Novel Bacteriophage Specific against Staphylococcus epidermidis and with Antibiofilm Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Bacteriophage
2.2. Morphological Analysis by Transmission Electron Microscopy
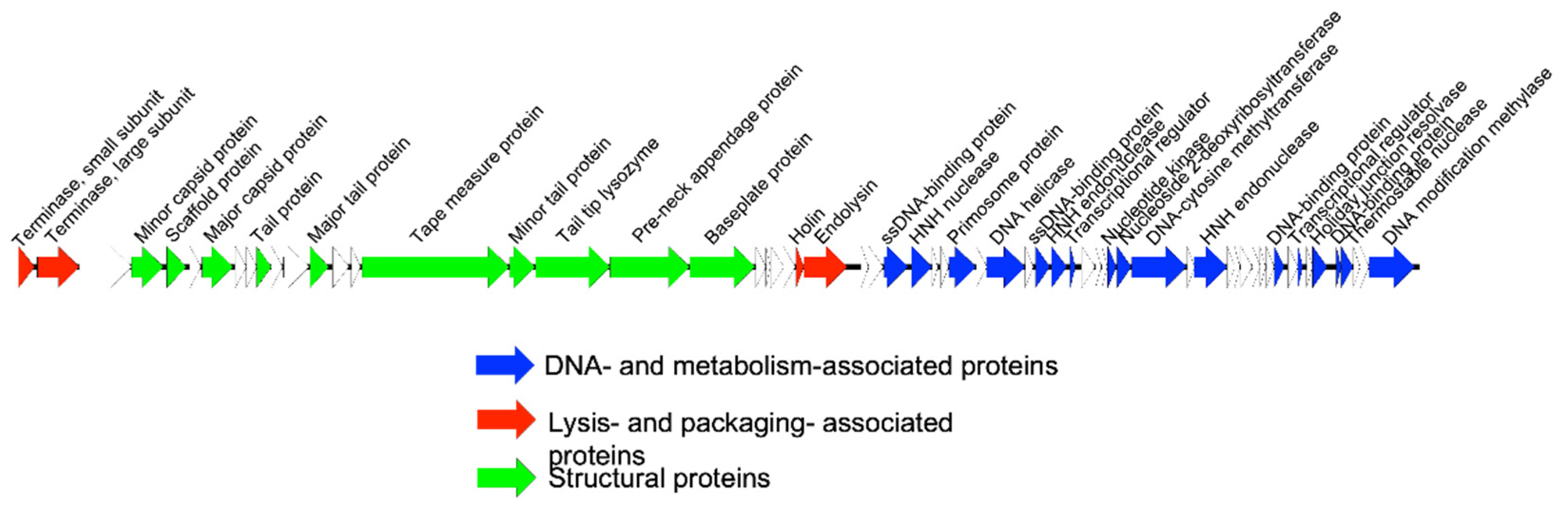
2.3. Genome Extraction, Sequencing, and Annotation
2.4. Host Range and Efficacy of Plating
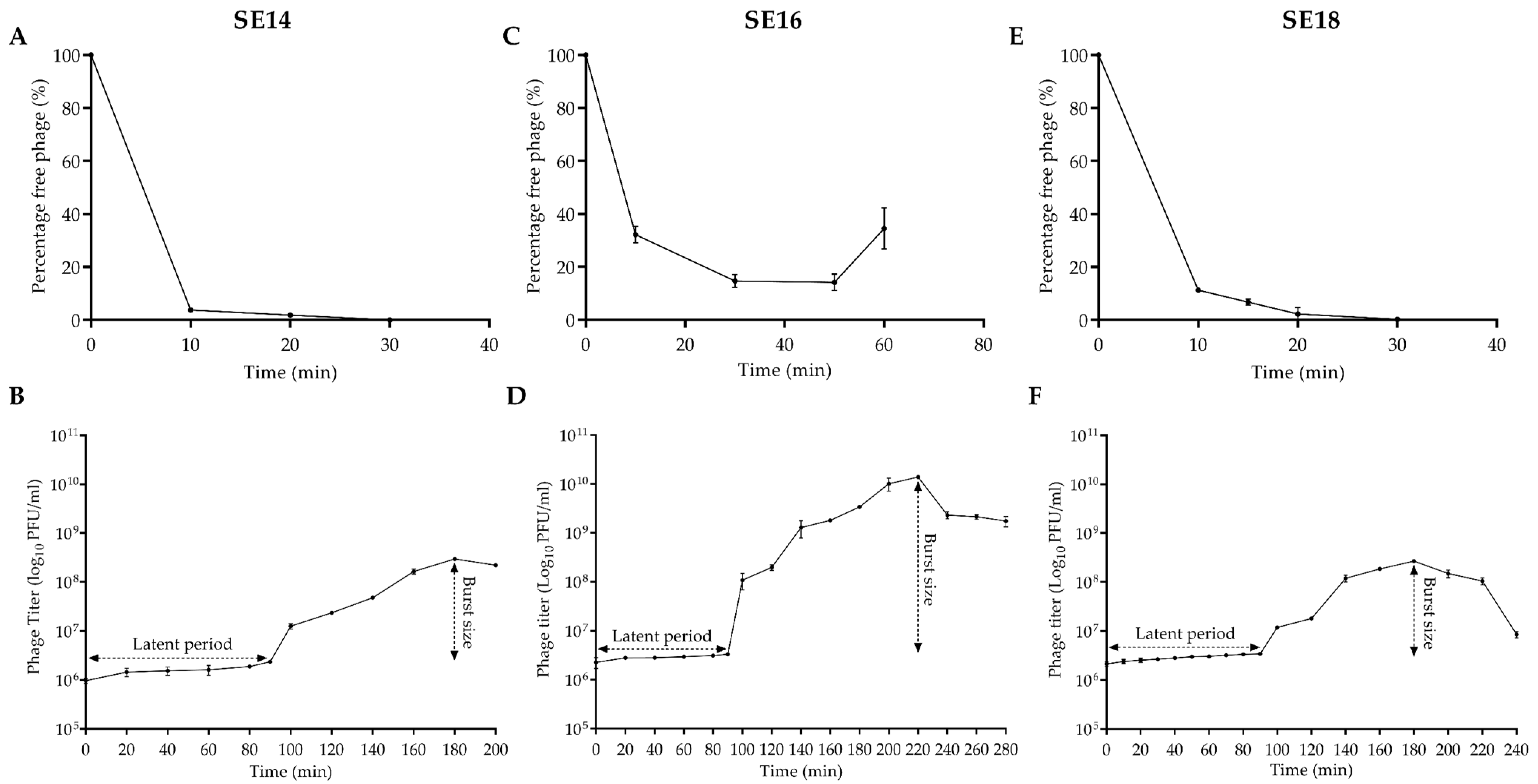
2.5. Adsorption and One-Step Growth Curve of CUB-EPI_14
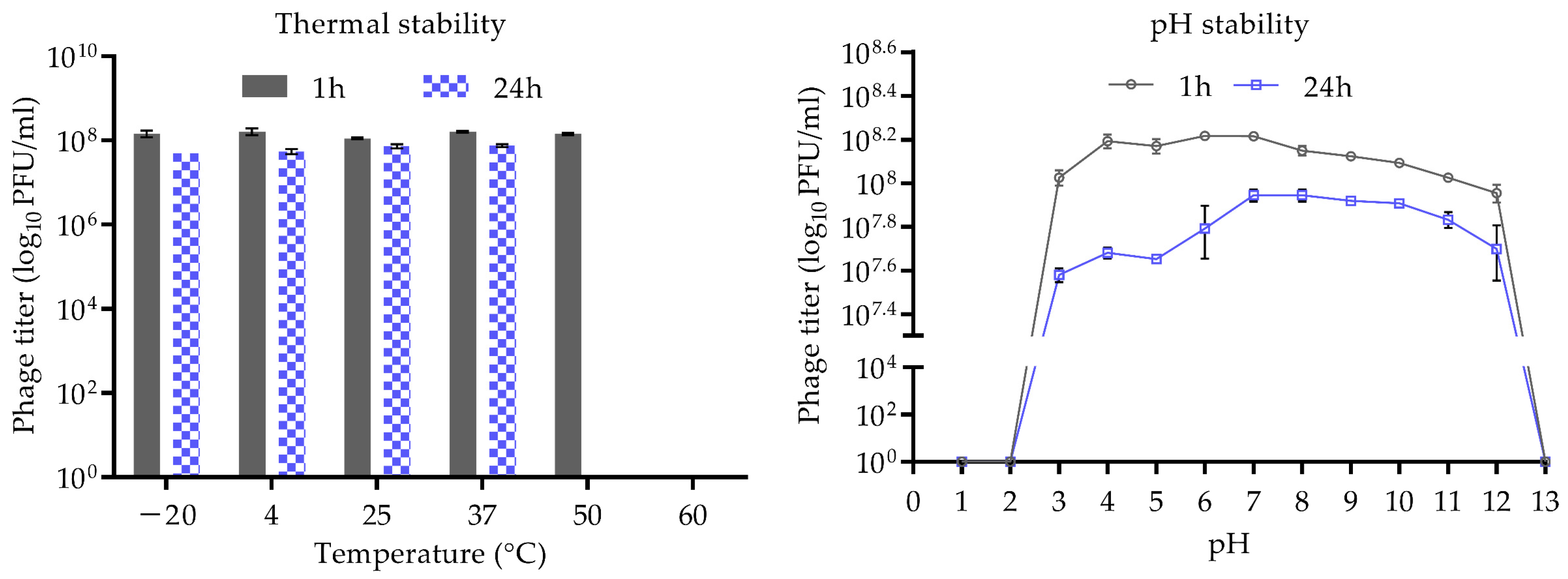
2.6. Thermal and pH Stability
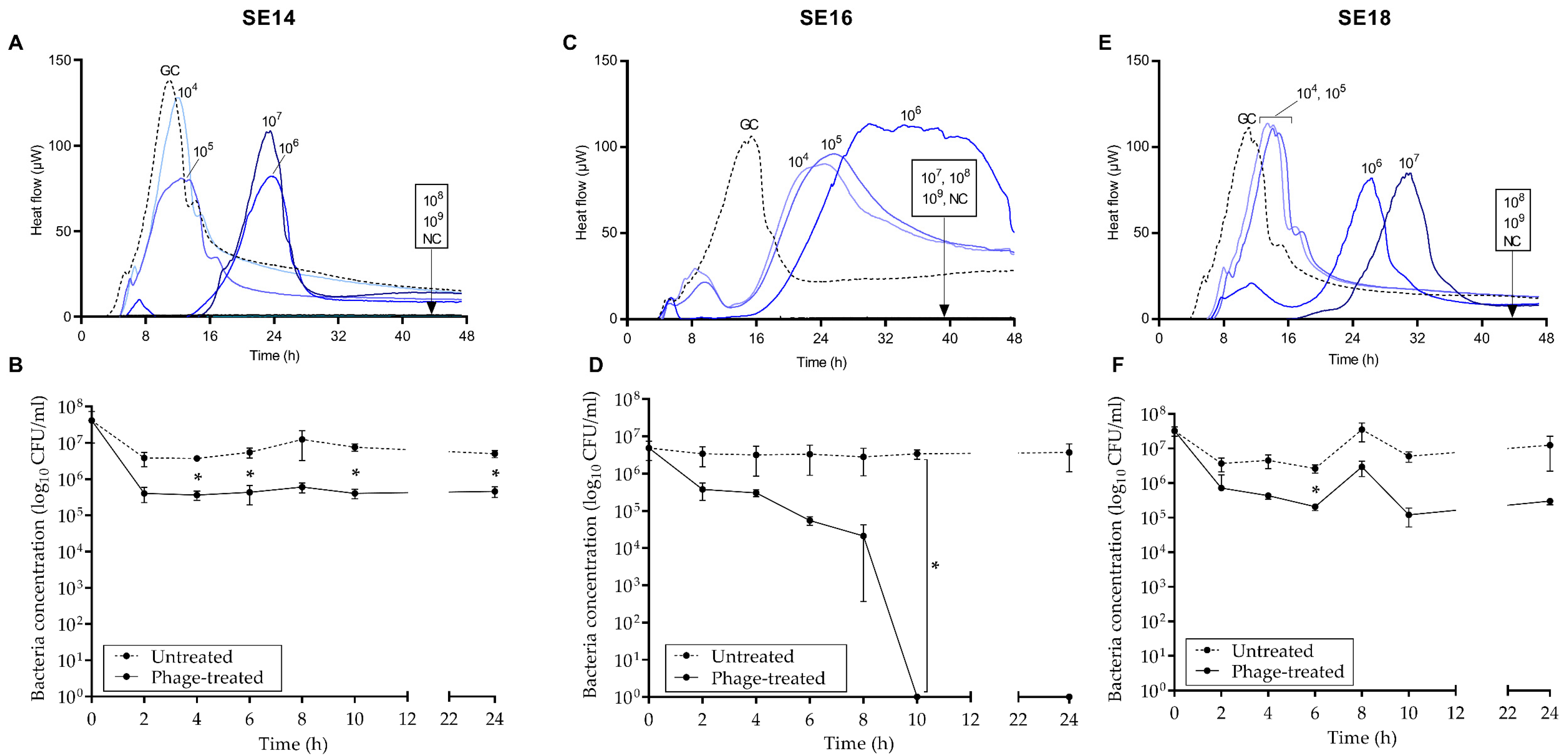
2.7. Antimicrobial Susceptibility Test by Isothermal Microcalorimetry
2.8. Biofilm Time-Killing Assay
2.9. Results Visualization and Analysis
3. Results
3.1. Bacteriophage Characterization
3.2. Stability of CUB-EPI_14 at Different pH and Temperatures
3.3. Antimicrobial Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, F.; Rohde, H.; Vilanova, M.; Cerca, N. Fighting Staphylococcus epidermidis Biofilm-Associated Infections: Can Iron Be the Key to Success? Front. Cell. Infect. Microbiol. 2021, 11, 798563. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus epidermidis--the ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espadinha, D.; Sobral, R.G.; Mendes, C.I.; Méric, G.; Sheppard, S.K.; Carriço, J.A.; de Lencastre, H.; Miragaia, M. Distinct Phenotypic and Genomic Signatures Underlie Contrasting Pathogenic Potential of Staphylococcus epidermidis Clonal Lineages. Front. Microbiol. 2019, 10, 1971. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.H.; Monk, I.R.; Gonçalves da Silva, A.; Seemann, T.; Chua, K.Y.L.; Kearns, A.; Hill, R.; Woodford, N.; Bartels, M.D.; Strommenger, B.; et al. Global spread of three multidrug-resistant lineages of Staphylococcus epidermidis. Nat. Microbiol. 2018, 3, 1175–1185. [Google Scholar] [CrossRef]
- Gonzalez-Moreno, M.; Morovic, P.; Tkhilaishvili, T.; Trampuz, A. Bacteriophages for Treatment of Biofilm Infections. In Bone and Joint Infections; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 65–80. [Google Scholar]
- Onsea, J.; Wagemans, J.; Pirnay, J.P.; Di Luca, M.; Gonzalez-Moreno, M.; Lavigne, R.; Trampuz, A.; Moriarty, T.F.; Metsemakers, W.J. Bacteriophage therapy as a treatment strategy for orthopaedic-device-related infections: Where do we stand? Eur. Cells Mater. 2020, 39, 193–210. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Koskella, B.; Meaden, S. Understanding bacteriophage specificity in natural microbial communities. Viruses 2013, 5, 806–823. [Google Scholar] [CrossRef] [Green Version]
- Topka-Bielecka, G.; Dydecka, A.; Necel, A.; Bloch, S.; Nejman-Faleńczyk, B.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage-Derived Depolymerases against Bacterial Biofilm. Antibiotics 2021, 10, 175. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Martínez, B.; Rodríguez, A.; García, P. Genomic characterization of two Staphylococcus epidermidis bacteriophages with anti-biofilm potential. BMC Genom. 2012, 13, 228. [Google Scholar] [CrossRef] [Green Version]
- Valente, L.G.; Pitton, M.; Fürholz, M.; Oberhaensli, S.; Bruggmann, R.; Leib, S.L.; Jakob, S.M.; Resch, G.; Que, Y.-A.; Cameron, D.R. Isolation and characterization of bacteriophages from the human skin microbiome that infect Staphylococcus epidermidis. FEMS Microbes 2021, 2, xtab003. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Vandenheuvel, D.; Martínez, B.; Rodríguez, A.; Lavigne, R.; García, P. Two Phages, phiIPLA-RODI and phiIPLA-C1C, Lyse Mono- and Dual-Species Staphylococcal Biofilms. Appl. Environ. Microbiol. 2015, 81, 3336–3348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, L.D.R.; Pinto, G.; Oliveira, F.; Vilas-Boas, D.; Almeida, C.; Sillankorva, S.; Cerca, N.; Azeredo, J. The Protective Effect of Staphylococcus epidermidis Biofilm Matrix against Phage Predation. Viruses 2020, 12, 76. [Google Scholar] [CrossRef]
- Manohar, P.; Tamhankar, A.J.; Lundborg, C.S.; Ramesh, N. Isolation, characterization and in vivo efficacy of Escherichia phage myPSH1131. PLoS ONE 2018, 13, e0206278. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wattam, A.R.; Davis, J.J.; Assaf, R.; Boisvert, S.; Brettin, T.; Bun, C.; Conrad, N.; Dietrich, E.M.; Disz, T.; Gabbard, J.L.; et al. Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res. 2017, 45, D535–D542. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Nishimura, Y.; Yoshida, T.; Kuronishi, M.; Uehara, H.; Ogata, H.; Goto, S. ViPTree: The viral proteomic tree server. Bioinformatics 2017, 33, 2379–2380. [Google Scholar] [CrossRef]
- Moraru, C.; Varsani, A.; Kropinski, A.M. VIRIDIC—A Novel Tool to Calculate the Intergenomic Similarities of Prokaryote-Infecting Viruses. Viruses 2020, 12, 1268. [Google Scholar] [CrossRef]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [Green Version]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, P.; Ji, W.; Fu, Q.; Wang, H.; Yan, Y.; Sun, J. SLPW: A Virulent Bacteriophage Targeting Methicillin-Resistant Staphylococcus aureus In vitro and In vivo. Front. Microbiol. 2016, 7, 934. [Google Scholar] [CrossRef] [Green Version]
- Viazis, S.; Akhtar, M.; Feirtag, J.; Brabban, A.D.; Diez-Gonzalez, F. Isolation and characterization of lytic bacteriophages against enterohaemorrhagic Escherichia coli. J. Appl. Microbiol. 2011, 110, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tkhilaishvili, T.; Bernal Andres, B.; Trampuz, A.; Gonzalez Moreno, M. Bacteriophage–antibiotic combinations against ciprofloxacin/ceftriaxone-resistant Escherichia coli in vitro and in an experimental Galleria mellonella model. Int. J. Antimicrob. Agents 2020, 56, 106200. [Google Scholar] [CrossRef] [PubMed]
- Nabergoj, D.; Modic, P.; Podgornik, A. Effect of bacterial growth rate on bacteriophage population growth rate. Microbiologyopen 2018, 7, e00558. [Google Scholar] [CrossRef] [Green Version]
- Tkhilaishvili, T.; Di Luca, M.; Abbandonato, G.; Maiolo, E.M.; Klatt, A.B.; Reuter, M.; Möncke-Buchner, E.; Trampuz, A. Real-time assessment of bacteriophage T3-derived antimicrobial activity against planktonic and biofilm-embedded Escherichia coli by isothermal microcalorimetry. Res. Microbiol. 2018, 169, 515–521. [Google Scholar] [CrossRef]
- Tisza, M.J.; Buck, C.B. A catalog of tens of thousands of viruses from human metagenomes reveals hidden associations with chronic diseases. Proc. Natl. Acad. Sci. USA 2021, 118, e2023202118. [Google Scholar] [CrossRef]
- Melo, L.D.R.; Sillankorva, S.; Ackermann, H.-W.; Kropinski, A.M.; Azeredo, J.; Cerca, N. Characterization of Staphylococcus epidermidis phage vB_SepS_SEP9-a unique member of the Siphoviridae family. Res. Microbiol. 2014, 165, 679–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aswani, V.H.; Tremblay, D.M.; Moineau, S.; Shukla, S.K. Complete Genome Sequence of a Staphylococcus epidermidis Bacteriophage Isolated from the Anterior Nares of Humans. Genome Announc. 2014, 2, e00549-14. [Google Scholar] [CrossRef] [Green Version]
- Merrill, B.D.; Ward, A.T.; Grose, J.H.; Hope, S. Software-based analysis of bacteriophage genomes, physical ends, and packaging strategies. BMC Genom. 2016, 17, 679. [Google Scholar] [CrossRef] [Green Version]
- Garneau, J.R.; Depardieu, F.; Fortier, L.-C.; Bikard, D.; Monot, M. PhageTerm: A tool for fast and accurate determination of phage termini and packaging mechanism using next-generation sequencing data. Sci. Rep. 2017, 7, 8292. [Google Scholar] [CrossRef]
- Tynecki, P.; Guziński, A.; Kazimierczak, J.; Jadczuk, M.; Dastych, J.; Onisko, A. PhageAI-Bacteriophage Life Cycle Recognition with Machine Learning and Natural Language Processing. bioRxiv 2020. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Göller, P.C.; Elsener, T.; Lorgé, D.; Radulovic, N.; Bernardi, V.; Naumann, A.; Amri, N.; Khatchatourova, E.; Coutinho, F.H.; Loessner, M.J.; et al. Multi-species host range of staphylococcal phages isolated from wastewater. Nat. Commun. 2021, 12, 6965. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Martínez, B.; Rodríguez, A.; García, P. Isolation and Characterization of Bacteriophages Infecting Staphylococcus epidermidis. Curr. Microbiol. 2010, 61, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Melo, L.D.R.; Sillankorva, S.; Ackermann, H.-W.; Kropinski, A.M.; Azeredo, J.; Cerca, N. Isolation and characterization of a new Staphylococcus epidermidis broad-spectrum bacteriophage. J. Gen. Virol. 2014, 95, 506–515. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Fernández, L.; Gutiérrez, D.; Campelo, A.B.; Rodríguez, A.; García, P. Analysis of Different Parameters Affecting Diffusion, Propagation and Survival of Staphylophages in Bacterial Biofilms. Front. Microbiol. 2018, 9, 2348. [Google Scholar] [CrossRef]
- Sutherland, I.W.; Hughes, K.A.; Skillman, L.C.; Tait, K. The interaction of phage and biofilms. FEMS Microbiol. Lett. 2004, 232, 1–6. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Wang, Z.; Wei, J.; Hu, T.; Si, J.; Tao, G.; Zhang, L.; Xie, L.; Abdalla, A.E.; et al. A combination therapy of Phages and Antibiotics: Two is better than one. Int. J. Biol. Sci. 2021, 17, 3573–3582. [Google Scholar] [CrossRef] [PubMed]

| Strain | EOP | Rank |
|---|---|---|
| SE16 | 2.55 ± 1.6 | high |
| SE18 | 0.2 ± 0.0001 | medium/low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanaei Pirlar, R.; Wagemans, J.; Ponce Benavente, L.; Lavigne, R.; Trampuz, A.; Gonzalez Moreno, M. Novel Bacteriophage Specific against Staphylococcus epidermidis and with Antibiofilm Activity. Viruses 2022, 14, 1340. https://doi.org/10.3390/v14061340
Fanaei Pirlar R, Wagemans J, Ponce Benavente L, Lavigne R, Trampuz A, Gonzalez Moreno M. Novel Bacteriophage Specific against Staphylococcus epidermidis and with Antibiofilm Activity. Viruses. 2022; 14(6):1340. https://doi.org/10.3390/v14061340
Chicago/Turabian StyleFanaei Pirlar, Rima, Jeroen Wagemans, Luis Ponce Benavente, Rob Lavigne, Andrej Trampuz, and Mercedes Gonzalez Moreno. 2022. "Novel Bacteriophage Specific against Staphylococcus epidermidis and with Antibiofilm Activity" Viruses 14, no. 6: 1340. https://doi.org/10.3390/v14061340
APA StyleFanaei Pirlar, R., Wagemans, J., Ponce Benavente, L., Lavigne, R., Trampuz, A., & Gonzalez Moreno, M. (2022). Novel Bacteriophage Specific against Staphylococcus epidermidis and with Antibiofilm Activity. Viruses, 14(6), 1340. https://doi.org/10.3390/v14061340









