ORFeome Phage Display Reveals a Major Immunogenic Epitope on the S2 Subdomain of SARS-CoV-2 Spike Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Serum Samples
2.2. Library Construction
2.3. Colony PCR for Library Quality Control
2.4. ORF Enrichment with Hyperphage
2.5. NGS Data Analysis
2.6. Identification of Immunogenic Epitopes
2.7. Phage Clone Production for Screening
2.8. Screening ELISA
2.9. Epitope Characterization by Peptide Microarray
2.10. Titration ELISA on Identified Peptides
3. Results
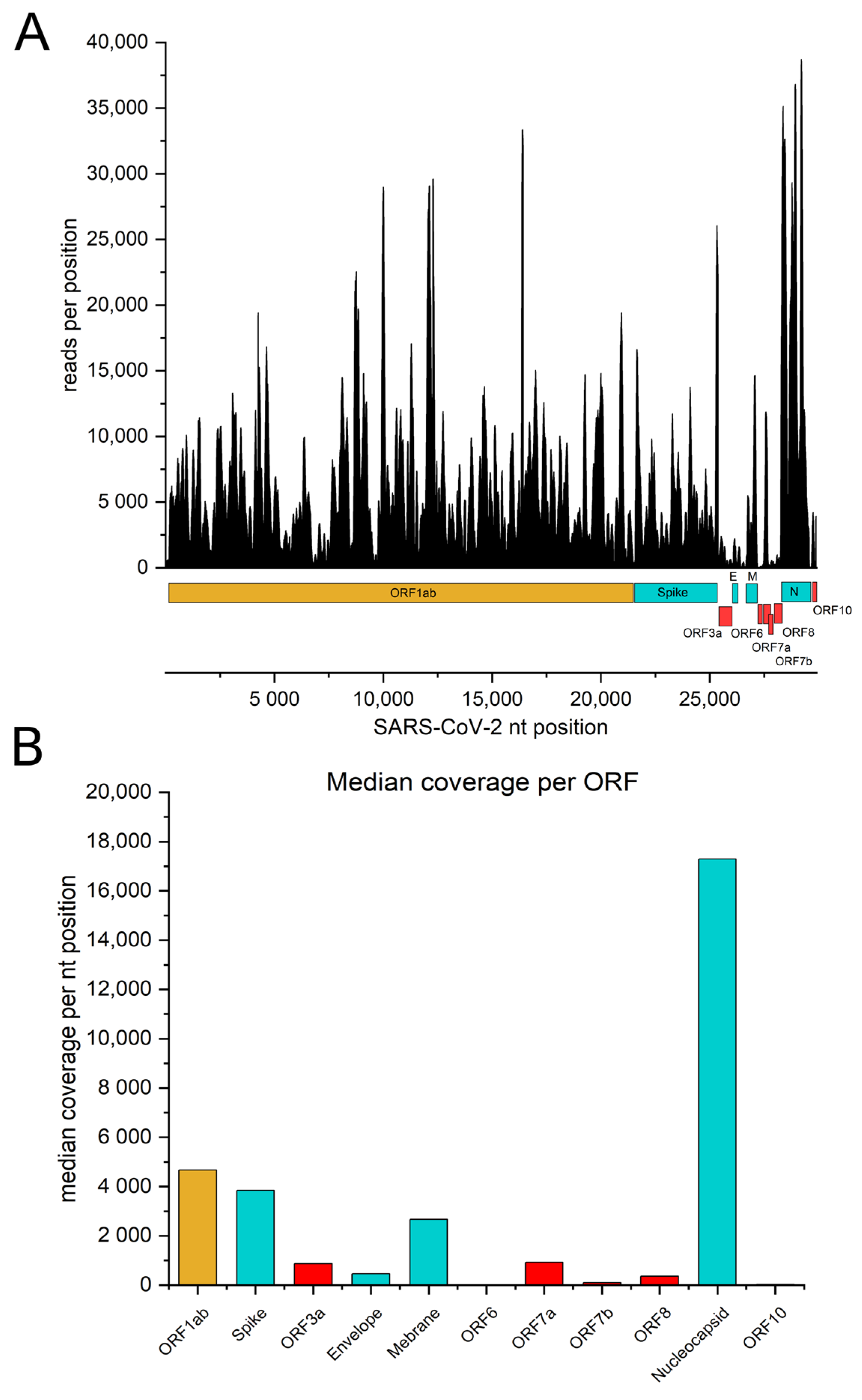
3.1. The Genome of SARS-CoV-2 Is Represented within the ORFeome Phage Display Library
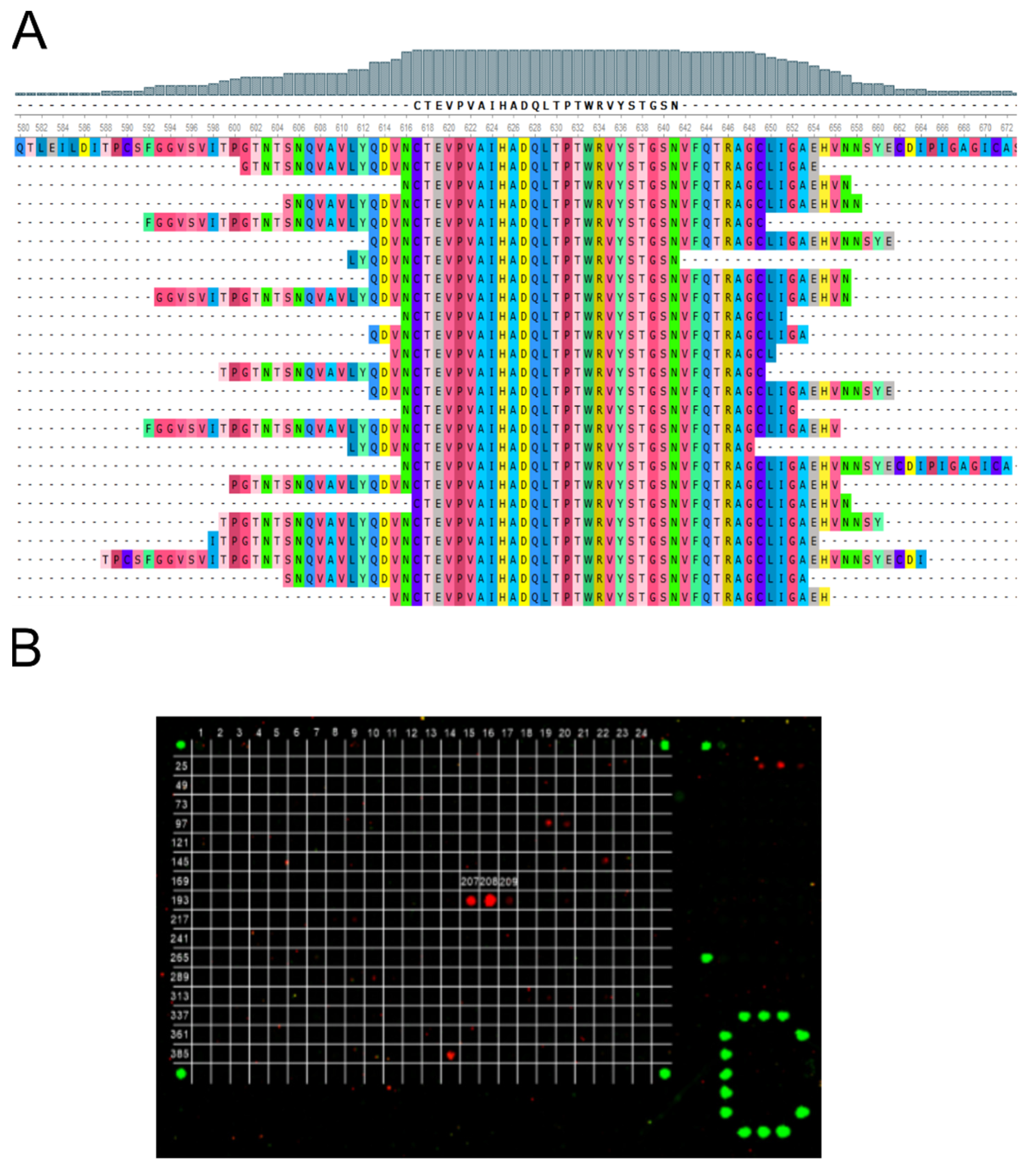
3.2. Epitope Identification of Monoclonal Antibodies
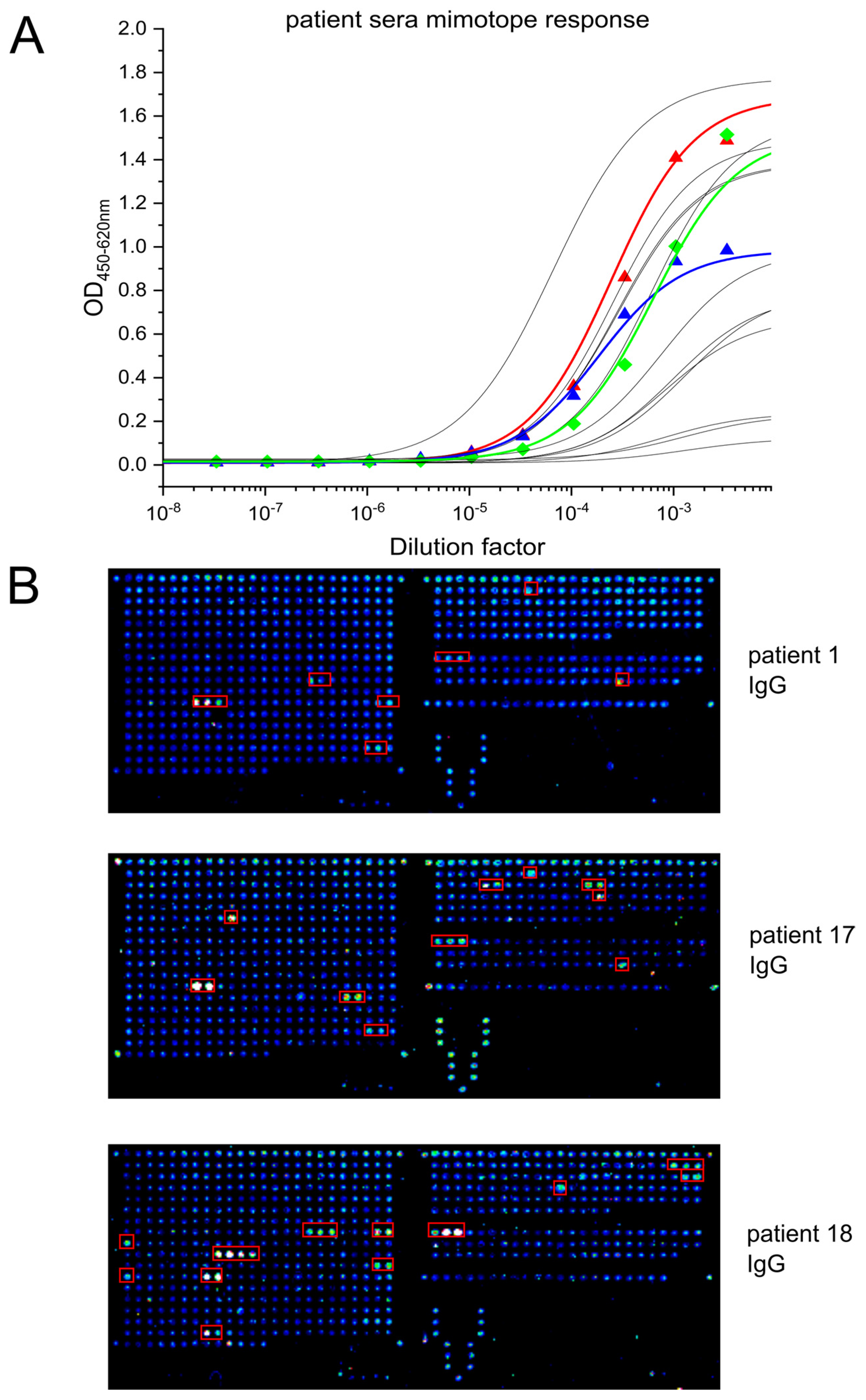
3.3. Immunogenic Epitope in the S2 Subdomain of the Spike Protein Is Common among COVID-19 Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Mallah, S.I.; Ghorab, O.K.; Al-Salmi, S.; Abdellatif, O.S.; Tharmaratnam, T.; Iskandar, M.A.; Sefen, J.A.N.; Sidhu, P.; Atallah, B.; El-Lababidi, R.; et al. COVID-19: Breaking down a global health crisis. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID 19 Public Health Emergency of International Concern (PHEIC) Global Research and Innovation Forum. Available online: https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum (accessed on 16 June 2022).
- Ellis, P.; Somogyvári, F.; Virok, D.P.; Noseda, M.; McLean, G.R. Decoding COVID-19 with the SARS-CoV-2 Genome. Curr. Genet. Med. Rep. 2021, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Menachery, V.D. Return of the Coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, M.; Pandey, N.; Shukla, A.; Singh, S.K. SARS coronavirus 2: From genome to infectome. Respir. Res. 2020, 21, 1–15. [Google Scholar] [CrossRef]
- Redondo, N.; Zaldívar-López, S.; Garrido, J.J.; Montoya, M. SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: Knowns and Unknowns. Front. Immunol. 2021, 12, 708264. [Google Scholar] [CrossRef]
- GISAID—hCoV-19 Reference Sequence. 2021. Available online: https://www.gisaid.org/references/hcov-19-reference-sequence/ (accessed on 2 December 2021).
- Bojkova, D.; Klann, K.; Koch, B.; Widera, M.; Krause, D.; Ciesek, S.; Cinatl, J.; Münch, C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 2020, 583, 469–472. [Google Scholar] [CrossRef]
- Satarker, S.; Nampoothiri, M. Structural Proteins in Severe Acute Respiratory Syndrome Coronavirus-2. Arch. Med Res. 2020, 51, 482–491. [Google Scholar] [CrossRef]
- Mariano, G.; Farthing, R.J.; Lale-Farjat, S.L.M.; Bergeron, J.R.C. Structural Characterization of SARS-CoV-2: Where We Are, and Where We Need to Be. Front. Mol. Biosci. 2020, 7, 605236. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Koppisetti, R.K.; Fulcher, Y.G.; Van Doren, S.R. Fusion Peptide of SARS-CoV-2 Spike Rearranges into a Wedge Inserted in Bilayered Micelles. J. Am. Chem. Soc. 2021, 143, 13205–13211. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, J.; Xiao, T.; Peng, H.; Sterling, S.M.; Walsh, R.M.; Rawson, S.; Rits-Volloch, S.; Chen, B. Distinct conformational states of SARS-CoV-2 spike protein. Science 2020, 369, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Pöhlmann, S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell 2020, 78, 779–784.e5. [Google Scholar] [CrossRef] [PubMed]
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.I.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE 2020, 15, e0244126. [Google Scholar] [CrossRef] [PubMed]
- Sherina, N.; Piralla, A.; Du, L.; Wan, H.; Kumagai-Braesch, M.; Andréll, J.; Braesch-Andersen, S.; Cassaniti, I.; Percivalle, E.; Sarasini, A.; et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6–8 months after the infection. Med 2021, 2, 281–295.e4. [Google Scholar] [CrossRef]
- Bertoglio, F.; Fühner, V.; Ruschig, M.; Heine, P.A.; Abassi, L.; Klünemann, T.; Rand, U.; Meier, D.; Langreder, N.; Steinke, S.; et al. A SARS-CoV-2 neutralizing antibody selected from COVID-19 patients binds to the ACE2-RBD interface and is tolerant to most known RBD mutations. Cell Rep. 2021, 36, 109433. [Google Scholar] [CrossRef]
- Hansen, J.; Baum, A.; Pascal, K.E.; Russo, V.; Giordano, S.; Wloga, E.; Fulton, B.O.; Yan, Y.; Koon, K.; Patel, K.; et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 2020, 369, 1010–1014. [Google Scholar] [CrossRef]
- Baum, A.; Fulton, B.O.; Wloga, E.; Copin, R.; Pascal, K.E.; Russo, V.; Giordano, S.; Lanza, K.; Negron, N.; Ni, M.; et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science 2020, 369, 1014–1018. [Google Scholar] [CrossRef]
- Pinto, D.; Park, Y.-J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef]
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 229–237. [Google Scholar] [CrossRef]
- Rees-Spear, C.; Muir, L.; Griffith, S.A.; Heaney, J.; Aldon, Y.; Snitselaar, J.L.; Thomas, P.; Graham, C.; Seow, J.; Lee, N.; et al. The effect of spike mutations on SARS-CoV-2 neutralization. Cell Rep. 2021, 34, 108890. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2021, 602, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Dobaño, C.; Santano, R.; Jiménez, A.; Vidal, M.; Chi, J.; Melero, N.R.; Popovic, M.; López-Aladid, R.; Fernández-Barat, L.; Tortajada, M.; et al. Immunogenicity and crossreactivity of antibodies to the nucleocapsid protein of SARS-CoV-2: Utility and limitations in seroprevalence and immunity studies. Transl. Res. 2021, 232, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.M.; Romero, C.; Cruz, M.; Vandeberg, P.; Merritt, W.K.; Pradenas, E.; Trinité, B.; Blanco, J.; Clotet, B.; Willis, T.; et al. Anti-Severe Acute Respiratory Syndrome Coronavirus 2 Hyperimmune Immunoglobulin Demonstrates Potent Neutralization and Antibody-Dependent Cellular Cytotoxicity and Phagocytosis Through N and S Proteins. J. Infect. Dis. 2021, 225, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, G.; Guo, Y.; Wang, P.; Nair, M.S.; Wang, M.; Huang, Y.; Yu, J.; Liu, L.; Katsamba, P.S.; Bahna, F.; et al. Neutralizing antibody 5-7 defines a distinct site of vulnerability in SARS-CoV-2 spike N-terminal domain. Cell Rep. 2021, 37, 450397. [Google Scholar] [CrossRef] [PubMed]
- Riemer, A.; Klinger, M.; Wagner, S.; Bernhaus, A.; Mazzucchelli, L.; Pehamberger, H.; Scheiner, O.; Zielinski, C.C.; Jensen-Jarolim, E. Generation of Peptide Mimics of the Epitope Recognized by Trastuzumab on the Oncogenic Protein Her-2/neu. J. Immunol. 2004, 173, 394–401. [Google Scholar] [CrossRef]
- Wang, H.; Wu, X.; Zhang, X.; Hou, X.; Liang, T.; Wang, D.; Teng, F.; Dai, J.; Duan, H.; Guo, S.; et al. SARS-CoV-2 Proteome Microarray for Mapping COVID-19 Antibody Interactions at Amino Acid Resolution. ACS Central Sci. 2020, 6, 2238–2249. [Google Scholar] [CrossRef]
- Li, Y.; Lai, D.-Y.; Zhang, H.-N.; Jiang, H.-W.; Tian, X.; Ma, M.-L.; Qi, H.; Meng, Q.-F.; Guo, S.-J.; Wu, Y.; et al. Linear epitopes of SARS-CoV-2 spike protein elicit neutralizing antibodies in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 1095–1097. [Google Scholar] [CrossRef]
- Kringelum, J.V.; Nielsen, M.; Padkjær, S.B.; Lund, O. Structural analysis of B-cell epitopes in antibody:protein complexes. Mol. Immunol. 2013, 53, 24–34. [Google Scholar] [CrossRef]
- Kügler, J.; Nieswandt, S.; Gerlach, G.F.; Meens, J.; Schirrmann, T.; Hust, M. Identification of immunogenic polypeptides from a Mycoplasma hyopneumoniae genome library by phage display. Appl. Microbiol. Biotechnol. 2008, 80, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Zantow, J.; Moreira, G.M.S.G.; Dübel, S.; Hust, M. ORFeome Phage Display. In Phage Display; Humana Press: New York, NY, USA, 2017; pp. 477–495. [Google Scholar] [CrossRef]
- Ramli, S.R.; Moreira, G.; Zantow, J.; Goris, M.G.A.; Nguyen, V.K.; Novoselova, N.; Pessler, F.; Hust, M. Discovery of Leptospira spp. seroreactive peptides using ORFeome phage display. PLOS Neglected Trop. Dis. 2019, 13, e0007131. [Google Scholar] [CrossRef] [PubMed]
- Rondot, S.; Koch, J.; Breitling, F.; Dübel, S. A helper phage to improve single-chain antibody presentation in phage display. Nat. Biotechnol. 2001, 19, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Pucker, B.; Brockington, S.F. Genome-wide analyses supported by RNA-Seq reveal non-canonical splice sites in plant genomes. BMC Genom. 2018, 19, 980. [Google Scholar] [CrossRef]
- Hotop, S.-K.; Reimering, S.; Shekhar, A.; Asgari, E.; Beutling, U.; Dahlke, C.; Fathi, A.; Khan, F.; Lütgehetmann, M.; Ballmann, R.; et al. Peptide microarrays coupled to machine learning reveal individual epitopes from human antibody responses with neutralizing capabilities against SARS-CoV-2. Emerg. Microbes Infect. 2022, 11, 1037–1048. [Google Scholar] [CrossRef]
- Hust, M.; Meysing, M.; Schirrmann, T.; Selke, M.; Meens, J.; Gerlach, G.-F.; Dübel, S. Enrichment of open reading frames presented on bacteriophage M13 using Hyperphage. BioTechniques 2006, 41, 335–342. [Google Scholar] [CrossRef]
- Bertoglio, F.; Meier, D.; Langreder, N.; Steinke, S.; Rand, U.; Simonelli, L.; Heine, P.A.; Ballmann, R.; Schneider, K.-T.; Roth, K.D.R.; et al. SARS-CoV-2 neutralizing human recombinant antibodies selected from pre-pandemic healthy donors binding at RBD-ACE2 interface. Nat. Commun. 2021, 12, 1577. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.-F.; Xu, W.; Liu, S.-W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Lai, A.L.; Millet, J.K.; Daniel, S.; Freed, J.H.; Whittaker, G.R. The SARS-CoV Fusion Peptide Forms an Extended Bipartite Fusion Platform that Perturbs Membrane Order in a Calcium-Dependent Manner. J. Mol. Biol. 2017, 429, 3875–3892. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Hu, Y.F.; Chen, L.L.; Tong, Y.G.; Hu, J.C.; Cai, J.P.; Chan, K.H.; Dou, Y.; Deng, J.; Gong, H.R.; et al. Mapping the Immunodominance Landscape of SARS-CoV-2 Spike Protein for the Design of Vaccines against COVID-19. bioRxiv 2020. [CrossRef]
- Poh, C.M.; Carissimo, G.; Wang, B.; Amrun, S.N.; Lee, C.Y.-P.; Chee, R.S.-L.; Fong, S.-W.; Yeo, N.K.-W.; Lee, W.-H.; Torres-Ruesta, A.; et al. Two linear epitopes on the SARS-CoV-2 spike protein that elicit neutralising antibodies in COVID-19 patients. Nat. Commun. 2020, 11, 2806. [Google Scholar] [CrossRef] [PubMed]
- Zamecnik, C.R.; Rajan, J.V.; Yamauchi, K.A.; Mann, S.A.; Loudermilk, R.P.; Sowa, G.M.; Zorn, K.C.; Alvarenga, B.D.; Gaebler, C.; Caskey, M.; et al. ReScan, a Multiplex Diagnostic Pipeline, Pans Human Sera for SARS-CoV-2 Antigens. Cell Rep. Med. 2020, 1, 100123. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Hahn, M.; Belaunzaran, P.; Ramos-Castañeda, J.; Nájera-Cancino, G.; Caballero-Sosa, S.; Fuentes, K.R.N.; Ruiz-Palacios, G.; Golding, H.; Beigel, J.H.; et al. Differential human antibody repertoires following Zika infection and the implications for serodiagnostics and disease outcome. Nat. Commun. 2019, 10, 1943. [Google Scholar] [CrossRef]
- Hoffmann, M.; Arora, P.; Groß, R.; Seidel, A.; Hörnich, B.F.; Hahn, A.S.; Krüger, N.; Graichen, L.; Hofmann-Winkler, H.; Kempf, A.; et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell 2021, 184, 2384–2393.e12. [Google Scholar] [CrossRef]
- Mahoney, M.; Damalanka, V.C.; Tartell, M.A.; Chung, D.H.; Lourenço, A.L.; Pwee, D.; Bridwell, A.E.M.; Hoffmann, M.; Voss, J.; Karmakar, P.; et al. A novel class of TMPRSS2 inhibitors potently block SARS-CoV-2 and MERS-CoV viral entry and protect human epithelial lung cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2108728118. [Google Scholar] [CrossRef]
- Böttcher-Friebertshäuser, E.; Freuer, C.; Sielaff, F.; Schmidt, S.; Eickmann, M.; Uhlendorff, J.; Steinmetzer, T.; Klenk, H.-D.; Garten, W. Cleavage of Influenza Virus Hemagglutinin by Airway Proteases TMPRSS2 and HAT Differs in Subcellular Localization and Susceptibility to Protease Inhibitors. J. Virol. 2010, 84, 5605–5614. [Google Scholar] [CrossRef]
- Sanda, M.; Morrison, L.; Goldman, R. N- and O-Glycosylation of the SARS-CoV-2 Spike Protein. Anal. Chem. 2021, 93, 2003–2009. [Google Scholar] [CrossRef]
| Sample | Selected Hits | Epitope Sequence |
|---|---|---|
| patient 1 | 5/8 | 811-PSKRSFIEDLLFNKVT-828 |
| 1/8 | 1143-ELDSFKEELDKYFKNHTSPDV-1165 | |
| patient 17 | 1 | 49- ASWFTALTQHGKEDLKFPRGQGVPINTNSSPDDQIGYYRRATRRIRGGDGKMKDLSPRWYFYYLGTGPEAGLPYGAN KDGIIWVATEGALNTPK DHIGTRNPANNAAIVL QLPQGTTLPKGFYAEGS -177 |
| patient 18 | 18/22 | 811-PSKRSFIEDLLFNKVT-828 |
| 2/22 | 1143-ELDSFKEELDKYFKN HTSPDVDLGDISGINASVVNIQKEIDR -1186 | |
| TUBS 21 | 11/67 | 553-ESNKKFLPFQQFGRDIA-571 |
| 33/67 | 810-KPSKRSFIEDLLFN-825 | |
| TUBS 42 | 18/29 | 809-SKPSKRSFIEDLLF-824 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballmann, R.; Hotop, S.-K.; Bertoglio, F.; Steinke, S.; Heine, P.A.; Chaudhry, M.Z.; Jahn, D.; Pucker, B.; Baldanti, F.; Piralla, A.; et al. ORFeome Phage Display Reveals a Major Immunogenic Epitope on the S2 Subdomain of SARS-CoV-2 Spike Protein. Viruses 2022, 14, 1326. https://doi.org/10.3390/v14061326
Ballmann R, Hotop S-K, Bertoglio F, Steinke S, Heine PA, Chaudhry MZ, Jahn D, Pucker B, Baldanti F, Piralla A, et al. ORFeome Phage Display Reveals a Major Immunogenic Epitope on the S2 Subdomain of SARS-CoV-2 Spike Protein. Viruses. 2022; 14(6):1326. https://doi.org/10.3390/v14061326
Chicago/Turabian StyleBallmann, Rico, Sven-Kevin Hotop, Federico Bertoglio, Stephan Steinke, Philip Alexander Heine, M. Zeeshan Chaudhry, Dieter Jahn, Boas Pucker, Fausto Baldanti, Antonio Piralla, and et al. 2022. "ORFeome Phage Display Reveals a Major Immunogenic Epitope on the S2 Subdomain of SARS-CoV-2 Spike Protein" Viruses 14, no. 6: 1326. https://doi.org/10.3390/v14061326
APA StyleBallmann, R., Hotop, S.-K., Bertoglio, F., Steinke, S., Heine, P. A., Chaudhry, M. Z., Jahn, D., Pucker, B., Baldanti, F., Piralla, A., Schubert, M., Čičin-Šain, L., Brönstrup, M., Hust, M., & Dübel, S. (2022). ORFeome Phage Display Reveals a Major Immunogenic Epitope on the S2 Subdomain of SARS-CoV-2 Spike Protein. Viruses, 14(6), 1326. https://doi.org/10.3390/v14061326









