Development of a Monoclonal Antibody to Pig CD69 Reveals Early Activation of T Cells in Pig after PRRSV and ASFV Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Cells, and Virus Strains
2.2. Porcine CD69 Polypeptide Immunization and Cell Fusion
2.3. Hybridoma Screening, Ascites Production, Purification, and Fluorescence Labeling of CD69 mAb
2.4. Cloning and Construction of pcDNA3.1-poCD69 Plasmid
2.5. Transfection and Indirect Immunofluorescence Assay
2.6. Western Blotting
2.7. Dot-ELISA
2.8. Infection Experiments with PRRSV and ASFV
2.9. Single-Cell Suspension Preparation and Culture
2.10. Flow Cytometry
2.11. Statistical Analysis
3. Results
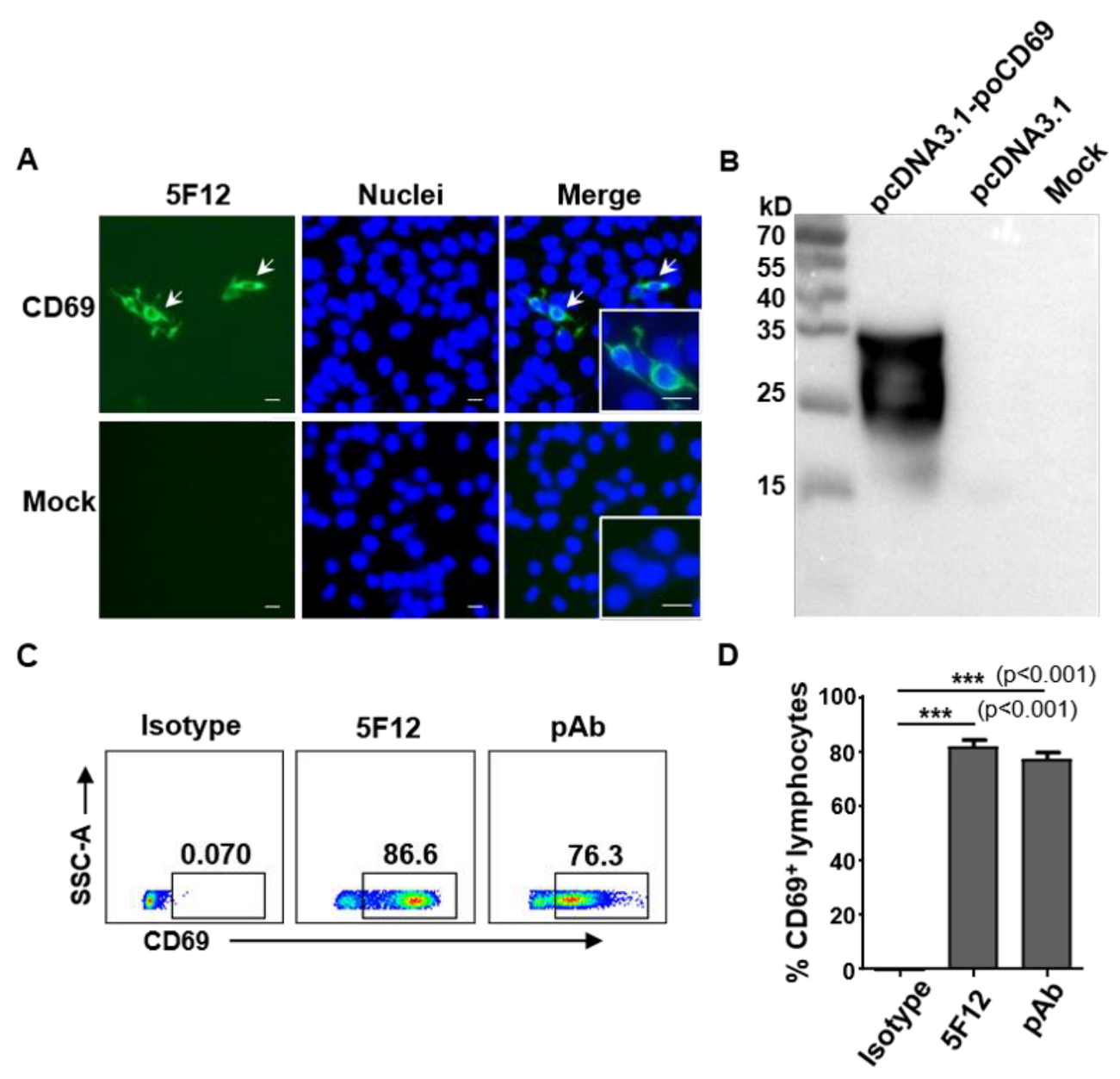
3.1. Generation and Characterization of Monoclonal Antibodies against Pig CD69
3.2. Anti-Pig CD69 mAb 5F12 Recognizes Epitope Residues 147–161 of Pig CD69
3.3. mAb 5F12 Shows Weak or No Cross-Reactivity with PBMCs from Bovine, Mice, and Chickens
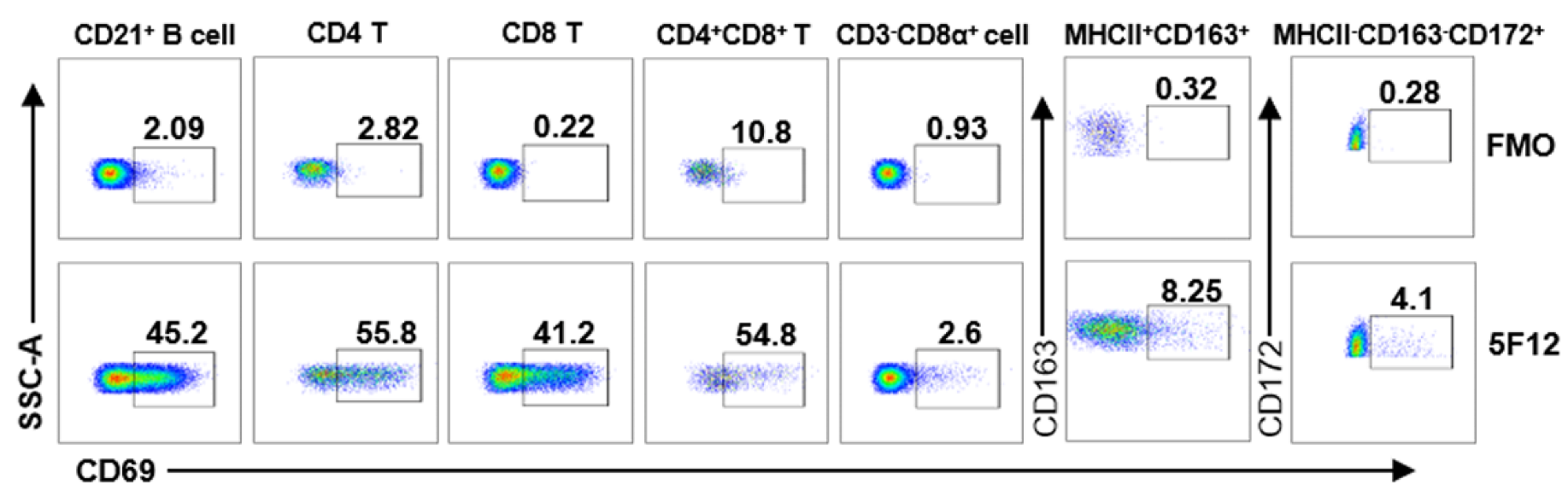
3.4. Detection of CD69 Expression on Different Leukocyte Subsets by Flow Cytometry
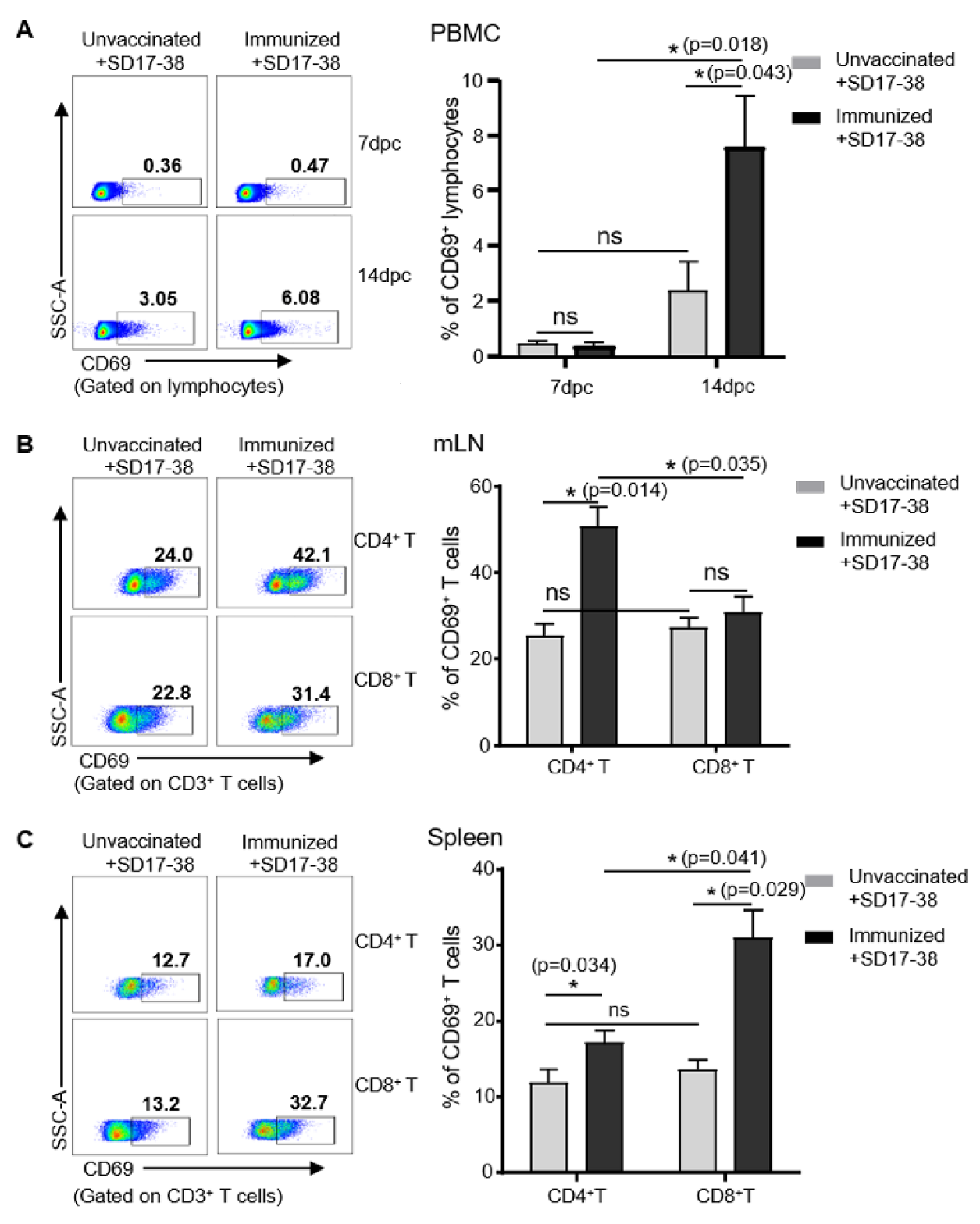
3.5. Detection of CD69 Expression on Lymphocytes in Pigs after Challenge with PRRSV
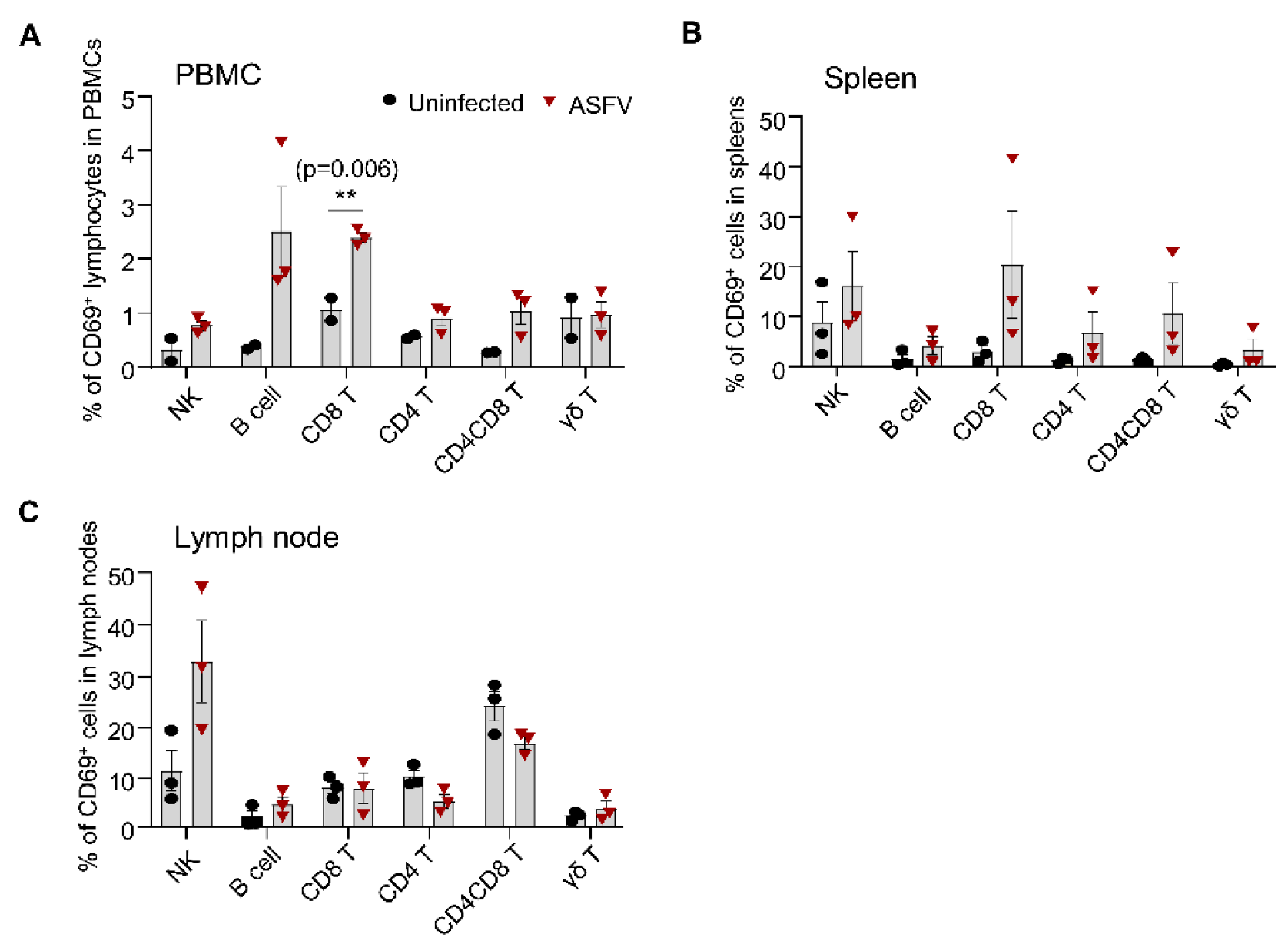
3.6. Detection of CD69 Expression on Different Lymphocyte Subsets in Pigs after ASFV Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamann, J.; Fiebig, H.; Strauss, M. Expression cloning of the early activation antigen CD69, a type II integral membrane protein with a C-type lectin domain. J. Immunol. 1993, 150, 4920–4927. [Google Scholar] [PubMed]
- Hara, T.; Jung, L.K.; Bjorndahl, J.M.; Fu, S.M. Human T cell activation. III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA 1) by 12-o-tetradecanoyl phorbol-13-acetate, mitogens, and antigens. J. Exp. Med. 1986, 164, 1988–2005. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L.; Buck, D.W.; Rhodes, L.; Ding, A.; Evans, E.; Barney, C.; Phillips, J.H. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J. Exp. Med. 1988, 167, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Mateos, P.; Cebrian, M.; Acevedo, A.; Lopez-Botet, M.; De Landazuri, M.O.; Sanchez-Madrid, F. Expression of a gp33/27,000 MW activation inducer molecule (AIM) on human lymphoid tissues. Induction of cell proliferation on thymocytes and B lymphocytes by anti-AIM antibodies. Immunology 1989, 68, 72–79. [Google Scholar]
- Werfel, T.; Boeker, M.; Kapp, A. Rapid expression of the CD69 antigen on T cells and natural killer cells upon antigenic stimulation of peripheral blood mononuclear cell suspensions. Allergy 1997, 52, 465–469. [Google Scholar] [CrossRef]
- Clausen, J.; Vergeiner, B.; Enk, M.; Petzer, A.L.; Gastl, G.; Gunsilius, E. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T cells. Immunobiology 2003, 207, 85–93. [Google Scholar] [CrossRef]
- Hayashi, Y.; Okutani, M.; Ogawa, S.; Tsukahara, T.; Inoue, R. Generation of anti-porcine CD69 monoclonal antibodies and their usefulness to evaluate early activation of cellular immunity by flow cytometric analysis. Anim. Sci. J. 2018, 89, 825–832. [Google Scholar] [CrossRef]
- Bodewes, R.; Fraaij, P.L.; Geelhoed-Mieras, M.M.; van Baalen, C.A.; Tiddens, H.A.; van Rossum, A.M.; van der Klis, F.R.; Fouchier, R.A.; Osterhaus, A.D.; Rimmelzwaan, G.F. Annual vaccination against influenza virus hampers development of virus-specific CD8(+) T cell immunity in children. J. Virol. 2011, 85, 11995–12000. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, S.E.; Reimer, J.M.; Karlsson, K.H.; Lilja, L.; Bengtsson, K.L.; Stertman, L. Immune enhancing properties of the novel Matrix-M adjuvant leads to potentiated immune responses to an influenza vaccine in mice. Vaccine 2013, 31, 1725–1733. [Google Scholar] [CrossRef]
- Shang, S.; Kats, D.; Cao, L.; Morgun, E.; Velluto, D.; He, Y.; Xu, Q.; Wang, C.R.; Scott, E.A. Induction of Mycobacterium Tuberculosis Lipid-Specific T Cell Responses by Pulmonary Delivery of Mycolic Acid-Loaded Polymeric Micellar Nanocarriers. Front. Immunol. 2018, 9, 2709. [Google Scholar] [CrossRef] [Green Version]
- Firstova, V.V.; Mokrievich, A.N.; Pavlov, V.M.; Gorbatov, A.A.; Kombarova, T.I.; Biketov, S.F.; Dyatlov, I.A. Immunological markers that correlate with protection immunity against tularemia infection. Adv. Exp. Med. Biol. 2014, 808, 15–23. [Google Scholar] [PubMed]
- Reiley, W.W.; Calayag, M.D.; Wittmer, S.T.; Huntington, J.L.; Pearl, J.E.; Fountain, J.J.; Martino, C.A.; Roberts, A.D.; Cooper, A.M.; Winslow, G.M.; et al. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc. Natl. Acad. Sci. USA 2008, 105, 10961–10966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, T.N.; Mooster, J.L.; Kilgore, A.M.; Osborn, J.F.; Nolz, J.C. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J. Exp. Med. 2016, 213, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Yim, D.; Sotiriadis, J.; Kim, K.S.; Shin, S.C.; Jie, H.B.; Rothschild, M.F.; Kim, Y.B. Molecular cloning, expression pattern and chromosomal mapping of pig CD69. Immunogenetics 2002, 54, 276–281. [Google Scholar] [CrossRef]
- Dawson, H.D.; Lunney, J.K. Porcine cluster of differentiation (CD) markers 2018 update. Res. Vet. Sci. 2018, 118, 199–246. [Google Scholar] [CrossRef]
- Feng, W.H.; Tompkins, M.B.; Xu, J.S.; Zhang, H.X.; McCaw, M.B. Analysis of constitutive cytokine expression by pigs infected in-utero with porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2003, 94, 35–45. [Google Scholar] [CrossRef]
- Klinge, K.L.; Vaughn, E.M.; Roof, M.B.; Bautista, E.M.; Murtaugh, M.P. Age-dependent resistance to Porcine reproductive and respiratory syndrome virus replication in swine. Virol. J. 2009, 6, 177. [Google Scholar] [CrossRef] [Green Version]
- Loving, C.L.; Osorio, F.A.; Murtaugh, M.P.; Zuckermann, F.A. Innate and adaptive immunity against Porcine Reproductive and Respiratory Syndrome Virus. Vet. Immunol. Immunopathol. 2015, 167, 1–14. [Google Scholar] [CrossRef]
- Bocard, L.V.; Kick, A.R.; Hug, C.; Lischer, H.E.L.; Kaser, T.; Summerfield, A. Systems Immunology Analyses Following Porcine Respiratory and Reproductive Syndrome Virus Infection and Vaccination. Front. Immunol. 2021, 12, 779747. [Google Scholar] [CrossRef]
- Meier, W.A.; Galeota, J.; Osorio, F.A.; Husmann, R.J.; Schnitzlein, W.M.; Zuckermann, F.A. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology 2003, 309, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Correas, I.; Osorio, F.A.; Steffen, D.; Pattnaik, A.K.; Vu, H.L.X. Cross reactivity of immune responses to porcine reproductive and respiratory syndrome virus infection. Vaccine 2017, 35, 782–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, L.; Canelli, E.; De Angelis, E.; Catella, A.; Ferrarini, G.; Ogno, G.; Bonati, L.; Nardini, R.; Borghetti, P.; Martelli, P. A highly pathogenic porcine reproductive and respiratory syndrome virus type 1 (PRRSV-1) strongly modulates cellular innate and adaptive immune subsets upon experimental infection. Vet. Microbiol. 2018, 216, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Canelli, E.; Catella, A.; Borghetti, P.; Ferrari, L.; Ogno, G.; De Angelis, E.; Bonilauri, P.; Guazzetti, S.; Nardini, R.; Martelli, P. Efficacy of a modified-live virus vaccine in pigs experimentally infected with a highly pathogenic porcine reproductive and respiratory syndrome virus type 1 (HP-PRRSV-1). Vet. Microbiol. 2018, 226, 89–96. [Google Scholar] [CrossRef]
- Cao, Q.M.; Tian, D.; Heffron, C.L.; Subramaniam, S.; Opriessnig, T.; Foss, D.L.; Calvert, J.G.; Meng, X.J. Cytotoxic T lymphocyte epitopes identified from a contemporary strain of porcine reproductive and respiratory syndrome virus enhance CD4+ CD8+ T, CD8+ T, and gammadelta T cell responses. Virology 2019, 538, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, H.; Pedrera, M.; Frossard, J.P.; Biffar, L.; Hammer, S.E.; Kvisgaard, L.K.; Larsen, L.E.; Stewart, G.R.; Somavarapu, S.; Steinbach, F.; et al. The Non-structural Protein 5 and Matrix Protein Are Antigenic Targets of T Cell Immunity to Genotype 1 Porcine Reproductive and Respiratory Syndrome Viruses. Front. Immunol. 2016, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Huhr, J.; Schafer, A.; Schwaiger, T.; Zani, L.; Sehl, J.; Mettenleiter, T.C.; Blome, S.; Blohm, U. Impaired T cell responses in domestic pigs and wild boar upon infection with a highly virulent African swine fever virus strain. Transbound. Emerg. Dis. 2020, 67, 3016–3032. [Google Scholar] [CrossRef]
- Schafer, A.; Zani, L.; Pikalo, J.; Huhr, J.; Sehl, J.; Mettenleiter, T.C.; Breithaupt, A.; Blome, S.; Blohm, U. T cell responses in domestic pigs and wild boar upon infection with the moderately virulent African swine fever virus strain ‘Estonia2014’. Transbound. Emerg. Dis. 2021, 68, 2733–2749. [Google Scholar] [CrossRef]
- Chen, N.; Li, S.; Tian, Y.; Li, X.; Li, S.; Li, J.; Qiu, M.; Sun, Z.; Xiao, Y.; Yan, X.; et al. Chimeric HP-PRRSV2 containing an ORF2-6 consensus sequence induces antibodies with broadly neutralizing activity and confers cross protection against virulent NADC30-like isolate. Vet. Res. 2021, 52, 74. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, R.; Zhang, X.; Li, F.; Wang, J.; Zhang, J.; Liu, X.; Wang, L.; Zhang, J.; Wu, X.; et al. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg. Microbes. Infect. 2019, 8, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; He, X.; Zhang, X.; Zhang, X.; Liu, L.; Guan, Y.; Zhang, Y.; Bu, Z. Genome sequences derived from pig and dried blood pig feed samples provide important insights into the transmission of African swine fever virus in China in 2018. Emerg. Microbes. Infect. 2019, 8, 303–306. [Google Scholar] [CrossRef]
- Hao, X.; Li, S.; Chen, L.; Dong, M.; Wang, J.; Hu, J.; Gu, M.; Wang, X.; Hu, S.; Peng, D.; et al. Establishing a Multicolor Flow Cytometry to Characterize Cellular Immune Response in Chickens Following H7N9 Avian Influenza Virus Infection. Viruses 2020, 12, 1396. [Google Scholar] [CrossRef] [PubMed]
- Maisonnasse, P.; Bouguyon, E.; Piton, G.; Ezquerra, A.; Urien, C.; Deloizy, C.; Bourge, M.; Leplat, J.J.; Simon, G.; Chevalier, C.; et al. The respiratory DC/macrophage network at steady-state and upon influenza infection in the swine biomedical model. Mucosal. Immunol. 2016, 9, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Craston, R.; Koh, M.; Mc Dermott, A.; Ray, N.; Prentice, H.G.; Lowdell, M.W. Temporal dynamics of CD69 expression on lymphoid cells. J. Immunol. Methods 1997, 209, 37–45. [Google Scholar] [CrossRef]
- Talker, S.C.; Koinig, H.C.; Stadler, M.; Graage, R.; Klingler, E.; Ladinig, A.; Mair, K.H.; Hammer, S.E.; Weissenbock, H.; Durrwald, R.; et al. Magnitude and kinetics of multifunctional CD4+ and CD8beta+ T cells in pigs infected with swine influenza A virus. Vet. Res. 2015, 46, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, S.F.; Levin, S.D.; Johnson, L.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Baker, E.; Sutherland, G.R.; Feldhaus, A.L.; Ramsdell, F. The mouse CD69 gene. Structure, expression, and mapping to the NK gene complex. J. Immunol. 1994, 152, 1228–1236. [Google Scholar]
- Bautista, E.M.; Molitor, T.W. Cell-mediated immunity to porcine reproductive and respiratory syndrome virus in swine. Viral Immunol. 1997, 10, 83–94. [Google Scholar] [CrossRef]
- Xiao, Z.; Batista, L.; Dee, S.; Halbur, P.; Murtaugh, M.P. The level of virus-specific T cell and macrophage recruitment in porcine reproductive and respiratory syndrome virus infection in pigs is independent of virus load. J. Virol. 2004, 78, 5923–5933. [Google Scholar] [CrossRef] [Green Version]
- Bankovich, A.J.; Shiow, L.R.; Cyster, J.G. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J. Biol. Chem. 2010, 285, 22328–22337. [Google Scholar] [CrossRef] [Green Version]
- Martini, V.; Edmans, M.; Gubbins, S.; Jayaraman, S.; Paudyal, B.; Morgan, S.; McNee, A.; Morin, T.; Rijal, P.; Gerner, W.; et al. Spatial, temporal and molecular dynamics of swine influenza virus-specific CD8 tissue resident memory T cells. Mucosal. Immunol. 2022, 15, 428–442. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Uldrich, A.P.; McCluskey, J.; Rossjohn, J.; Moody, D.B. The burgeoning family of unconventional T cells. Nat. Immunol. 2015, 16, 1114–1123. [Google Scholar] [CrossRef]
- Piriou-Guzylack, L.; Salmon, H. Membrane markers of the immune cells in swine: An update. Vet. Res. 2008, 39, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiromizu, C.M.; Jancic, C.C. gammadelta T Lymphocytes: An Effector Cell in Autoimmunity and Infection. Front. Immunol. 2018, 9, 2389. [Google Scholar] [CrossRef] [PubMed]


| Peptides | Sequence | Start | End | Length * |
|---|---|---|---|---|
| CD69-peptide | CLKQHVGRAEHWIGLKNEDGQTWKWSNGKE | 133 | 161 | 30 |
| Pep 1 | CLKQHVGRAEHWIGLK | 133 | 147 | 16 |
| Pep 2 | CAEHWIGLKNEDGQTW | 140 | 154 | 16 |
| Pep 3 | CKNEDGQTWKWSNGKE | 147 | 161 | 16 |
| Antigen | Clone | Isotype | Conjugate | Source |
|---|---|---|---|---|
| CD3 | BB23-8E6-8C8 | Mouse IgG2a | PerCP-cy5.5 | BD |
| CD4 | 74-12-4 | Mouse IgG2b | PE-Cy™7 | BD Pharmingen |
| CD8α | 76-2-11 | Mouse IgG2a | biotin | Southernbiotech |
| CD21 | BB6-11C9.6 | Mouse IgG1 | AlexaFluor®488 | Southernbiotech |
| γδTCR | MAC320 | Rat PVG IgG2a | PE | BD |
| CD69 | 5F12 | Mouse IgG1 | Dylight®755 | in-house |
| CD163 | 2A10/11 | Mouse IgG1 | RPE | Bio-rad |
| CD172a | 74-22-15 | Mouse IgG1 | FITC | Southernbiotech |
| SLA II DR | 2E9/13 | Mouse IgG2b | APC | Bio-rad |
| Mouse IgG | Poly4053 | Goat polyclonal IgG | AlexaFluor®488 | Biolegend |
| Streptavidin | — | — | BV 510™ | Biolegend |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Hao, Y.; Dong, M.; Li, S.; Wang, D.; Jiang, F.; Wang, Q.; Hao, X.; Yang, Y.; Chen, N.; et al. Development of a Monoclonal Antibody to Pig CD69 Reveals Early Activation of T Cells in Pig after PRRSV and ASFV Infection. Viruses 2022, 14, 1343. https://doi.org/10.3390/v14061343
Tian Y, Hao Y, Dong M, Li S, Wang D, Jiang F, Wang Q, Hao X, Yang Y, Chen N, et al. Development of a Monoclonal Antibody to Pig CD69 Reveals Early Activation of T Cells in Pig after PRRSV and ASFV Infection. Viruses. 2022; 14(6):1343. https://doi.org/10.3390/v14061343
Chicago/Turabian StyleTian, Yunfei, Yuxin Hao, Maoli Dong, Shuai Li, Dongyue Wang, Fei Jiang, Qingqing Wang, Xiaoli Hao, Yi Yang, Nanhua Chen, and et al. 2022. "Development of a Monoclonal Antibody to Pig CD69 Reveals Early Activation of T Cells in Pig after PRRSV and ASFV Infection" Viruses 14, no. 6: 1343. https://doi.org/10.3390/v14061343
APA StyleTian, Y., Hao, Y., Dong, M., Li, S., Wang, D., Jiang, F., Wang, Q., Hao, X., Yang, Y., Chen, N., Zhu, J., Guo, J., Wu, J., Shang, S., & Zhou, J. (2022). Development of a Monoclonal Antibody to Pig CD69 Reveals Early Activation of T Cells in Pig after PRRSV and ASFV Infection. Viruses, 14(6), 1343. https://doi.org/10.3390/v14061343







