Multiple Receptors Involved in Invasion and Neuropathogenicity of Canine Distemper Virus: A Review
Abstract
1. Introduction
2. Cell-to-Cell Fusion Mediated by CDV H/F Protein Complex
3. Receptors That Enable CDV Entry and Spread
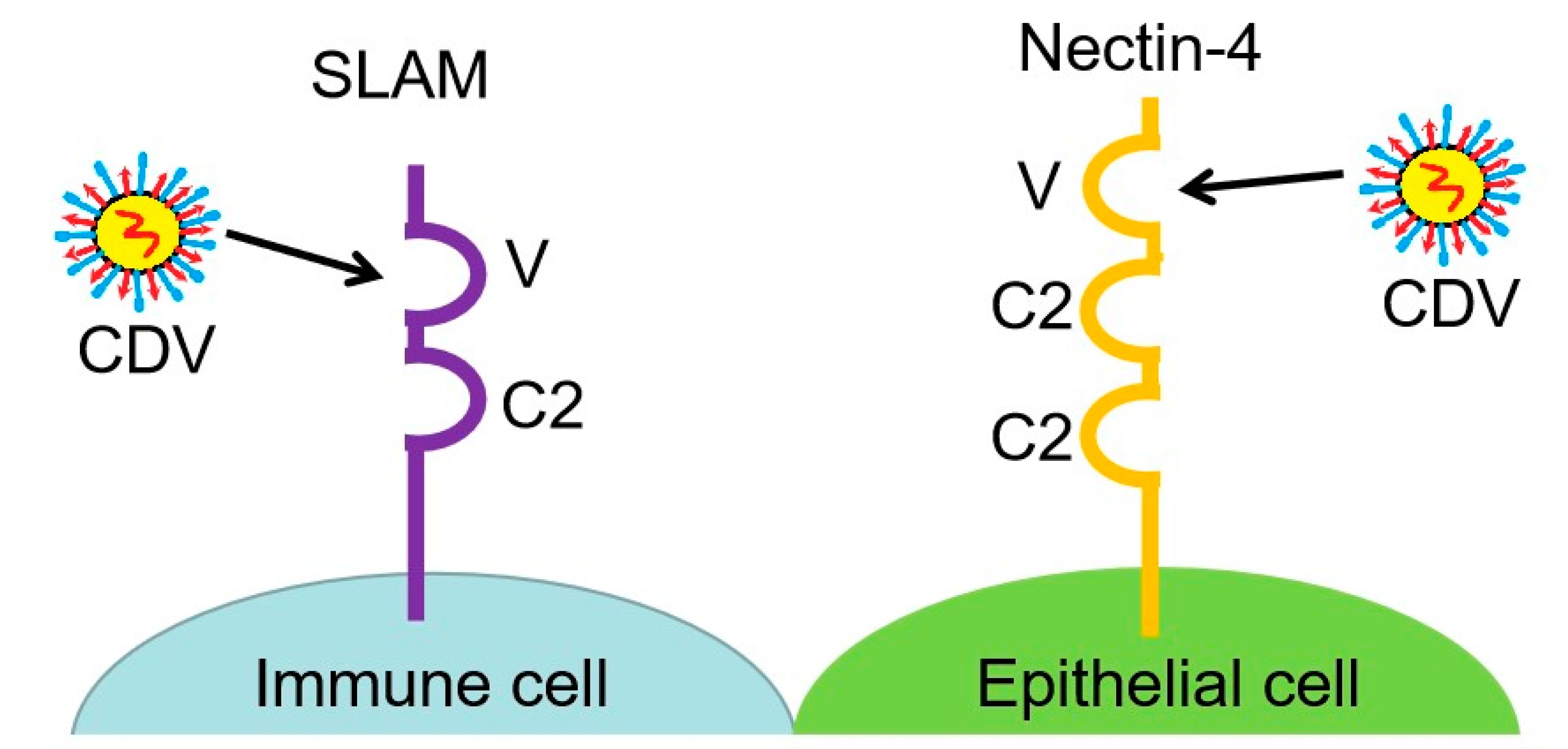
3.1. SLAM, a Lymphocyte Receptor Involved in Host Immune Suppression
3.2. Nectin-4, an Epithelial Cell Receptor Associated with CDV Neurovirulence
3.3. GliaR, an Unknown Glial Cell Receptor
4. CDV Invasion and Pathogenicity in the CNS
5. Anti-CDV Immune Response in the CNS
6. Neurotropic Viruses in Humans and Animals and Their Receptors
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez-Gutierrez, M.; Ruiz-Saenz, J. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, A.; Ye, H.; Shi, Y.; Hu, Z.; Zeng, L. Natural infection with canine distemper virus in hand-feeding Rhesus monkeys in China. Vet. Microbiol. 2010, 141, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Nagata, N.; Ami, Y.; Seki, F.; Suzaki, Y.; Iwata-Yoshikawa, N.; Suzuki, T.; Fukushi, S.; Mizutani, T.; Yoshikawa, T.; et al. Lethal canine distemper virus outbreak in cynomolgus monkeys in Japan in 2008. J. Virol. 2013, 87, 1105–1114. [Google Scholar] [CrossRef]
- Munoz-Alia, M.A.; Russell, S.J. Probing morbillivirus antisera neutralization using functional chimerism between measles virus and canine distemper virus envelope glycoproteins. Viruses 2019, 11, 688. [Google Scholar] [CrossRef] [PubMed]
- Duque-Valencia, J.; Sarute, N.; Olarte-Castillo, X.A.; Ruiz-Saenz, J. Evolution and interspecies transmission of canine distemper virus-an outlook of the diverse evolutionary landscapes of a multi-host virus. Viruses 2019, 11, 582. [Google Scholar] [CrossRef]
- da Fontoura Budaszewski, R.; Streck, A.F.; Nunes Weber, M.; Maboni Siqueira, F.; Muniz Guedes, R.L.; Wageck Canal, C. Influence of vaccine strains on the evolution of canine distemper virus. Infect. Genet. Evol. 2016, 41, 262–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.; Zhang, L.; Zhao, J.; Ji, P.; Li, Y.; Liu, B.; Zhang, J.; Zhao, Q.; Sun, Y.; et al. Development of a double monoclonal antibody-based sandwich enzyme-linked immunosorbent assay for detecting canine distemper virus. Appl. Microbiol. Biotechnol. 2020, 104, 10725–10735. [Google Scholar] [CrossRef]
- Svitek, N.; Gerhauser, I.; Goncalves, C.; Grabski, E.; Doring, M.; Kalinke, U.; Anderson, D.E.; Cattaneo, R.; von Messling, V. Morbillivirus control of the interferon response: Relevance of STAT2 and mda5 but not STAT1 for canine distemper virus virulence in ferrets. J. Virol. 2014, 88, 2941–2950. [Google Scholar] [CrossRef]
- von Messling, V.; Svitek, N.; Cattaneo, R. Receptor (SLAM [CD150]) recognition and the V protein sustain swift lymphocyte-based invasion of mucosal tissue and lymphatic organs by a morbillivirus. J. Virol. 2006, 80, 6084–6092. [Google Scholar] [CrossRef]
- Smith, E.C.; Popa, A.; Chang, A.; Masante, C.; Dutch, R.E. Viral entry mechanisms: The increasing diversity of paramyxovirus entry. FEBS J. 2009, 276, 7217–7227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Hu, B.; Gong, C.; Shi, N.; Liu, M.; Yan, X.; Bai, X.; Zhao, J. Mink SLAM V-region V74I substitutions contribute to the formation of syncytia induced by canine distemper virus. Front. Vet. Sci. 2020, 7, 570283. [Google Scholar] [CrossRef] [PubMed]
- Pratakpiriya, W.; Seki, F.; Otsuki, N.; Sakai, K.; Fukuhara, H.; Katamoto, H.; Hirai, T.; Maenaka, K.; Techangamsuwan, S.; Lan, N.T.; et al. Nectin4 is an epithelial cell receptor for canine distemper virus and involved in neurovirulence. J. Virol. 2012, 86, 10207–10210. [Google Scholar] [CrossRef] [PubMed]
- von Messling, V.; Milosevic, D.; Cattaneo, R. Tropism illuminated: Lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc. Natl. Acad. Sci. USA 2004, 101, 14216–14221. [Google Scholar] [CrossRef]
- Rendon-Marin, S.; da Fontoura Budaszewski, R.; Canal, C.W.; Ruiz-Saenz, J. Tropism and molecular pathogenesis of canine distemper virus. Virol. J. 2019, 16, 30. [Google Scholar] [CrossRef]
- Sawatsky, B.; Cattaneo, R.; von Messling, V. Canine distemper virus spread and transmission to naive ferrets: Selective pressure on signaling lymphocyte activation molecule-dependent entry. J. Virol. 2018, 92, e00669-18. [Google Scholar] [CrossRef]
- Sawatsky, B.; Wong, X.X.; Hinkelmann, S.; Cattaneo, R.; von Messling, V. Canine distemper virus epithelial cell infection is required for clinical disease but not for immunosuppression. J. Virol. 2012, 86, 3658–3666. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, Y.; Chen, J.; Zheng, J.; Sun, D. Viral pathogenesis, recombinant vaccines, and oncolytic virotherapy: Applications of the canine distemper virus reverse genetics system. Viruses 2020, 12, 339. [Google Scholar] [CrossRef]
- Klemens, J.; Ciurkiewicz, M.; Chludzinski, E.; Iseringhausen, M.; Klotz, D.; Pfankuche, V.M.; Ulrich, R.; Herder, V.; Puff, C.; Baumgartner, W.; et al. Neurotoxic potential of reactive astrocytes in canine distemper demyelinating leukoencephalitis. Sci. Rep. 2019, 9, 11689. [Google Scholar] [CrossRef]
- Amude, A.M.; Alfieri, A.A.; Alfieri, A.F. Clinicopathological findings in dogs with distemper encephalomyelitis presented without characteristic signs of the disease. Res. Vet. Sci. 2007, 82, 416–422. [Google Scholar] [CrossRef]
- Lempp, C.; Spitzbarth, I.; Puff, C.; Cana, A.; Kegler, K.; Techangamsuwan, S.; Baumgartner, W.; Seehusen, F. New aspects of the pathogenesis of canine distemper leukoencephalitis. Viruses 2014, 6, 2571–2601. [Google Scholar] [CrossRef]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgartner, W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunopathol. 2009, 127, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.A.; Bastien-Hamel, L.E.; von Messling, V. Acute canine distemper encephalitis is associated with rapid neuronal loss and local immune activation. J. Gen. Virol. 2010, 91 Pt 4, 980–989. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, N.; Sun, Y.; Martella, V.; Nikolin, V.; Zhu, C.; Zhang, H.; Hu, B.; Bai, X.; Yan, X. Pathogenesis of canine distemper virus in experimentally infected raccoon dogs, foxes, and minks. Antivir. Res. 2015, 122, 1–11. [Google Scholar] [CrossRef]
- Summers, B.A.; Greisen, H.A.; Appel, M.J. Canine distemper encephalomyelitis: Variation with virus strain. J. Comp. Pathol. 1984, 94, 65–75. [Google Scholar] [CrossRef]
- Wenzlow, N.; Plattet, P.; Wittek, R.; Zurbriggen, A.; Grone, A. Immunohistochemical demonstration of the putative canine distemper virus receptor CD150 in dogs with and without distemper. Vet. Pathol. 2007, 44, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Khosravi, M.; Avila, M.; Ader-Ebert, N.; Bringolf, F.; Zurbriggen, A.; Vandevelde, M.; Plattet, P. SLAM- and nectin-4-independent noncytolytic spread of canine distemper virus in astrocytes. J. Virol. 2015, 89, 5724–5733. [Google Scholar] [CrossRef] [PubMed]
- Pratakpiriya, W.; Ping Teh, A.P.; Radtanakatikanon, A.; Pirarat, N.; Thi Lan, N.; Takeda, M.; Techangamsuwan, S.; Yamaguchi, R. Expression of canine distemper virus receptor nectin-4 in the central nervous system of dogs. Sci. Rep. 2017, 7, 349. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Fluehmann, G.; Zurbriggen, A.; Vandevelde, M.; Plattet, P. Canine distemper virus persistence in demyelinating encephalitis by swift intracellular cell-to-cell spread in astrocytes is controlled by the viral attachment protein. Acta Neuropathol. 2010, 119, 617–630. [Google Scholar] [CrossRef]
- Munoz-Alia, M.A.; Muller, C.P.; Russell, S.J. Hemagglutinin-specific neutralization of subacute sclerosing panencephalitis viruses. PLoS ONE 2018, 13, e0192245. [Google Scholar] [CrossRef]
- Tatsuo, H.; Ono, N.; Tanaka, K.; Yanagi, Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature 2000, 406, 893–897. [Google Scholar] [CrossRef]
- Delpeut, S.; Noyce, R.S.; Richardson, C.D. The tumor-associated marker, PVRL4 (nectin-4), is the epithelial receptor for morbilliviruses. Viruses 2014, 6, 2268–2286. [Google Scholar] [CrossRef]
- Tahara, M.; Takeda, M.; Seki, F.; Hashiguchi, T.; Yanagi, Y. Multiple amino acid substitutions in hemagglutinin are necessary for wild-type measles virus to acquire the ability to use receptor CD46 efficiently. J. Virol. 2007, 81, 2564–2572. [Google Scholar] [CrossRef]
- Jiang, Y.; Qin, Y.; Chen, M. Host-pathogen interactions in measles virus replication and anti-viral immunity. Viruses 2016, 8, 308. [Google Scholar] [CrossRef]
- Ader-Ebert, N.; Khosravi, M.; Herren, M.; Avila, M.; Alves, L.; Bringolf, F.; Orvell, C.; Langedijk, J.P.; Zurbriggen, A.; Plemper, R.K.; et al. Sequential conformational changes in the morbillivirus attachment protein initiate the membrane fusion process. PLoS Pathog. 2015, 11, e1004880. [Google Scholar] [CrossRef]
- Avila, M.; Alves, L.; Khosravi, M.; Ader-Ebert, N.; Origgi, F.; Schneider-Schaulies, J.; Zurbriggen, A.; Plemper, R.K.; Plattet, P. Molecular determinants defining the triggering range of prefusion F complexes of canine distemper virus. J. Virol. 2014, 88, 2951–2966. [Google Scholar] [CrossRef]
- Khosravi, M.; Bringolf, F.; Rothlisberger, S.; Bieringer, M.; Schneider-Schaulies, J.; Zurbriggen, A.; Origgi, F.; Plattet, P. Canine distemper virus fusion activation: Critical role of residue E123 of CD150/SLAM. J. Virol. 2016, 90, 1622–1637. [Google Scholar] [CrossRef]
- Brindley, M.A.; Takeda, M.; Plattet, P.; Plemper, R.K. Triggering the measles virus membrane fusion machinery. Proc. Natl. Acad. Sci. USA 2012, 109, E3018–E3027. [Google Scholar] [CrossRef]
- Lin, L.T.; Richardson, C.D. The host cell receptors for measles virus and their interaction with the viral hemagglutinin (h) protein. Viruses 2016, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Burrell, C.E.; Anchor, C.; Ahmed, N.; Landolfi, J.; Jarosinski, K.W.; Terio, K.A. Characterization and comparison of SLAM/CD150 in free-ranging coyotes, raccoons, and skunks in illinois for elucidation of canine distemper virus disease. Pathogens 2020, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Yoneda, M.; Honda, T.; Kai, C. Morbillivirus receptors and tropism: Multiple pathways for infection. Front. Microbiol. 2012, 3, 75. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Ikeda, W.; Ogita, H.; Rikitake, Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu. Rev. Cell Dev. Biol. 2008, 24, 309–342. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Miyoshi, J.; Ikeda, W.; Ogita, H. Nectins and nectin-like molecules: Roles in contact inhibition of cell movement and proliferation. Nat. Rev. Mol. Cell Biol. 2008, 9, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Kurita, S.; Ogita, H.; Takai, Y. Cooperative role of nectin-nectin and nectin-afadin interactions in formation of nectin-based cell-cell adhesion. J. Biol. Chem. 2011, 286, 36297–36303. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Gromeier, M.; Herndon, J.E., 2nd; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Prajapati, M.; Alfred, N.; Dou, Y.; Yin, X.; Prajapati, R.; Li, Y.; Zhang, Z. Host cellular receptors for the peste des petits ruminant virus. Viruses 2019, 11, 729. [Google Scholar] [CrossRef]
- Takano, A.; Ishikawa, N.; Nishino, R.; Masuda, K.; Yasui, W.; Inai, K.; Nishimura, H.; Ito, H.; Nakayama, H.; Miyagi, Y.; et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009, 69, 6694–6703. [Google Scholar] [CrossRef]
- Delpeut, S.; Noyce, R.S.; Richardson, C.D. The V domain of dog PVRL4 (nectin-4) mediates canine distemper virus entry and virus cell-to-cell spread. Virology 2014, 454, 109–117. [Google Scholar] [CrossRef]
- Navaratnarajah, C.K.; Generous, A.R.; Yousaf, I.; Cattaneo, R. Receptor-mediated cell entry of paramyxoviruses: Mechanisms, and consequences for tropism and pathogenesis. J. Biol. Chem. 2020, 295, 2771–2786. [Google Scholar] [CrossRef]
- Takenaka, A.; Sato, H.; Ikeda, F.; Yoneda, M.; Kai, C. Infectious progression of canine distemper virus from circulating cerebrospinal fluid into the central nervous system. J. Virol. 2016, 90, 9285–9292. [Google Scholar] [CrossRef]
- Ludlow, M.; Nguyen, D.T.; Silin, D.; Lyubomska, O.; de Vries, R.D.; von Messling, V.; McQuaid, S.; De Swart, R.L.; Duprex, W.P. Recombinant canine distemper virus strain Snyder Hill expressing green or red fluorescent proteins causes meningoencephalitis in the ferret. J. Virol. 2012, 86, 7508–7519. [Google Scholar] [CrossRef]
- Fujita, K.; Miura, R.; Yoneda, M.; Shimizu, F.; Sato, H.; Muto, Y.; Endo, Y.; Tsukiyama-Kohara, K.; Kai, C. Host range and receptor utilization of canine distemper virus analyzed by recombinant viruses: Involvement of heparin-like molecule in CDV infection. Virology 2007, 359, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Singethan, K.; Muller, N.; Schubert, S.; Luttge, D.; Krementsov, D.N.; Khurana, S.R.; Krohne, G.; Schneider-Schaulies, S.; Thali, M.; Schneider-Schaulies, J. CD9 clustering and formation of microvilli zippers between contacting cells regulates virus-induced cell fusion. Traffic 2008, 9, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Schmid, E.; Zurbriggen, A.; Gassen, U.; Rima, B.; ter Meulen, V.; Schneider-Schaulies, J. Antibodies to CD9, a tetraspan transmembrane protein, inhibit canine distemper virus-induced cell-cell fusion but not virus-cell fusion. J. Virol. 2000, 74, 7554–7561. [Google Scholar] [CrossRef] [PubMed]
- Singethan, K.; Topfstedt, E.; Schubert, S.; Duprex, W.P.; Rima, B.K.; Schneider-Schaulies, J. CD9-dependent regulation of Canine distemper virus-induced cell-cell fusion segregates with the extracellular domain of the haemagglutinin. J. Gen. Virol. 2006, 87 Pt 6, 1635–1642. [Google Scholar] [CrossRef]
- Chen, J.; Liang, X.; Chen, P.F. Canine distemper virus utilizes different receptors to infect chicken embryo fibroblasts and vero cells. Virol. Sin. 2011, 26, 139–145. [Google Scholar] [CrossRef]
- Mateo, M.; Navaratnarajah, C.K.; Syed, S.; Cattaneo, R. The measles virus hemagglutinin beta-propeller head beta4-beta5 hydrophobic groove governs functional interactions with nectin-4 and CD46 but not those with the signaling lymphocytic activation molecule. J. Virol. 2013, 87, 9208–9216. [Google Scholar] [CrossRef]
- Dorig, R.E.; Marcil, A.; Richardson, C.D. CD46, a primate-specific receptor for measles virus. Trends Microbiol. 1994, 2, 312–318. [Google Scholar] [CrossRef]
- Munoz-Alia, M.A.; Nace, R.A.; Tischer, A.; Zhang, L.; Bah, E.S.; Auton, M.; Russell, S.J. MeV-Stealth: A CD46-specific oncolytic measles virus resistant to neutralization by measles-immune human serum. PLoS Pathog. 2021, 17, e1009283. [Google Scholar] [CrossRef]
- Generous, A.R.; Harrison, O.J.; Troyanovsky, R.B.; Mateo, M.; Navaratnarajah, C.K.; Donohue, R.C.; Pfaller, C.K.; Alekhina, O.; Sergeeva, A.P.; Indra, I.; et al. Trans-endocytosis elicited by nectins transfers cytoplasmic cargo, including infectious material, between cells. J. Cell Sci. 2019, 132, jcs235507. [Google Scholar] [CrossRef]
- Ludlow, M.; Lemon, K.; de Vries, R.D.; McQuaid, S.; Millar, E.L.; van Amerongen, G.; Yuksel, S.; Verburgh, R.J.; Osterhaus, A.D.; de Swart, R.L.; et al. Measles virus infection of epithelial cells in the macaque upper respiratory tract is mediated by subepithelial immune cells. J. Virol. 2013, 87, 4033–4042. [Google Scholar] [CrossRef]
- Sato, Y.; Watanabe, S.; Fukuda, Y.; Hashiguchi, T.; Yanagi, Y.; Ohno, S. Cell-to-cell measles virus spread between human neurons is dependent on hemagglutinin and hyperfusogenic fusion protein. J. Virol. 2018, 92, e02166-17. [Google Scholar] [CrossRef] [PubMed]
- Shirogane, Y.; Hashiguchi, T.; Yanagi, Y. Weak cis and trans interactions of the hemagglutinin with receptors trigger fusion proteins of neuropathogenic measles virus isolates. J. Virol. 2020, 94, e01727-19. [Google Scholar] [CrossRef]
- Mathieu, C.; Ferren, M.; Jurgens, E.; Dumont, C.; Rybkina, K.; Harder, O.; Stelitano, D.; Madeddu, S.; Sanna, G.; Schwartz, D.; et al. Measles Virus Bearing Measles Inclusion Body Encephalitis-Derived Fusion Protein Is Pathogenic after Infection via the Respiratory Route. J. Virol. 2019, 93, e01862-18. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.D.; Ludlow, M.; de Jong, A.; Rennick, L.J.; Verburgh, R.J.; van Amerongen, G.; van Riel, D.; van Run, P.; Herfst, S.; Kuiken, T.; et al. Delineating morbillivirus entry, dissemination and airborne transmission by studying in vivo competition of multicolor canine distemper viruses in ferrets. PLoS Pathog. 2017, 13, e1006371. [Google Scholar] [CrossRef] [PubMed]
- Attig, F.; Spitzbarth, I.; Kalkuhl, A.; Deschl, U.; Puff, C.; Baumgartner, W.; Ulrich, R. Reactive oxygen species are key mediators of demyelination in canine distemper leukoencephalitis but not in theiler’s murine encephalomyelitis. Int. J. Mol. Sci. 2019, 20, 3217. [Google Scholar] [CrossRef] [PubMed]
- Rubin, S.; Eckhaus, M.; Rennick, L.J.; Bamford, C.G.; Duprex, W.P. Molecular biology, pathogenesis and pathology of mumps virus. J. Pathol. 2015, 235, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Bonami, F.; Rudd, P.A.; von Messling, V. Disease duration determines canine distemper virus neurovirulence. J. Virol. 2007, 81, 12066–12070. [Google Scholar] [CrossRef]
- Rudd, P.A.; Cattaneo, R.; von Messling, V. Canine distemper virus uses both the anterograde and the hematogenous pathway for neuroinvasion. J. Virol. 2006, 80, 9361–9370. [Google Scholar] [CrossRef]
- Miller, K.D.; Matullo, C.M.; Milora, K.A.; Williams, R.M.; O’Regan, K.J.; Rall, G.F. Immune-mediated control of a dormant neurotropic RNA virus infection. J. Virol. 2019, 93, e00241-19. [Google Scholar] [CrossRef]
- Poelaert, K.C.K.; Williams, R.M.; Matullo, C.M.; Rall, G.F. Noncanonical transmission of a measles virus vaccine strain from neurons to astrocytes. mBio 2021, 12, e00288-21. [Google Scholar] [CrossRef]
- Makhortova, N.R.; Askovich, P.; Patterson, C.E.; Gechman, L.A.; Gerard, N.P.; Rall, G.F. Neurokinin-1 enables measles virus trans-synaptic spread in neurons. Virology 2007, 362, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Schobesberger, M.; Zurbriggen, A.; Doherr, M.G.; Weissenbock, H.; Vandevelde, M.; Lassmann, H.; Griot, C. Demyelination precedes oligodendrocyte loss in canine distemper virus-induced encephalitis. Acta Neuropathol. 2002, 103, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Techangamsuwan, S.; Haas, L.; Rohn, K.; Baumgartner, W.; Wewetzer, K. Distinct cell tropism of canine distemper virus strains to adult olfactory ensheathing cells and Schwann cells in vitro. Virus Res. 2009, 144, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Miele, J.A.; Krakowka, S. Antibody responses to virion polypeptides in gnotobiotic dogs infected with canine distemper virus. Infect. Immun. 1983, 41, 869–871. [Google Scholar] [CrossRef]
- Bieringer, M.; Han, J.W.; Kendl, S.; Khosravi, M.; Plattet, P.; Schneider-Schaulies, J. Experimental adaptation of wild-type canine distemper virus (CDV) to the human entry receptor CD150. PLoS ONE 2013, 8, e57488. [Google Scholar] [CrossRef]
- Plattet, P.; Rivals, J.P.; Zuber, B.; Brunner, J.M.; Zurbriggen, A.; Wittek, R. The fusion protein of wild-type canine distemper virus is a major determinant of persistent infection. Virology 2005, 337, 312–326. [Google Scholar] [CrossRef][Green Version]
- Seehusen, F.; Al-Azreg, S.A.; Raddatz, B.B.; Haist, V.; Puff, C.; Spitzbarth, I.; Ulrich, R.; Baumgartner, W. Accumulation of extracellular matrix in advanced lesions of canine distemper demyelinating encephalitis. PLoS ONE 2016, 11, e0159752. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, S.; Li, P.; Yue, F.; Zhang, Y.; Pan, B.; Liu, X. Apoptotic investigation of brain tissue cells in dogs naturally infected by canine distemper virus. Virol. J. 2021, 18, 165. [Google Scholar] [CrossRef]
- Cavanaugh, S.E.; Holmgren, A.M.; Rall, G.F. Homeostatic interferon expression in neurons is sufficient for early control of viral infection. J. Neuroimmunol. 2015, 279, 11–19. [Google Scholar] [CrossRef]
- Patterson, C.E.; Lawrence, D.M.; Echols, L.A.; Rall, G.F. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J. Virol. 2002, 76, 4497–4506. [Google Scholar] [CrossRef]
- Waisman, A.; Johann, L. Antigen-presenting cell diversity for T cell reactivation in central nervous system autoimmunity. J. Mol. Med. 2018, 96, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Markus, S.; Failing, K.; Baumgartner, W. Increased expression of pro-inflammatory cytokines and lack of up-regulation of anti-inflammatory cytokines in early distemper CNS lesions. J. Neuroimmunol. 2002, 125, 30–41. [Google Scholar] [CrossRef]
- Arii, J.; Goto, H.; Suenaga, T.; Oyama, M.; Kozuka-Hata, H.; Imai, T.; Minowa, A.; Akashi, H.; Arase, H.; Kawaoka, Y.; et al. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature 2010, 467, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Vilela, M.C.; Lima, G.K.; Rodrigues, D.H.; Lacerda-Queiroz, N.; Pedroso, V.S.; Miranda, A.S.; Rachid, M.A.; Kroon, E.G.; Campos, M.A.; Teixeira, M.M.; et al. Absence of CCR5 increases neutrophil recruitment in severe herpetic encephalitis. BMC Neurosci. 2013, 14, 19. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Son, Y. Astrocytes stimulate microglial proliferation and M2 polarization in vitro through crosstalk between astrocytes and microglia. Int. J. Mol. Sci. 2021, 22, 8800. [Google Scholar] [CrossRef]
- Welsch, J.C.; Charvet, B.; Dussurgey, S.; Allatif, O.; Aurine, N.; Horvat, B.; Gerlier, D.; Mathieu, C. Type I interferon receptor signaling drives selective permissiveness of astrocytes and microglia to measles virus during brain infection. J. Virol. 2019, 93, e00618-19. [Google Scholar] [CrossRef]
- Al-Obaidi, M.M.J.; Bahadoran, A.; Wang, S.M.; Manikam, R.; Raju, C.S.; Sekaran, S.D. Disruption of the blood brain barrier is vital property of neurotropic viral infection of the central nervous system. Acta Virol. 2018, 62, 16–27. [Google Scholar] [CrossRef]
- Ghosh, D.; Basu, A. Japanese encephalitis—A pathological and clinical perspective. PLoS Negl. Trop. Dis. 2009, 3, e437. [Google Scholar] [CrossRef]
- Nazmi, A.; Dutta, K.; Basu, A. Antiviral and neuroprotective role of octaguanidinium dendrimer-conjugated morpholino oligomers in Japanese encephalitis. PLoS Negl. Trop. Dis. 2010, 4, e892. [Google Scholar] [CrossRef]
- Lepoutre, V.; Jain, P.; Quann, K.; Wigdahl, B.; Khan, Z.K. Role of resident CNS cell populations in HTLV-1-associated neuroinflammatory disease. Front. Biosci. 2009, 14, 1152–1168. [Google Scholar] [CrossRef]
- Madhu, B.P.; Singh, K.P.; Saminathan, M.; Singh, R.; Shivasharanappa, N.; Sharma, A.K.; Malik, Y.S.; Dhama, K.; Manjunatha, V. Role of nitric oxide in the regulation of immune responses during rabies virus infection in mice. Virusdisease 2016, 27, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Nazmi, A.; Dutta, K.; Basu, A. Neurons under viral attack: Victims or warriors? Neurochem. Int. 2010, 56, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, H.A.; Amancherla, K.; Johnson, R.W. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J. Neurosci. 2012, 32, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Liu, R.S.; Wu, M.F.; Lin, Y.L.; Chen, S.Y.; Tan, D.T.; Chou, T.Y.; Tsai, I.S.; Li, L.; Hsieh, S.L. CLEC5A regulates Japanese encephalitis virus-induced neuroinflammation and lethality. PLoS Pathog. 2012, 8, e1002655. [Google Scholar] [CrossRef]
- Singh, R.; Singh, K.P.; Cherian, S.; Saminathan, M.; Kapoor, S.; Manjunatha Reddy, G.B.; Panda, S.; Dhama, K. Rabies-epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: A comprehensive review. Vet. Q. 2017, 37, 212–251. [Google Scholar] [CrossRef]
- Detje, C.N.; Meyer, T.; Schmidt, H.; Kreuz, D.; Rose, J.K.; Bechmann, I.; Prinz, M.; Kalinke, U. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J. Immunol. 2009, 182, 2297–2304. [Google Scholar] [CrossRef]
- Mori, I.; Nishiyama, Y.; Yokochi, T.; Kimura, Y. Olfactory transmission of neurotropic viruses. J. Neurovirol. 2005, 11, 129–137. [Google Scholar] [CrossRef]
- van Riel, D.; Verdijk, R.; Kuiken, T. The olfactory nerve: A shortcut for influenza and other viral diseases into the central nervous system. J. Pathol. 2015, 235, 277–287. [Google Scholar] [CrossRef]
- Albertini, A.A.; Ruigrok, R.W.; Blondel, D. Rabies virus transcription and replication. Adv. Virus Res. 2011, 79, 1–22. [Google Scholar]
- Young, V.A.; Rall, G.F. Making it to the synapse: Measles virus spread in and among neurons. Measles 2009, 330, 3–30. [Google Scholar]
- Davis, B.M.; Fensterl, V.; Lawrence, T.M.; Hudacek, A.W.; Sen, G.C.; Schnell, M.J. Ifit2 is a restriction factor in rabies virus pathogenicity. J. Virol. 2017, 91, e00889-17. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.A.; Edgar, C.R.; Prevost, J.; Trothen, S.M.; Lurie, A.; Mumby, M.J.; Galbraith, A.; Kirchhoff, F.; Haeryfar, S.M.M.; Finzi, A.; et al. The HIV-1 accessory protein Nef increases surface expression of the checkpoint receptor Tim-3 in infected CD4(+) T cells. J. Biol. Chem. 2021, 297, 101042. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; He, J.J. Cell-cell contact viral transfer contributes to HIV infection and persistence in astrocytes. J. Neurovirol. 2015, 21, 66–80. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, S.M.; Rotta, I.; Vidal, L.R.R.; Dos Santos, J.S.; Nath, A.; Johnson, K.; Letendre, S.; Ellis, R.J.; Group, H.I.V.N.R.C. HIV-1C and HIV-1B Tat protein polymorphism in Southern Brazil. J. Neurovirol. 2021, 27, 126–136. [Google Scholar] [CrossRef]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef]
- Filatov, A.; Sharma, P.; Hindi, F.; Espinosa, P.S. Neurological complications of coronavirus disease (COVID-19): Encephalopathy. Cureus 2020, 12, e7352. [Google Scholar] [CrossRef]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2020, 218, e20202135. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Y.; Zhou, Z.; Zhang, Z.; Xiao, X.; Liu, Z.; Chen, A.; Dong, X.; Tian, F.; Chen, S.; et al. Genome-wide CRISPR activation screen identifies candidate receptors for SARS-CoV-2 entry. Sci. China Life Sci. 2021, 65, 701–717. [Google Scholar] [CrossRef]
- Ranganathan, S.; Noyes, N.C.; Migliorini, M.; Winkles, J.A.; Battey, F.D.; Hyman, B.T.; Smith, E.; Yepes, M.; Mikhailenko, I.; Strickland, D.K. LRAD3, a novel low-density lipoprotein receptor family member that modulates amyloid precursor protein trafficking. J. Neurosci. 2011, 31, 10836–10846. [Google Scholar] [CrossRef]
- Carvalho, O.V.; Botelho, C.V.; Ferreira, C.G.; Scherer, P.O.; Soares-Martins, J.A.; Almeida, M.R.; Silva Junior, A. Immunopathogenic and neurological mechanisms of canine distemper virus. Adv. Virol. 2012, 2012, 163860. [Google Scholar] [CrossRef]
- Watanabe, S.; Ohno, S.; Shirogane, Y.; Suzuki, S.O.; Koga, R.; Yanagi, Y. Measles virus mutants possessing the fusion protein with enhanced fusion activity spread effectively in neuronal cells, but not in other cells, without causing strong cytopathology. J. Virol. 2015, 89, 2710–2717. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Shirogane, Y.; Suzuki, S.O.; Ikegame, S.; Koga, R.; Yanagi, Y. Mutant fusion proteins with enhanced fusion activity promote measles virus spread in human neuronal cells and brains of suckling hamsters. J. Virol. 2013, 87, 2648–2659. [Google Scholar] [CrossRef] [PubMed]


| Infection Phase | Clinical Manifestations | Neurological Symptoms | Pathology of Non-Neuronal Tissues | Pathology of Nervous Distemper |
|---|---|---|---|---|
| Acute phase | Cutaneous rash; Serous nasal and ocular discharge; Conjunctivitis; Anorexia | Myoclonus; Nystagmus; Ataxia; Tetraparesis or plegia | Cytoplasmic and intranuclear inclusion bodies; Mucopurulent rhinitis; Interstitial pneumonia; Necrotizing bronchiolitis; Catarrhal enteritis; Hyper- and parakeratosis | Neuronal necrosis; Intranuclear inclusion bodies in neurons and astrocytes; Focal vacuolization of the white matter; Mild gliosis |
| Late phase | Subtle early clinical signs | Persistent myoclonus; CNS disturbances | Suppurative bronchopneumonia | Progressive perivascular mononuclear infiltrations; Astrogliosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Ren, Y. Multiple Receptors Involved in Invasion and Neuropathogenicity of Canine Distemper Virus: A Review. Viruses 2022, 14, 1520. https://doi.org/10.3390/v14071520
Zhao J, Ren Y. Multiple Receptors Involved in Invasion and Neuropathogenicity of Canine Distemper Virus: A Review. Viruses. 2022; 14(7):1520. https://doi.org/10.3390/v14071520
Chicago/Turabian StyleZhao, Jianjun, and Yanrong Ren. 2022. "Multiple Receptors Involved in Invasion and Neuropathogenicity of Canine Distemper Virus: A Review" Viruses 14, no. 7: 1520. https://doi.org/10.3390/v14071520
APA StyleZhao, J., & Ren, Y. (2022). Multiple Receptors Involved in Invasion and Neuropathogenicity of Canine Distemper Virus: A Review. Viruses, 14(7), 1520. https://doi.org/10.3390/v14071520






