SARS-CoV-2, Placental Histopathology, Gravity of Infection and Immunopathology: Is There an Association?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Controls
2.3. Procedure
2.4. Statistical Analysis
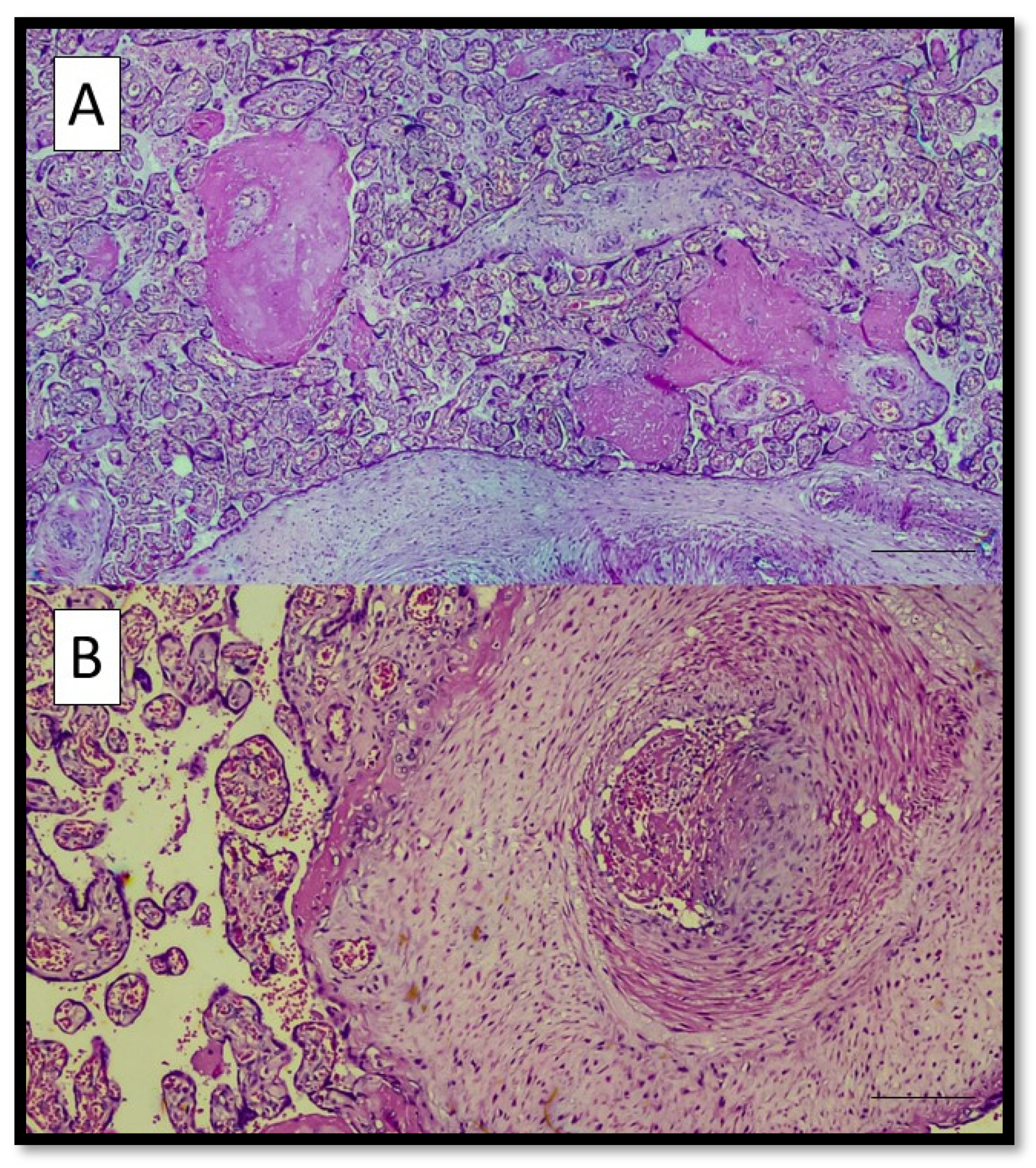
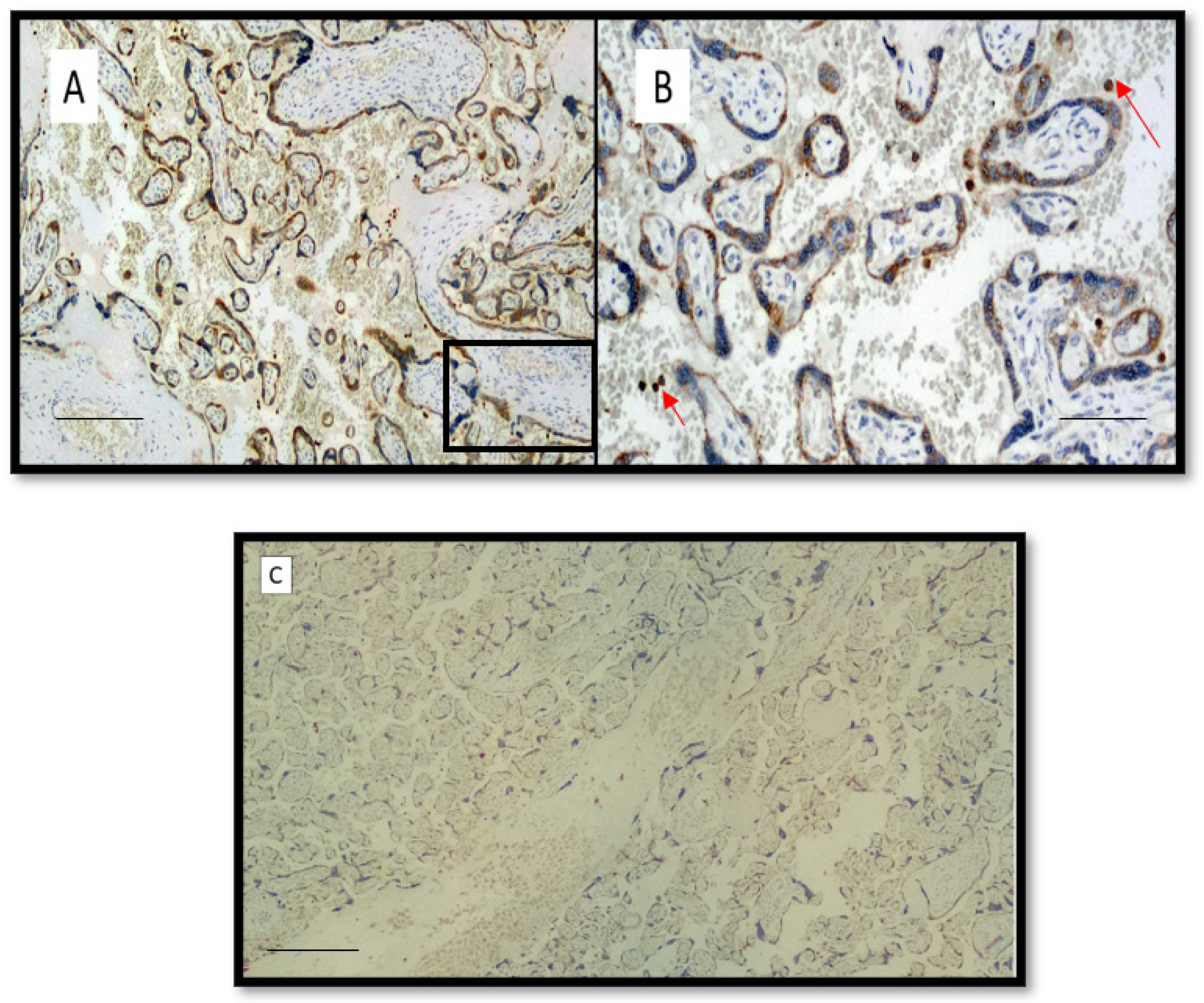
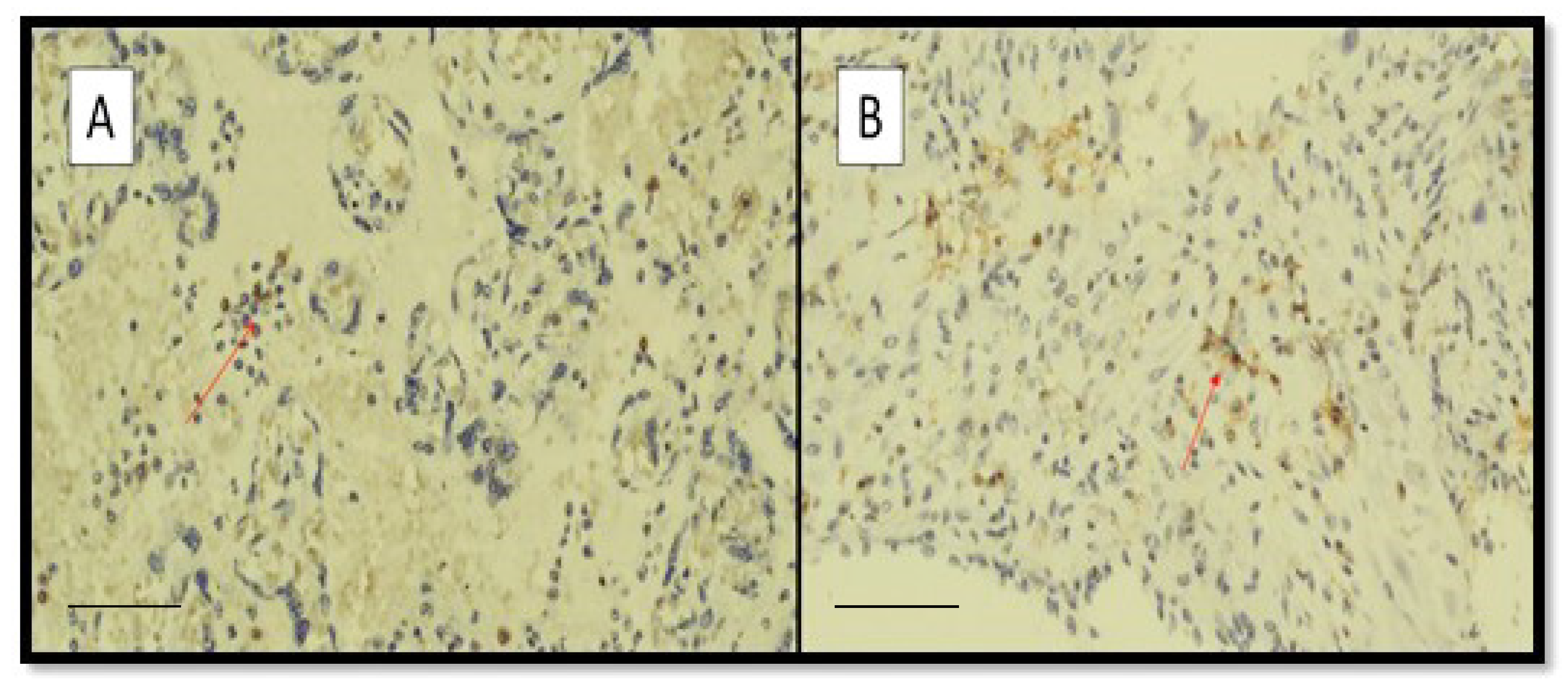
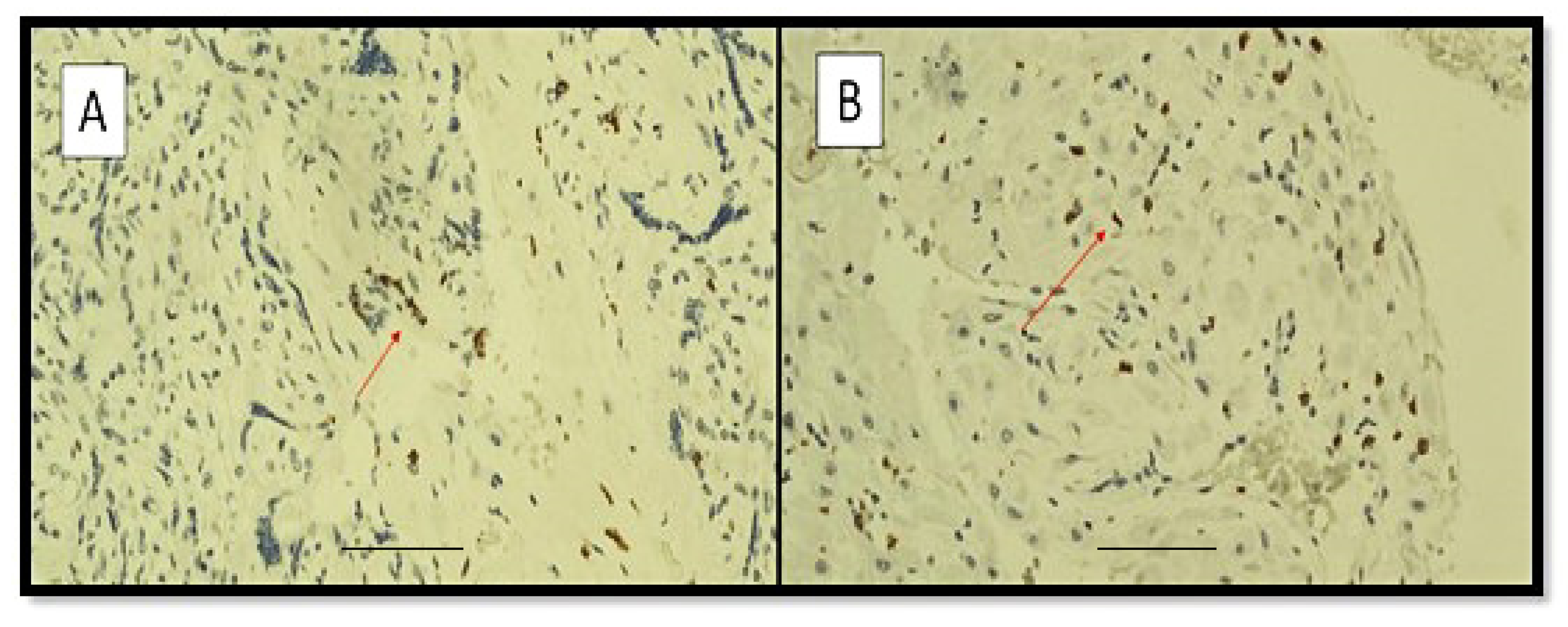
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Giovanetti, M.; Benedetti, F.; Campisi, G.; Ciccozzi, A.; Fabris, S.; Ceccarelli, G.; Tambone, V.; Caruso, A.; Angeletti, S.; Zella, D.; et al. Evolution patterns of SARS-CoV-2: Snapshot on its genome variants. Biochem. Biophys. Res. Commun. 2021, 29, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Kirtipal, N.; Bharadwaj, S.; Kang, S.G. From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect. Genet. Evol. 2020, 85, 104502. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, G.; Foti, C.; Colagrande, A.; Cimmino, A.; Scarcella, S.; Cicco, G.; Sablone, S.; Arezzo, F.; Romita, P.; Lettini, T.; et al. Skin Manifestation of SARS-CoV-2: The Italian Experience. J. Clin. Med. 2021, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, G.; Mazzia, G.; Cimmino, A.; Colagrande, A.; Sablone, S.; Lettini, T.; Rossi, R.; Santarella, N.; Elia, R.; Nacchiero, E.; et al. SARS-CoV-2 and Skin: The Pathologist’s Point of View. Biomolecules 2021, 4, 838. [Google Scholar] [CrossRef]
- Ingravallo, G.; Mazzotta, F.; Resta, L.; Sablone, S.; Cazzato, G.; Cimmino, A.; Rossi, R.; Colagrande, A.; Ferrante, B.; Troccoli, T.; et al. Inflammatory Skin Lesions in Three SARS-CoV-2 Swab-Negative Adolescents: A Possible COVID-19 Sneaky Manifestation? Pediatr. Rep. 2021, 9, 181–188. [Google Scholar] [CrossRef]
- Cazzato, G.; Romita, P.; Foti, C.; Cimmino, A.; Colagrande, A.; Arezzo, F.; Sablone, S.; Barile, A.; Lettini, T.; Resta, L.; et al. Purpuric Skin Rash in a Patient Undergoing Pfizer-BioNTech COVID-19 Vaccination: Histological Evaluation and Perspectives. Vaccines 2021, 8, 760. [Google Scholar] [CrossRef]
- Resta, L.; Vimercati, A.; Cazzato, G.; Mazzia, G.; Cicinelli, E.; Colagrande, A.; Fanelli, M.; Scarcella, S.V.; Ceci, O.; Rossi, R. SARS-CoV-2 and Placenta: New Insights and Perspectives. Viruses 2021, 13, 723. [Google Scholar] [CrossRef]
- Resta, L.; Vimercati, A.; Sablone, S.; Marzullo, A.; Cazzato, G.; Ingravallo, G.; Mazzia, G.; Arezzo, F.; Colagrande, A.; Rossi, R. Is the First of the Two Born Saved? A Rare and Dramatic Case of Double Placental Damage from SARS-CoV-2. Viruses 2021, 26, 995. [Google Scholar] [CrossRef]
- CDC 2019—Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. 2020. Available online: https://www.fda.gov/media/134922/download (accessed on 15 April 2022).
- Cepheid. Xpert® Xpress SARS-CoV-2 Instructions for Use for Labs. 2020. Available online: https://www.fda.gov/media/136314/download (accessed on 14 September 2021).
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Della Penna, S.L.; Kouyoumdzian, N.M.; Choi, M.R.; Gorzalczany, S.; Fernández, B.E.; Toblli, J.E.; Rosón, M.I. Immunohistochemical expression of intrarenal renin angiotensin system components in response to tempol in rats fed a high salt diet. World J. Nephrol. 2017, 6, 29–40. [Google Scholar] [CrossRef]
- Schwartz, D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab. Med. 2020, 144, 799–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menter, T.; Mertz, K.D.; Jiang, S.; Chen, H.; Monod, C.; Tzankov, A.; Waldvogel, S.; Schulzke, S.M.; Hösli, I.; Bruder, E. Placental Pathology Findings during and after SARS-CoV-2 Infection: Features of Villitis and Malperfusion. Pathobiology 2020, 88, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Baergen, R.N.; Heller, D.S. Placental Pathology in COVID-19 Positive Mothers: Preliminary Findings. Pediatr. Dev. Pathol. 2020, 23, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Zaigham, M.; Andersson, O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020, 99, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Robbins, J.R.; Bakardjiev, A.I. Pathogens and the placental fortress. Curr. Opin. Microbiol. 2012, 15, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Wenling, Y.; Junchao, Q.; Xiao, Z.; Ouyang, S. Pregnancy and COVID-19: Management and challenges. Rev. Inst. Med. Trop Sao Paulo 2020, 62, e62. [Google Scholar] [CrossRef]
- Tan, C.; Li, S.; Liang, Y.; Chen, M.; Liu, J. SARS-CoV-2 viremia may predict rapid deterioration of COVID-19 patients. Braz. J. Infect. Dis. 2020, 24, 565–569. [Google Scholar] [CrossRef]
- Penfield, C.A.; Brubaker, S.G.; Limaye, M.A.; Lighter, J.; Ratner, A.J.; Thomas, K.M.; Meyer, J.A.; Roman, A.S. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM 2020, 2, 100133. [Google Scholar] [CrossRef]
- Hosier, H.; Farhadian, S.F.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.; Casanovas-Massana, A.; Vijayakumar, P.; et al. SARS-CoV-2 infection of the placenta. J. Clin. Investig. 2020, 130, 4947–4953. [Google Scholar] [CrossRef]
- Tang, L.; Yin, Z.; Hu, Y.; Mei, H. Controlling Cytokine Storm Is Vital in COVID-19. Front. Immunol. 2020, 11, 570993. [Google Scholar] [CrossRef]
- Kreis, N.N.; Ritter, A.; Louwen, F.; Yuan, J. A Message from the Human Placenta: Structural and Immunomodulatory Defense against SARS-CoV-2. Cells 2020, 25, 1777. [Google Scholar] [CrossRef]
- Iba, T.; Connors, J.M.; Levy, J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020, 69, 1181–1189. [Google Scholar] [CrossRef]
- Flores-Pliego, A.; Miranda, J.; Vega-Torreblanca, S.; Valdespino-Vázquez, Y.; Helguera-Repetto, C.; Espejel-Nuñez, A.; Borboa-Olivares, H.; Espino, Y.; Sosa, S.; Mateu-Rogell, P.; et al. Molecular Insights into the Thrombotic and Microvascular Injury in Placental Endothelium of Women with Mild or Severe COVID-19. Cells 2021, 10, 364. [Google Scholar] [CrossRef]
- Espino-y-Sosa, S.; Martinez-Portilla, R.J.; Torres-Torres, J.; Solis-Paredes, J.M.; Estrada-Gutierrez, G.; Hernandez-Pacheco, J.A.; Espejel-Nuñez, A.; Mateu-Rogell, P.; Juarez-Reyes, A.; Lopez-Ceh, F.E.; et al. Novel Ratio Soluble Fms-like Tyrosine Kinase-1/Angiotensin-II (sFlt-1/ANG-II) in Pregnant Women Is Associated with Critical Illness in COVID-19. Viruses 2021, 23, 1906. [Google Scholar] [CrossRef]
- Bertero, L.; Borella, F.; Botta, G.; Carosso, A.; Cosma, S.; Bovetti, M.; Carosso, M.; Abbona, G.; Collemi, G.; Papotti, M.; et al. Placenta histopathology in SARS-CoV-2 infection: Analysis of a consecutive series and comparison with control cohorts. Virchows Arch. 2021, 479, 715–728. [Google Scholar] [CrossRef]
- Bukowska-Ośko, I.; Popiel, M.; Kowalczyk, P. The Immunological Role of the Placenta in SARS-CoV-2 Infection-Viral Transmission, Immune Regulation, and Lactoferrin Activity. Int. J. Mol. Sci. 2021, 28, 5799. [Google Scholar] [CrossRef]
- Facchetti, F.; Bugatti, M.; Drera, E.; Tripodo, C.; Sartori, E.; Cancila, V.; Papaccio, M.; Castellani, R.; Casola, S.; Boniotti, M.; et al. SARS-CoV-2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of placenta. EBioMedicine 2020, 59, 102951. [Google Scholar] [CrossRef]
- Kirtsman, M.; Diambomba, Y.; Poutanen, S.M.; Malinowski, A.K.; Vlachodimitropoulou, E.; Parks, W.T.; Erdman, L.; Morris, S.K.; Shah, P.S. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ 2020, 192, E647–E650. [Google Scholar] [CrossRef]
- Mongula, J.E.; Frenken, M.W.E.; van Lijnschoten, G.; Arents, N.L.A.; de Wit-Zuurendonk, L.D.; Schimmel-de Kok, A.P.A.; van Runnard Heimel, P.J.; Porath, M.M.; Goossens, S. COVID-19 during pregnancy: Non-reassuring fetal heart rate, placental pathology and coagulopathy. Ultrasound Obstet. Gynecol. 2020, 56, 773–776. [Google Scholar] [CrossRef]
- Richtmann, R.; Torloni, M.R.; Oyamada Otani, A.R.; Levi, J.E.; Crema Tobara, M.; de Almeida Silva, C.; Dias, L.; Miglioli-Galvão, L.; Martins Silva, P.; Macoto Kondo, M. Fetal deaths in pregnancies with SARS-CoV-2 infection in brazil: A case series. Case Rep. Womens Health 2020, 27, e00243. [Google Scholar] [CrossRef]
- Sisman, J.; Jaleel, M.A.; Moreno, W.; Rajaram, V.; Collins, R.R.J.; Savani, R.C.; Rakheja, D.; Evans, A.S. Intrauterine transmission of SARS-CoV-2 infection in a preterm infant. Pediatr. Infect. Dis. 2020, 39, e265–e267. [Google Scholar] [CrossRef]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Do Cao, J.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef]
- Bloise, E.; Zhang, J.; Nakpu, J.; Hamada, H.; Dunk, C.E.; Li, S.; Imperio, G.E.; Nadeem, L.; Kibschull, M.; Lye, P.; et al. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am. J. Obstet. Gynecol. 2021, 224, 298.e1–298.e8. [Google Scholar] [PubMed]
- Faure-Bardon, V.; Isnard, P.; Roux, N.; Leruez-Ville, M.; Molina, T.; Bessieres, B.; Ville, Y. Protein expression of angiotensin-converting enzyme 2, a SARS-CoV-2-specific receptor, in fetal and placental tissues throughout gestation: New insight for perinatal counseling. Ultrasound Obstet. Gynecol. 2021, 57, 242–247. [Google Scholar] [CrossRef]
- Verma, S.; Joshi, C.S.; Silverstein, R.B.; He, M.; Carter, E.B.; Mysorekar, I.U. SARS-CoV-2 colonization of maternal and fetal cells of the human placenta promotes alteration of local renin-angiotensin system. Med 2021, 2, 575–590.e5. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Liu, Y.; Jiang, X.; Ding, C.; Poon, L.C.; Wang, H.; Yang, H. Single-cell RNA expression profiling of SARS-CoV-2-related ACE2 and TMPRSS2 in human trophectoderm and placenta. Ultrasound Obstet. Gynecol. 2021, 57, 248–256. [Google Scholar] [CrossRef]
- Lamba, V.; Lien, J.; Desai, J.; Talati, A.J. Management and short-term outcomes of neonates born to mothers with active perinatal SARS-CoV-2 infection. BMC Pediatr. 2021, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Juan, J.; Gil, M.M.; Rong, Z.; Zhang, Y.; Yang, H.; Poon, L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: Systematic review. Ultrasound Obstet. Gynecol. 2020, 56, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Rad, H.S.; Röhl, J.; Stylianou, N.; Allenby, M.C.; Bazaz, S.R.; Warkiani, M.E.; Guimaraes, F.S.; Clifton, V.L.; Kulasinghe, A. The Effects of COVID-19 on the Placenta During Pregnancy. Front. Immunol. 2021, 12, 743022. [Google Scholar] [CrossRef]
- Theiler, R.N.; Wick, M.; Mehta, R.; Weaver, A.L.; Virk, A.; Swift, M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am. J. Obstet. Gynecol. MFM 2021, 3, 100467. [Google Scholar] [CrossRef]
- Wong, Y.P.; Khong, T.Y.; Tan, G.C. The Effects of COVID-19 on Placenta and Pregnancy: What Do We Know So Far? Diagnostics 2021, 8, 94. [Google Scholar] [CrossRef]
- Blitz, M.J.; Gerber, R.P.; Gulersen, M.; Shan, W.; Rausch, A.C.; Prasannan, L.; Meirowitz, N.; Rochelson, B. Preterm birth among women with and without severe acute respiratory syndrome coronavirus 2 infection. Acta Obstet. Gynecol. Scand. 2021, 100, 2253–2259. [Google Scholar] [CrossRef]
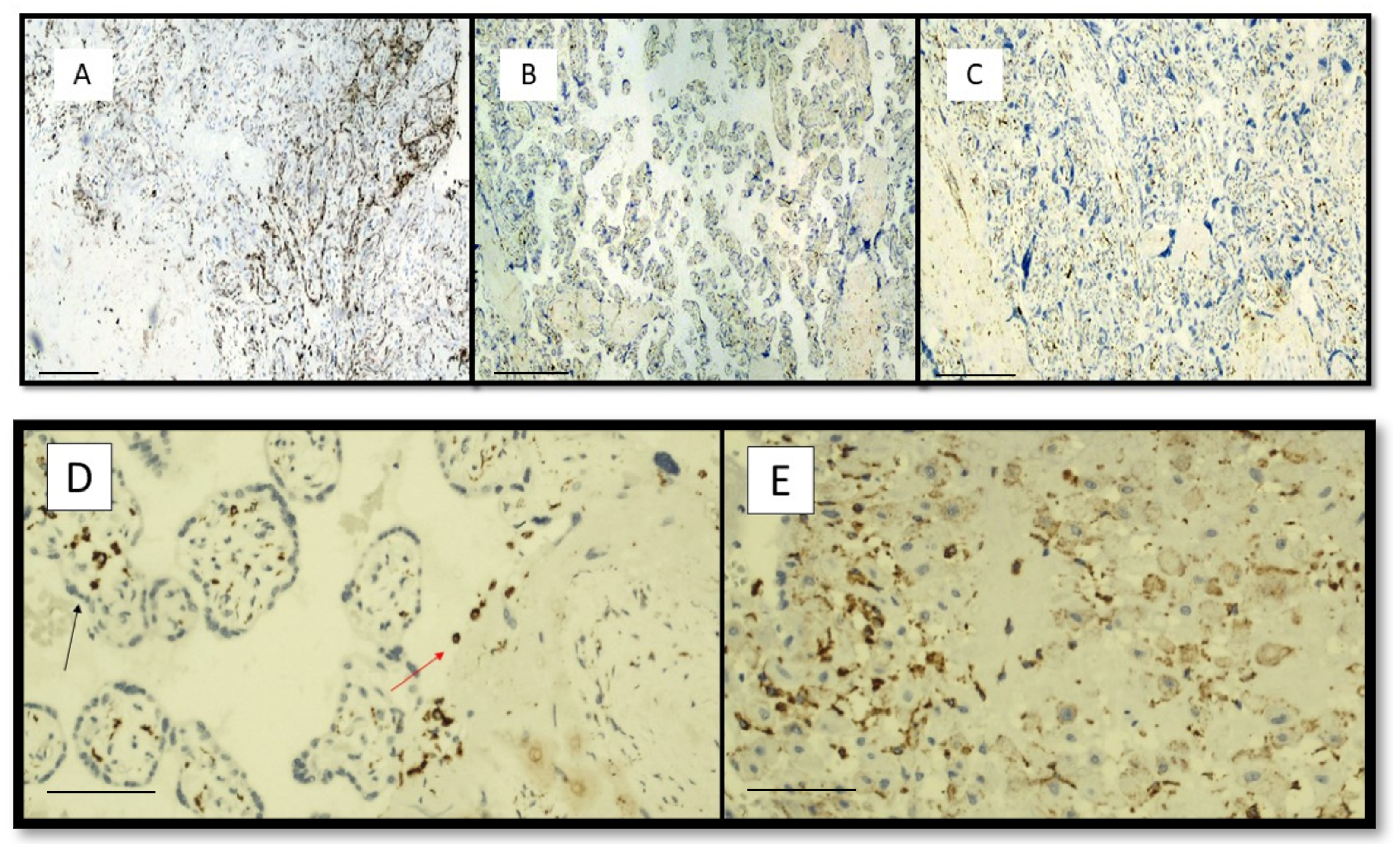
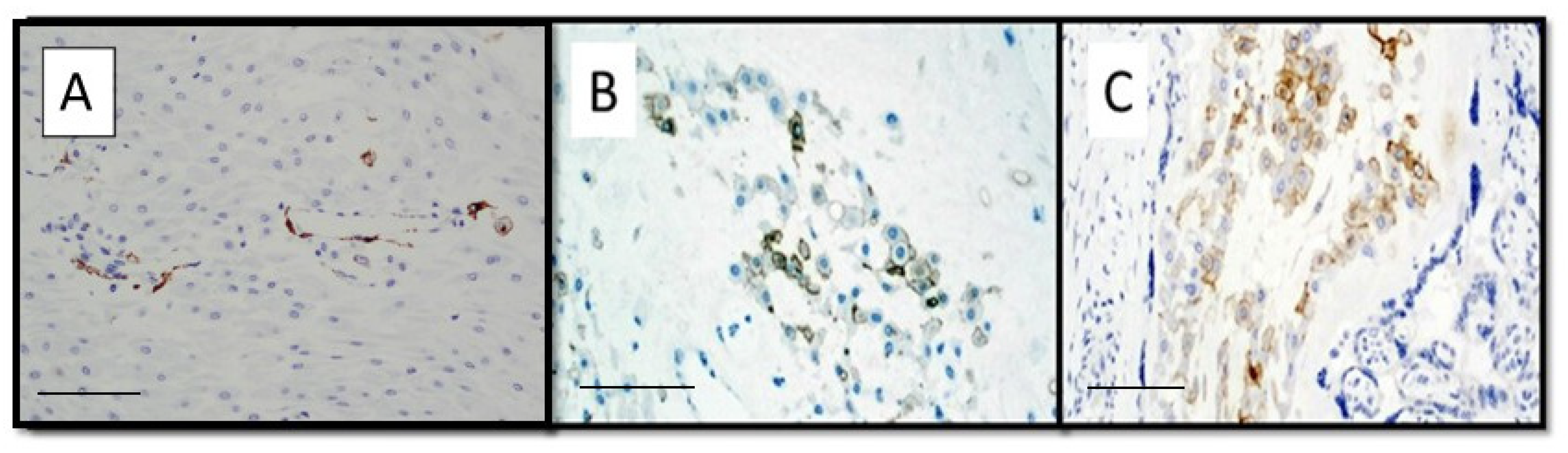
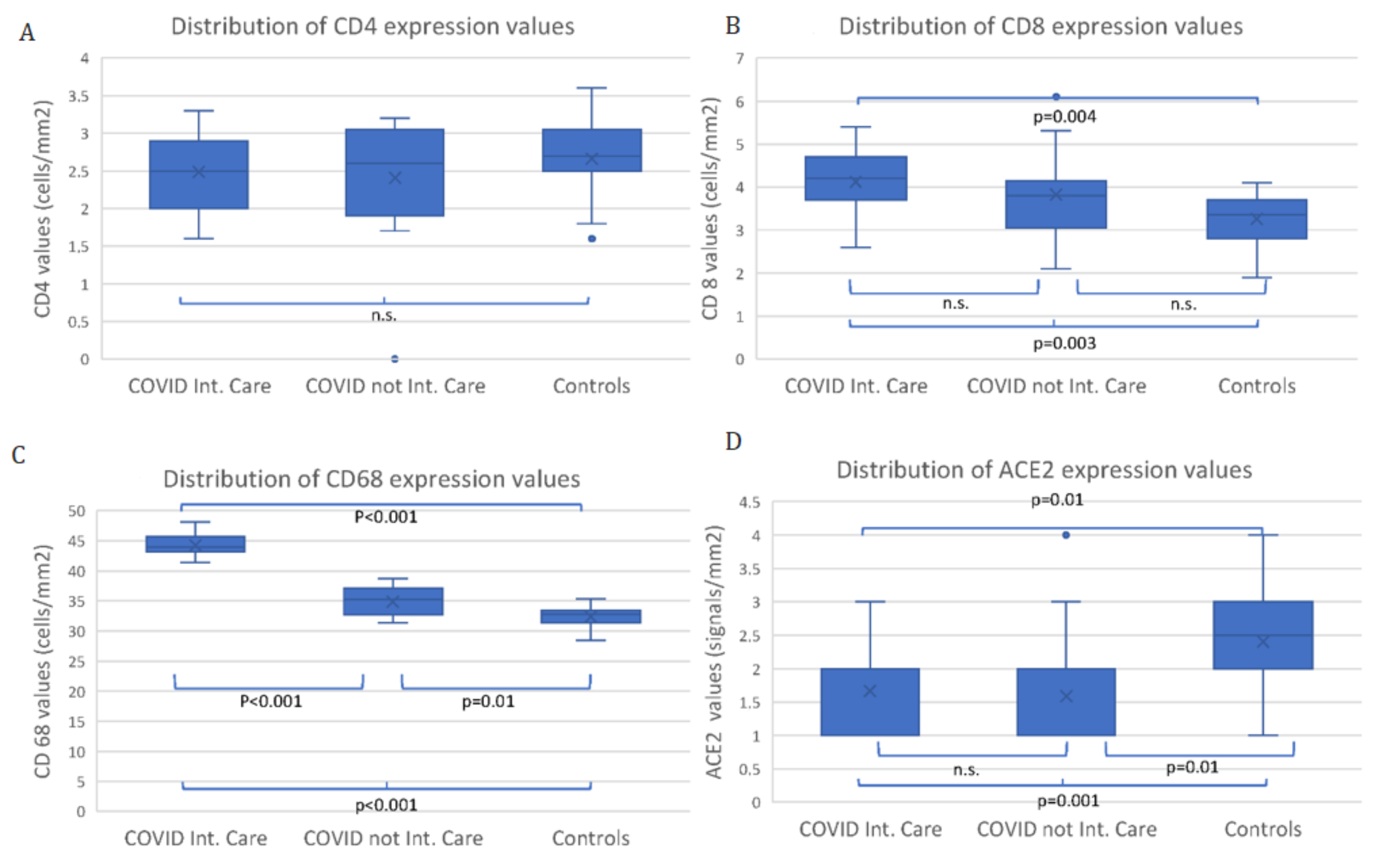
| Maternal and Pregnancy Features | COVID Intensive Care (13 Mothers) | COVID non Intensive Care (16 Mothers) | Control Group (28 Mothers) | p Values |
|---|---|---|---|---|
| Gestational age (week), means ± sd | 35.2 ± 4.4 | 36.9 ± 1.8 | 38 ± 2 | 0.020 |
| Preterm, n (%) | 7 (53.8) | 9 (56.2) | 4 (14.3) | 0.005 |
| Primiparous, n (%) | 6 (50.0) • | 6 (40.0) • | 17 (60.7) | 0.420 |
| Cesarean Section, n (%) | 9 (81.8) •• | 10 (71.4) • § | 26 (92.8) | 0.180 |
| Placental Finding | COVID Intensive Care (13 Placentae) | COVID non Intensive Care (16 Placentae) | Control Group (28 Placentae) | FDR Corrected p Values | OR (95%CI) |
|---|---|---|---|---|---|
| Weight (grams), means ± sd | 445.8 ± 104.9 | 547.2 ± 100 | 533 ± 188 | 0.02 | - |
| Maternal malperfusion, n (%) | 13 (100.0) | 11 (68.7) | 11 (39.3) | 0.001 | 1.5 (1.1–2.2) § |
| Decidual arteriopathy, n (%) | 12 (92.3) | 7 (43.7) | 1 (3.6) | <0.001 | 15.4 (1.6–148.8) |
| Fetal malperfusion, n (%) | 6 (46.2) | 6 (37.5) | 0 | 0.002 | 1.4 (0.3–6.3) |
| Decidual inflammation, n (%) | 11 (84.6) | 10 (62.5) | 0 | <0.001 | 3.3 (0.5–20.3) |
| Perivillous fibrin deposition, n (%) | 12 (100.0) • | 11 (68.7) | 3 (11) | <0.001 | 1.4 (1–2) § |
| Terminal villous hyperplasia n (%) | 8 (61.5) | 3 (18.8) | 5 (17.9) | 0.01 | 6.9 (1.3–37.2) |
| Villous hypervascularization, n (%) | 4 (30.8) | 2 (12.5) | 12 (42.9) | 0.11 | 3.1 (0.5–20.6) |
| Thrombi in fetal vessels, n (%) | 12 (92.3) | 5 (31.3) | 0 | <0.001 | 26.4 (2.6–262) |
| Syncytial nodes, n (%) | 2 (15.4) | 5 (31.3) | 14 (50.0) | 0.10 | 0.4 (0.06–2.5) |
| Chorioamnionitis, n (%) | 1 (7.7) | 4 (25.0) | 0 | 0.02 | 0.25 (0.02–2.6) |
| Perivillary histiocytosis, n (%) | 11 (84.6) | 9 (56.3) | 5 (17.9) | <0.001 | 4.3 (0.7–25) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resta, L.; Vimercati, A.; Cazzato, G.; Fanelli, M.; Scarcella, S.V.; Ingravallo, G.; Colagrande, A.; Sablone, S.; Stolfa, M.; Arezzo, F.; et al. SARS-CoV-2, Placental Histopathology, Gravity of Infection and Immunopathology: Is There an Association? Viruses 2022, 14, 1330. https://doi.org/10.3390/v14061330
Resta L, Vimercati A, Cazzato G, Fanelli M, Scarcella SV, Ingravallo G, Colagrande A, Sablone S, Stolfa M, Arezzo F, et al. SARS-CoV-2, Placental Histopathology, Gravity of Infection and Immunopathology: Is There an Association? Viruses. 2022; 14(6):1330. https://doi.org/10.3390/v14061330
Chicago/Turabian StyleResta, Leonardo, Antonella Vimercati, Gerardo Cazzato, Margherita Fanelli, Sara Vincenza Scarcella, Giuseppe Ingravallo, Anna Colagrande, Sara Sablone, Mary Stolfa, Francesca Arezzo, and et al. 2022. "SARS-CoV-2, Placental Histopathology, Gravity of Infection and Immunopathology: Is There an Association?" Viruses 14, no. 6: 1330. https://doi.org/10.3390/v14061330
APA StyleResta, L., Vimercati, A., Cazzato, G., Fanelli, M., Scarcella, S. V., Ingravallo, G., Colagrande, A., Sablone, S., Stolfa, M., Arezzo, F., Lettini, T., & Rossi, R. (2022). SARS-CoV-2, Placental Histopathology, Gravity of Infection and Immunopathology: Is There an Association? Viruses, 14(6), 1330. https://doi.org/10.3390/v14061330













