Takada, A.; Ito, T.; Imai, M.; Takakuwa, H.; Hatta, M.; Ozaki, H.; Tanizaki, T.; Nagano, T.; Ninomiya, A.; et al. Precursor genes of future pandemic influenza viruses are perpetuated in ducks nesting in Siberia. Arch. Virol. 2000, 145, 885–893. [Google Scholar] [CrossRef]
- Ohishi, K.; Kishida, N.; Ninomiya, A.; Kida, H.; Takada, Y.; Miyazaki, N.; Boltunov, A.N.; Maruyama, T. Antibodies to human-related H3 influenza A virus in Baikal seals (Phoca sibirica) and ringed seals (Phoca hispida) in Russia. Microbiol. Immunol. 2004, 48, 905–909. [Google Scholar] [CrossRef]
- Nielsen, O.; Clavijo, A.; Boughen, J.A. Serologic evidence of influenza A infection in marine mammals of arctic Canada. J. Wildl. Dis. 2001, 37, 820–825. [Google Scholar] [CrossRef]
- Morin, C.W.; Stoner-Duncan, B.; Winker, K.; Scotch, M.; Hess, J.J.; Meschke, J.S.; Ebi, K.L.; Rabinowitz, P.M. Avian influenza virus ecology and evolution through a climatic lens. Environ. Int. 2018, 119, 241–249. [Google Scholar] [CrossRef]
- Lang, A.S.; Lebarbenchon, C.; Ramey, A.M.; Robertson, G.J.; Waldenström, J.; Wille, M. Assessing the Role of Seabirds in the Ecology of Influenza A Viruses. Avian Dis. 2016, 60, 378–386. [Google Scholar] [CrossRef]
- Liberda, E.M.R.; Charania, N.; Davey, R.; Tsuji, L.J. Avian influenza prevalence among hunter-harvested birds in a remote Canadian First Nation community. Avian influenza prevalence among hunter-harvested birds in a remote Canadian First Nation community. Rural Remote Health 2017, 17, 3864. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stallknecht, D.E.; Kienzle-Dean, C.; Davis-Fields, N.; Jennelle, C.S.; Bowman, A.S.; Nolting, J.M.; Boyce, W.M.; Crum, J.M.; Santos, J.J.S.; Brown, J.D.; et al. Limited detection of antibodies to clade 2.3.4.4 a/goose/guangdong/1/1996 lineage highly pathogenic h5 avian influenza virus in north american waterfowl. J. Wildl. Dis. 2020, 56, 47–57, 11. [Google Scholar] [CrossRef] [PubMed]
- Meltofte, H.; Huntington, H.; Barry, T. Arctic Biodiversity Assessment: Synthesis; Conservation of Arctic Flora and Fauna (CAFF). 2013. Available online: https://oaarchive.arctic-council.org/handle/11374/232 (accessed on 16 May 2022).
- Yu, H.; Xiao, X.; Domenech, J.; Lubroth, J.; Martin, V.; Slingenbergh, J. Anatidae Migration in the Western Palearctic and Spread of Highly Pathogenic Avian Influenza H5N1 Virus. Emerg. Infect. Dis. J. 2006, 12, 1650. [Google Scholar] [CrossRef]
- Girard, Y.A.; Runstadler, J.A.; Aldehoff, F.; Boyce, W. Genetic structure of Pacific Flyway avian influenza viruses is shaped by geographic location, host species, and sampling period. Virus Genes 2012, 44, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Toennessen, R.; Germundsson, A.; Jonassen, C.M.; Haugen, I.; Berg, K.; Barrett, R.T.; Rimstad, E. Virological and serological surveillance for type A influenza in the black-legged kittiwake (Rissa tridactyla). Virol. J. 2011, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Uher-Koch, B.D.; Spivey, T.J.; Van Hemert, C.R.; Schmutz, J.A.; Jiang, K.; Wan, X.-F.; Ramey, A.M. Serologic Evidence for Influenza a Virus Exposure in Three Loon Species Breeding in Alaska, USA. J. Wildl. Dis. 2019, 55, 862–867, 866. [Google Scholar] [CrossRef] [PubMed]
- Lindh, E.; Ek-Kommonen, C.; Isomursu, M.; Alasaari, J.; Vaheri, A.; Vapalahti, O.; Huovilainen, A. Genetic characterization of h13 and h16 influenza a viruses in gulls (Larus spp.) with clinically severe disease and concurrent circovirus infection. J. Wildl. Dis. 2017, 53, 561–571. [Google Scholar] [CrossRef]
- Ramey, A.M.; Uher-Koch, B.D.; Reeves, A.B.; Schmutz, J.A.; Poulson, R.L.; Stallknecht, D.E. Emperor geese (Anser canagicus) are exposed to a diversity of influenza A viruses, are infected during the non-breeding period and contribute to intercontinental viral dispersal. Transbound Emerg. Dis. 2019, 66, 1958–1970. [Google Scholar] [CrossRef]
- Wille, M.; Huang, Y.; Robertson, G.J.; Ryan, P.; Wilhelm, S.I.; Fifield, D.; Bond, A.L.; Granter, A.; Munro, H.; Buxton, R.; et al. Evaluation of Seabirds in Newfoundland and Labrador, Canada, as Hosts of Influenza A Viruses. J. Wildl. Dis. 2014, 50, 98–103. [Google Scholar] [CrossRef]
- Ip, H.S.; Flint, P.L.; Franson, J.C.; Dusek, R.J.; Derksen, D.V.; Gill, R.E.; Ely, C.R.; Pearce, J.M.; Lanctot, R.B.; Matsuoka, S.M.; et al. Prevalence of Influenza A viruses in wild migratory birds in Alaska: Patterns of variation in detection at a crossroads of intercontinental flyways. Virol. J. 2008, 5, 71. [Google Scholar] [CrossRef]
- Huang, Y.; Robertson, G.J.; Ojkic, D.; Whitney, H.; Lang, A.S. Diverse inter-continental and host lineage reassortant avian influenza A viruses in pelagic seabirds. Infect. Genet. Evol. 2014, 22, 103–111. [Google Scholar] [CrossRef]
- Hartby, C.M.; Krog, J.S.; Merkel, F.; Holm, E.; Larsen, L.E.; Hjulsager, C.K. First Characterization of Avian Influenza Viruses from Greenland 2014. Avian Dis. 2016, 60, 302–310. [Google Scholar] [CrossRef]
- Runstadler, J.A.; Happ, G.M.; Slemons, R.D.; Sheng, Z.M.; Gundlach, N.; Petrula, M.; Senne, D.; Nolting, J.; Evers, D.L.; Modrell, A.; et al. Using RRT-PCR analysis and virus isolation to determine the prevalence of avian influenza virus infections in ducks at Minto Flats State Game Refuge, Alaska, during August 2005. Arch. Virol. 2007, 152, 1901–1910. [Google Scholar] [CrossRef]
- Wahlgren, J.; Waldenström, J.; Sahlin, S.; Haemig, P.D.; Fouchier, R.A.; Osterhaus, A.D.; Pinhassi, J.; Bonnedahl, J.; Pisareva, M.; Grudinin, M.; et al. Gene segment reassortment between American and Asian lineages of avian influenza virus from waterfowl in the Beringia area. Vector Borne Zoonotic Dis. 2008, 8, 783–790. [Google Scholar] [CrossRef]
- Ramey, A.M.; Pearce, J.M.; Reeves, A.B.; Franson, J.C.; Petersen, M.R.; Ip, H.S. Evidence for limited exchange of avian influenza viruses between seaducks and dabbling ducks at Alaska Peninsula coastal lagoons. Arch. Virol. 2011, 156, 1813. [Google Scholar] [CrossRef]
- Hill, N.; Takekawa, J.; Ackerman, J.; Hobson, K.; Herring, G.; Cardona, C.; Runstadler, J.; Boyce, W. Migration strategy affects avian influenza dynamics in mallards (Anas platyrhynchos). Mol. Ecol. 2012, 21, 5986–5999. [Google Scholar] [CrossRef]
- Ramey, A.M.; Reeves, A.B.; Poulson, R.L.; Wasley, J.; Esler, D.; Stallknecht, D.E. Sampling of Sea Ducks for Influenza A Viruses in Alaska during Winter Provides Lack of Evidence for Epidemiologic Peak of Infection. J. Wildl. Dis. 2015, 51, 938–941. [Google Scholar] [CrossRef]
- Reeves, A.B.; Hall, J.S.; Poulson, R.L.; Donnelly, T.; Stallknecht, D.E.; Ramey, A.M. Influenza A virus recovery, diversity, and intercontinental exchange: A multi-year assessment of wild bird sampling at Izembek National Wildlife Refuge, Alaska. PLoS ONE 2018, 13, e0195327. [Google Scholar] [CrossRef]
- Ramey, A.M.; Pearce, J.M.; Reeves, A.B.; Poulson, R.L.; Dobson, J.; Lefferts, B.; Spragens, K.; Stallknecht, D.E. Surveillance for Eurasian-origin and intercontinental reassortant highly pathogenic influenza A viruses in Alaska, spring and summer 2015. Virol. J. 2016, 13, 55. [Google Scholar] [CrossRef]
- Ramey, A.; Reeves, A.; TeSlaa, J.; Nashold, S.; Donnelly, T.; Bahl, J.; Hall, J. Evidence for common ancestry among viruses isolated from wild birds in Beringia and highly pathogenic intercontinental reassortant H5N1 and H5N2 influenza A viruses. Infect. Genet. Evol. 2016, 40, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ramey, A.; Reeves, A.; Sonsthagen, S.; TeSlaa, J.; Nashold, S.; Donnelly, T.; Casler, B.; Hall, J. Dispersal of H9N2 influenza A viruses between East Asia and North America by wild birds. Virology 2015, 482, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, V.Y.; Susloparov, I.M.; Kolosova, N.P.; Goncharova, N.I.; Shipovalov, A.V.; Durymanov, A.G.; Ilyicheva, T.N.; Budatsirenova, L.V.; Ivanova, V.K.; Ignatyev, G.A.; et al. Influenza A(H5N8) virus isolation in Russia, 2014. Arch. Virol. 2015, 160, 2857–2860. [Google Scholar] [CrossRef] [PubMed]
- Spivey, T.J.; Lindberg, M.S.; Meixell, B.W.; Smith, K.R.; Puryear, W.B.; Davis, K.R.; Runstadler, J.A.; Stallknecht, D.E.; Ramey, A.M. Maintenance of influenza A viruses and antibody response in mallards (Anas platyrhynchos) sampled during the non-breeding season in Alaska. PLoS ONE 2017, 12, e0183505. [Google Scholar] [CrossRef]
- Parmley, E.J.; Bastien, N.; Booth, T.F.; Bowes, V.; Buck, P.A.; Breault, A.; Caswell, D.; Daoust, P.-Y.; Davies, J.C.; Elahi, S.M.; et al. Wild bird influenza survey, Canada, 2005. Emerg. Infect. Dis. 2008, 14, 84–87. [Google Scholar] [CrossRef]
- Lee, D.H.; Torchetti, M.K.; Killian, M.L.; DeLiberto, T.J.; Swayne, D.E. Reoccurrence of Avian Influenza A(H5N2) Virus Clade 2.3.4.4 in Wild Birds, Alaska, USA, 2016. Emerg. Infect. Dis. 2017, 23, 365–367. [Google Scholar] [CrossRef]
- Gaidet, N.; Leclercq, I.; Batéjat, C.; Grassin, Q.; Daufresne, T.; Manuguerra, J.-C. Avian Influenza Virus Surveillance in High Arctic Breeding Geese, Greenland. Avian Dis. 2018, 62, 237–240, 234. [Google Scholar] [CrossRef]
- Samuel, M.D.; Hall, J.S.; Brown, J.D.; Goldberg, D.R.; Ip, H.S.; Baranyuk, V.V. The dynamics of avian influenza in western Arctic snow geese: Implications for annual and migratory infection patterns. Ecol. Appl. 2015, 25, 1851–1859. [Google Scholar] [CrossRef]
- Hall, J.S.; Russell, R.E.; Franson, J.C.; Soos, C.; Dusek, R.J.; Allen, R.B.; Nashold, S.W.; TeSlaa, J.L.; Jónsson, J.E.; Ballard, J.R.; et al. Avian Influenza Ecology in North Atlantic Sea Ducks: Not All Ducks Are Created Equal. PLoS ONE 2015, 10, e0144524. [Google Scholar] [CrossRef]
- Hall, J.S.; Hallgrimsson, G.T.; Suwannanarn, K.; Sreevatsen, S.; Ip, H.S.; Magnusdottir, E.; TeSlaa, J.L.; Nashold, S.W.; Dusek, R.J. Avian influenza virus ecology in Iceland shorebirds: Intercontinental reassortment and movement. Infect. Genet. Evol. 2014, 28, 130–136. [Google Scholar] [CrossRef]
- Caliendo, V.; Lewis, N.S.; Pohlmann, A.; Baillie, S.R.; Banyard, A.C.; Beer, M.; Brown, I.H.; Fouchier, R.A.M.; Hansen, R.D.E.; Lameris, T.K.; et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. bioRxiv 2022. [Google Scholar] [CrossRef]
- Zohari, S.; Neimanis, A.; Härkönen, T.; Moraeus, C.; Valarcher, J.F. Avian influenza A(H10N7) virus involvement in mass mortality of harbour seals (Phoca vitulina) in Sweden, March through October 2014. Eurosurveillance 2014, 19, 20967. [Google Scholar] [CrossRef]
- Samelius, G.; Alisauskas, R.T. Deterring arctic fox predation: The role of parental nest attendance by lesser snow geese. Can. J. Zool. 2001, 79, 861–866. [Google Scholar] [CrossRef]
- McDonald, R.S.; Roth, J.D.; Baldwin, F.B. Goose persistence in fall strongly influences Arctic fox diet, but not reproductive success, in the southern Arctic. Polar Res. 2017, 36, 5. [Google Scholar] [CrossRef]
- Liebezeit, J.R.; Zack, S. Point Counts Underestimate the Importance of Arctic Foxes as Avian Nest Predators: Evidence from Remote Video Cameras in Arctic Alaskan Oil Fields. Arctic 2008, 61, 153–161. [Google Scholar] [CrossRef]
- Runstadler, J.A.; Puryear, W. A Brief Introduction to Influenza A Virus in Marine Mammals. In Animal Influenza Virus: Methods and Protocols; Spackman, E., Ed.; Springer: New York, NY, USA, 2020; pp. 429–450. [Google Scholar]
- Danner, G.R.; McGregor, M.W.; Zarnke, R.L.; Olsen, C.W. Serologic evidence of influenza virus infection in a ringed seal (Phoca hispida) from alaska. Mar. Mammal. Sci. 1998, 14, 380–384. [Google Scholar] [CrossRef]
- Calle, P.P.; Seagars, D.J.; McClave, C.; Senne, D.; House, C.; House, J.A. Viral and bacterial serology of free-ranging Pacific walrus. J. Wildl. Dis. 2002, 38, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Have, P.; Osterhaus, A.; Arnemo, J.; Moustgaard, A. Serological investigation of virus infections in harp seals (Phoca groenlandica) and hooded seals (Cystophora cristata). Vet. Rec. 1994, 134, 502. [Google Scholar] [CrossRef]
- Measures, L.N.; Fouchier, R.A.M. Antibodies against influenza virus types a and b in canadian seals. J. Wildl. Dis. 2021, 57, 808–819. [Google Scholar] [CrossRef]
- Goertz, C.E.C.; Reichmuth, C.; Thometz, N.M.; Ziel, H.; Boveng, P. Comparative Health Assessments of Alaskan Ice Seals. Front. Vet. Sci. 2019, 6, 4. [Google Scholar] [CrossRef]
- Ramey, A.M.; Cleveland, C.A.; Hilderbrand, G.V.; Joly, K.; Gustine, D.D.; Mangipane, B.; Leacock, W.B.; Crupi, A.P.; Hill, D.E.; Dubey, J.P.; et al. Exposure of alaska brown bears (Ursus arctos) to bacterial, viral, and parasitic agents varies spatiotemporally and may be influenced by age. J. Wildl. Dis. 2019, 55, 576–588. [Google Scholar] [CrossRef]
- Coombe, M.; Iwasawa, S.; Byers, K.A.; Prystajecky, N.; Hsiao, W.; Patrick, D.M.; Himsworth, C.G. A systematic review and narrative synthesis of the use of environmental samples for the surveillance of avian influenza viruses in wild waterbirds. J. Wildl. Dis. 2021, 57, 18. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Slingenbergh, J.; Xiao, X. Climate change and avian influenza. Rev. Sci. Tech. 2008, 27, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Causey, D.; Edwards, S.V. Ecology of Avian Influenza Virus in Birds. J. Infect. Dis. 2008, 197, S29–S33. [Google Scholar] [CrossRef]
- Ito, T.; Okazaki, K.; Kawaoka, Y.; Takada, A.; Webster, R.G.; Kida, H. Perpetuation of influenza A viruses in Alaskan waterfowl reservoirs. Arch. Virol. 1995, 140, 1163–1172. [Google Scholar] [CrossRef]
- Zhang, G.; Shoham, D.; Gilichinsky, D.; Davydov, S.; Castello, J.D.; Rogers, S.O. Evidence of Influenza A Virus RNA in Siberian Lake Ice. J. Virol. 2006, 80, 12229–12235. [Google Scholar] [CrossRef]
- Ramey, A.M.; Reeves, A.B.; Drexler, J.Z.; Ackerman, J.T.; De La Cruz, S.; Lang, A.S.; Leyson, C.; Link, P.; Prosser, D.J.; Robertson, G.J.; et al. Influenza A viruses remain infectious for more than seven months in northern wetlands of North America. Proc. Biol. Sci. 2020, 287, 20201680. [Google Scholar] [CrossRef] [PubMed]
- Ramey, A.M.; Reeves, A.B.; Lagassé, B.J.; Patil, V.; Hubbard, L.E.; Kolpin, D.W.; McCleskey, R.B.; Repert, D.A.; Stallknecht, D.E.; Poulson, R.L. Evidence for interannual persistence of infectious influenza A viruses in Alaska wetlands. Sci. Total Environ. 2022, 803, 150078. [Google Scholar] [CrossRef]
- Ramey, A.M.; Pearce, J.M.; Flint, P.L.; Ip, H.S.; Derksen, D.V.; Franson, J.C.; Petrula, M.J.; Scotton, B.D.; Sowl, K.M.; Wege, M.L.; et al. Intercontinental reassortment and genomic variation of low pathogenic avian influenza viruses isolated from northern pintails (Anas acuta) in Alaska: Examining the evidence through space and time. Virology 2010, 401, 179–189. [Google Scholar] [CrossRef]
- Lang, A.S.; Kelly, A.; Runstadler, J.A. Prevalence and diversity of avian influenza viruses in environmental reservoirs. J. Gen. Virol. 2008, 89, 509–519. [Google Scholar] [CrossRef]
- Marchenko, V.; Goncharova, N.; Susloparov, I.; Kolosova, N.; Gudymo, A.; Svyatchenko, S.; Danilenko, A.; Durymanov, A.; Gavrilova, E.; Maksyutov, R.; et al. Isolation and characterization of H5Nx highly pathogenic avian influenza viruses of clade 2.3.4.4 in Russia. Virology 2018, 525, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.; Ramey, A.; Poulson, R.; Stallknecht, D. The Genome Sequence of an H6N5 Influenza A Virus Strain Isolated from a Northern Pintail (Anas acuta) Sampled in Alaska, USA, Shares High Identity with That of a South Korean Wild Bird Strain. Microbiol. Resour. Announc. 2020, 9, e00639-20. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, G.A. The spring migration pattern of arctic birds in southwest Iceland, as recorded by radar. Ibis 1993, 135, 166–176. [Google Scholar] [CrossRef]
- Mine, J.; Uchida, Y.; Sharshov, K.; Sobolev, I.; Shestopalov, A.; Saito, T. Phylogeographic evidence for the inter- and intracontinental dissemination of avian influenza viruses via migration flyways. PLoS ONE 2019, 14, e0218506. [Google Scholar] [CrossRef]
- Pearce, J.M.; Reeves, A.B.; Ramey, A.M.; Hupp, J.W.; Ip, H.S.; Bertram, M.; Petrula, M.J.; Scotton, B.D.; Trust, K.A.; Meixell, B.W.; et al. Interspecific exchange of avian influenza virus genes in Alaska: The influence of trans-hemispheric migratory tendency and breeding ground sympatry. Mol. Ecol. 2011, 20, 1015–1025. [Google Scholar] [CrossRef]
- Claes, F.; Morzaria, S.P.; Donis, R.O. Emergence and dissemination of clade 2.3.4.4 H5Nx influenza viruses-how is the Asian HPAI H5 lineage maintained. Curr. Opin. Virol. 2016, 16, 158–163. [Google Scholar] [CrossRef]
- Antigua, K.J.C.; Choi, W.-S.; Baek, Y.H.; Song, M.-S. The Emergence and Decennary Distribution of Clade 2.3.4.4 HPAI H5Nx. Microorganisms 2019, 7, 156. [Google Scholar] [CrossRef]
- Yamaji, R.; Saad, M.D.; Davis, C.T.; Swayne, D.E.; Wang, D.; Wong, F.Y.K.; McCauley, J.W.; Peiris, J.S.M.; Webby, R.J.; Fouchier, R.A.M.; et al. Pandemic potential of highly pathogenic avian influenza clade 2.3.4.4 A(H5) viruses. Rev. Med. Virol. 2020, 30, e2099. [Google Scholar] [CrossRef]
- Reeves, A.B.; Ip, H.S. H9N2 Influenza A Virus Isolated from a Greater White-Fronted Wild Goose (Anser albifrons) in Alaska Has a Mutation in the PB2 Gene, Which Is Associated with Pathogenicity in Human Pandemic 2009 H1N1. Genome Announc. 2016, 4, e00869-16. [Google Scholar] [CrossRef]
- Rijks, J.M.; Hesselink, H.; Lollinga, P.; Wesselman, R.; Prins, P.; Weesendorp, E.; Engelsma, M.; Heutink, R.; Harders, F.; Kik, M.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus in Wild Red Foxes, the Netherlands, 2021. Emerg. Infect. Dis. 2021, 27, 2960–2962. [Google Scholar] [CrossRef]
- Postel, A.; King, J.; Kaiser, F.K.; Kennedy, J.; Lombardo, M.S.; Reineking, W.; de le Roi, M.; Harder, T.; Pohlmann, A.; Gerlach, T.; et al. Infections with highly pathogenic avian influenza A virus (HPAIV) H5N8 in harbor seals at the German North Sea coast, 2021. Emerg. Microbes Infect. 2022, 11, 725–729. [Google Scholar] [CrossRef]
- Shin, D.-L.; Siebert, U.; Lakemeyer, J.; Grilo, M.; Pawliczka, I.; Wu, N.-H.; Valentin-Weigand, P.; Haas, L.; Herrler, G. Highly Pathogenic Avian Influenza A(H5N8) Virus in Gray Seals, Baltic Sea. Emerg. Infect. Dis. 2019, 25, 2295–2298. [Google Scholar] [CrossRef]
- Ramey, A.M.; Pearce, J.M.; Ely, C.R.; Guy, L.M.; Irons, D.B.; Derksen, D.V.; Ip, H.S. Transmission and reassortment of avian influenza viruses at the Asian-North American interface. Virology 2010, 406, 352–359. [Google Scholar] [CrossRef]
- Hinzman, L.D.; Bettez, N.D.; Bolton, W.R.; Chapin, F.S.; Dyurgerov, M.B.; Fastie, C.L.; Griffith, B.; Hollister, R.D.; Hope, A.; Huntington, H.P.; et al. Evidence and Implications of Recent Climate Change in Northern Alaska and Other Arctic Regions. Clim. Change 2005, 72, 251–298. [Google Scholar] [CrossRef]
- Yon, L.; Duff, J.P.; Ågren, E.O.; Erdélyi, K.; Ferroglio, E.; Godfroid, J.; Hars, J.; Hestvik, G.; Horton, D.; Kuiken, T.; et al. Recent changes in infectious diseases in european wildlife. J. Wildl. Dis. 2019, 55, 3–43. [Google Scholar] [CrossRef]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef]
- Napp, S.; Majó, N.; Sánchez-Gónzalez, R.; Vergara-Alert, J. Emergence and spread of highly pathogenic avian influenza A(H5N8) in Europe in 2016–2017. Transbound. Emerg. Dis. 2018, 65, 1217–1226. [Google Scholar] [CrossRef]
- Hoye, B.J.; Munster, V.J.; Nishiura, H.; Klaassen, M.; Fouchier, R.A.M. Surveillance of wild birds for avian influenza virus. Emerg. Infect. Dis. 2010, 16, 1827–1834. [Google Scholar] [CrossRef]
- Hill, N.; Ma, E.; Meixell, B.; Lindberg, M.; Boyce, W.; Runstadler, J. Transmission of influenza reflects seasonality of wild birds across the annual cycle. Ecol. Lett. 2016, 19, 915–925. [Google Scholar] [CrossRef]
- Peterson, A.T.; Benz, B.W.; Papeş, M. Highly pathogenic H5N1 avian influenza: Entry pathways into North America via bird migration. PLoS ONE 2007, 2, e261. [Google Scholar] [CrossRef]
- Ramey, A.M.; Reeves, A.B. Ecology of Influenza A Viruses in Wild Birds and Wetlands of Alaska. Avian Dis. 2020, 64, 109–122, 114. [Google Scholar] [CrossRef]

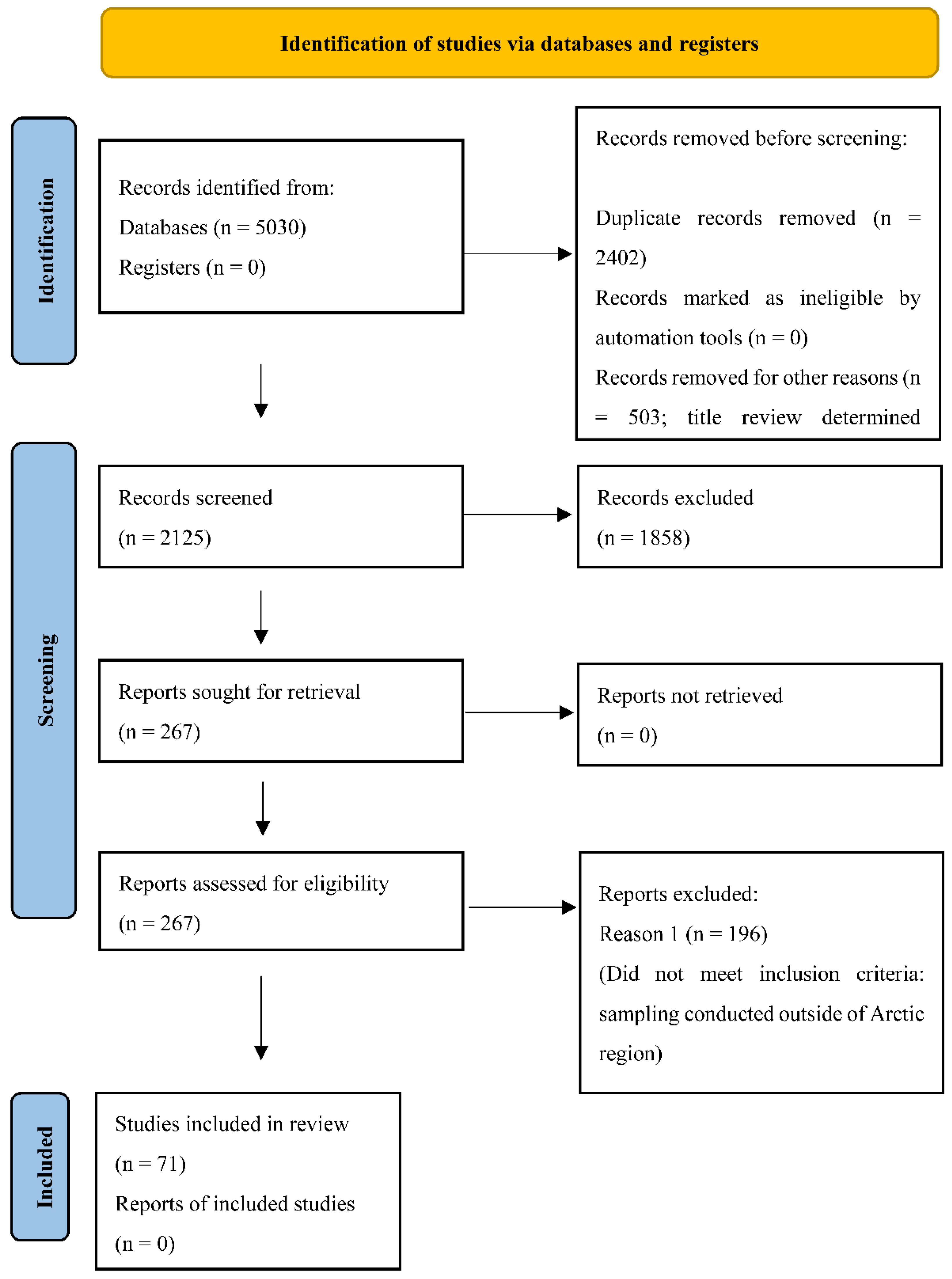
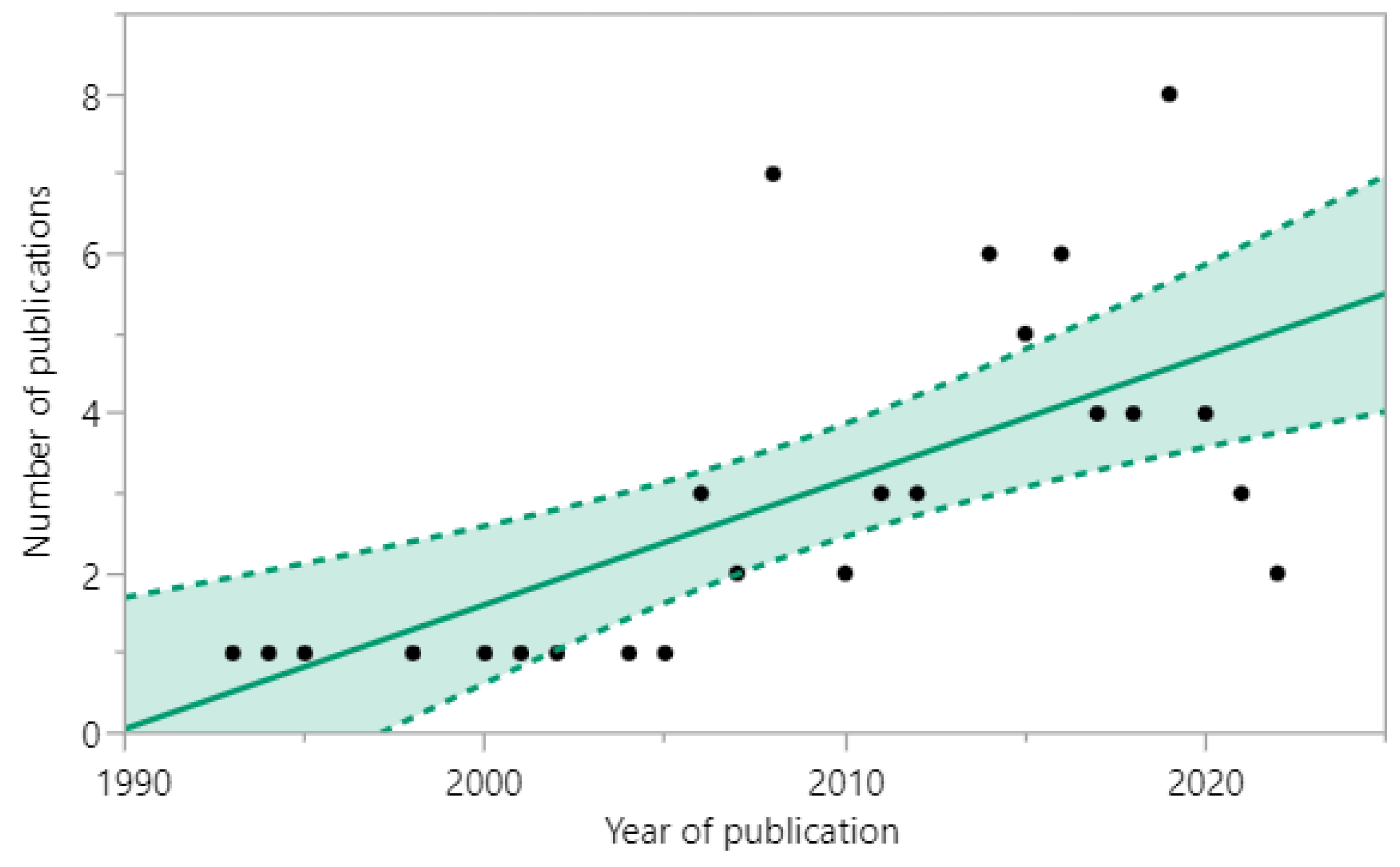

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gass, J.D., Jr.; Kellogg, H.K.; Hill, N.J.; Puryear, W.B.; Nutter, F.B.; Runstadler, J.A. Epidemiology and Ecology of Influenza A Viruses among Wildlife in the Arctic. Viruses 2022, 14, 1531. https://doi.org/10.3390/v14071531
Gass JD Jr., Kellogg HK, Hill NJ, Puryear WB, Nutter FB, Runstadler JA. Epidemiology and Ecology of Influenza A Viruses among Wildlife in the Arctic. Viruses. 2022; 14(7):1531. https://doi.org/10.3390/v14071531
Chicago/Turabian StyleGass, Jonathon D., Jr., Hunter K. Kellogg, Nichola J. Hill, Wendy B. Puryear, Felicia B. Nutter, and Jonathan A. Runstadler. 2022. "Epidemiology and Ecology of Influenza A Viruses among Wildlife in the Arctic" Viruses 14, no. 7: 1531. https://doi.org/10.3390/v14071531
APA StyleGass, J. D., Jr., Kellogg, H. K., Hill, N. J., Puryear, W. B., Nutter, F. B., & Runstadler, J. A. (2022). Epidemiology and Ecology of Influenza A Viruses among Wildlife in the Arctic. Viruses, 14(7), 1531. https://doi.org/10.3390/v14071531






