Cardiac Complications of COVID-19 in Low-Risk Patients
Abstract
:1. Introduction
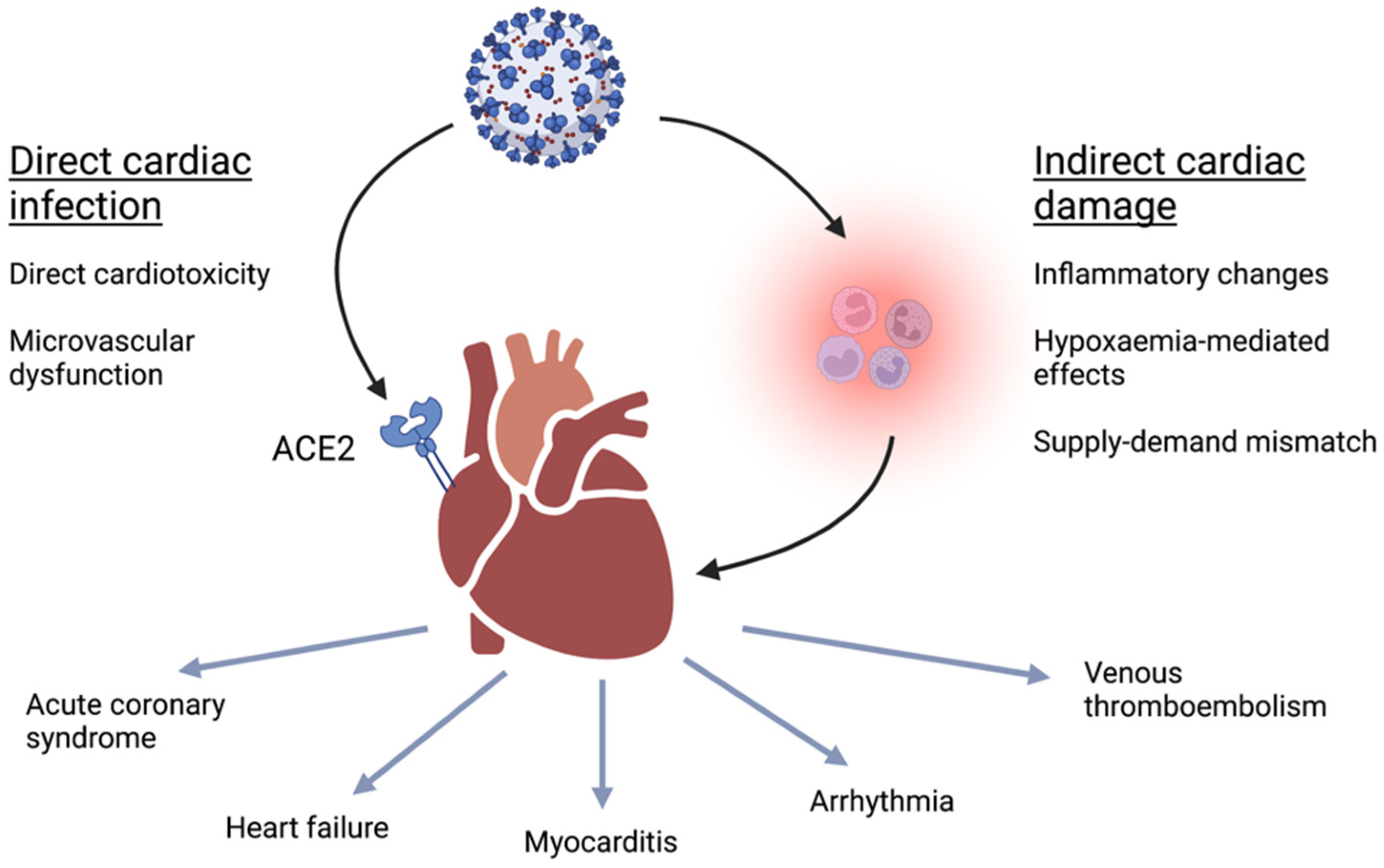
2. Pathophysiology of Cardiac Complications
3. Acute Cardiac Complications
3.1. Low-Risk Adults
3.2. Children
4. Post-Acute Cardiac Sequelae
Predictive Factors for Post-COVID Syndrome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| ARDS | Acute respiratory distress syndrome |
| COVID-19 | Coronavirus disease 2019 |
| NICE | National Institute for Health and Care Excellence |
| PIMS | Paediatric inflammatory multisystem syndrome |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Map. Available online: https://coronavirus.jhu.edu/map.html (accessed on 28 March 2022).
- Drake, T.M.; Riad, A.M.; Fairfield, C.J.; Egan, C.; Knight, S.R.; Pius, R.; Hardwick, H.E.; Norman, L.; Shaw, C.A.; McLean, K.A.; et al. Characterisation of In-Hospital Complications Associated with COVID-19 Using the ISARIC WHO Clinical Characterisation Protocol UK: A Prospective, Multicentre Cohort Study. Lancet 2021, 398, 223–237. [Google Scholar] [CrossRef]
- Gabarre, P.; Dumas, G.; Dupont, T.; Darmon, M.; Azoulay, E.; Zafrani, L. Acute Kidney Injury in Critically Ill Patients with COVID-19. Intensive Care Med. 2020, 46, 1339–1348. [Google Scholar] [CrossRef]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, Clinical Course, and Outcomes of Critically Ill Adults with COVID-19 in New York City: A Prospective Cohort Study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Vakili, K.; Fathi, M.; Pezeshgi, A.; Mohamadkhani, A.; Hajiesmaeili, M.; Rezaei-Tavirani, M.; Sayehmiri, F. Critical Complications of COVID-19: A Descriptive Meta-Analysis Study. Rev. Cardiovasc. Med. 2020, 21, 433–442. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-Term Cardiovascular Outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Collard, D.; Nurmohamed, N.S.; Kaiser, Y.; Reeskamp, L.F.; Dormans, T.; Moeniralam, H.; Simsek, S.; Douma, R.; Eerens, A.; Reidinga, A.C.; et al. Cardiovascular Risk Factors and COVID-19 Outcomes in Hospitalised Patients: A Prospective Cohort Study. BMJ Open 2021, 11, e045482. [Google Scholar] [CrossRef]
- Whittaker, E.; Bamford, A.; Kenny, J.; Kaforou, M.; Jones, C.E.; Shah, P.; Ramnarayan, P.; Fraisse, A.; Miller, O.; Davies, P.; et al. Clinical Characteristics of 58 Children with a Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2. JAMA 2020, 324, 259–269. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Elezkurtaj, S.; Greuel, S.; Ihlow, J.; Michaelis, E.G.; Bischoff, P.; Kunze, C.A.; Sinn, B.V.; Gerhold, M.; Hauptmann, K.; Ingold-Heppner, B.; et al. Causes of Death and Comorbidities in Hospitalized Patients with COVID-19. Sci. Rep. 2021, 11, 4263. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Huang, Z.; Lin, L.; Lv, J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Am. Heart Assoc. 2020, 9, e016219. [Google Scholar] [CrossRef]
- Zeng, J.-H.; Wu, W.-B.; Qu, J.-X.; Wang, Y.; Dong, C.-F.; Luo, Y.-F.; Zhou, D.; Feng, W.-X.; Feng, C. Cardiac Manifestations of COVID-19 in Shenzhen, China. Infection 2020, 48, 861–870. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-Cell RNA-Seq Data Analysis on the Receptor ACE2 Expression Reveals the Potential Risk of Different Human Organs Vulnerable to 2019-NCoV Infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural Basis of Receptor Recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.H.; Li, T.Y.; He, Z.C.; Ping, Y.F.; Liu, H.W.; Yu, S.C.; Mou, H.M.; Wang, L.H.; Zhang, H.R.; Fu, W.J.; et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi 2020, 49, 411–417. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, X.R.; Chen, H.-Y.; Penninger, J.M.; Lan, H.Y. Loss of Angiotensin-Converting Enzyme 2 Enhances TGF-β/Smad-Mediated Renal Fibrosis and NF-ΚB-Driven Renal Inflammation in a Mouse Model of Obstructive Nephropathy. Lab. Investig. 2012, 92, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.-F.; Zhang, L.-H.; Bai, F.; Wang, N.-P.; Garner, R.E.; McKallip, R.J.; Zhao, Z.-Q. Attenuation of Myocardial Fibrosis with Curcumin Is Mediated by Modulating Expression of Angiotensin II AT1/AT2 Receptors and ACE2 in Rats. Drug Des. Devel. Ther. 2015, 9, 6043–6054. [Google Scholar] [CrossRef] [PubMed]
- Kazbanov, I.V.; ten Tusscher, K.H.W.J.; Panfilov, A.V. Effects of Heterogeneous Diffuse Fibrosis on Arrhythmia Dynamics and Mechanism. Sci. Rep. 2016, 6, 20835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Webb, B.J.; Peltan, I.D.; Jensen, P.; Hoda, D.; Hunter, B.; Silver, A.; Starr, N.; Buckel, W.; Grisel, N.; Hummel, E.; et al. Clinical Criteria for COVID-19-Associated Hyperinflammatory Syndrome: A Cohort Study. Lancet Rheumatol. 2020, 2, e754–e763. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Madjid, M.; Musher, D.M. Role of Acute Infection in Triggering Acute Coronary Syndromes. Lancet Infect. Dis. 2010, 10, 83–92. [Google Scholar] [CrossRef]
- Doyen, D.; Moceri, P.; Ducreux, D.; Dellamonica, J. Myocarditis in a Patient with COVID-19: A Cause of Raised Troponin and ECG Changes. Lancet 2020, 395, 1516. [Google Scholar] [CrossRef]
- Trpkov, C.; MacMullan, P.; Feuchter, P.; Kachra, R.; Heydari, B.; Merchant, N.; Bristow, M.S.; White, J.A. Rapid Response to Cytokine Storm Inhibition Using Anakinra in a Patient With COVID-19 Myocarditis. CJC Open 2021, 3, 210–213. [Google Scholar] [CrossRef]
- Pinto, J.M.B.; Boyden, P.A. Electrical Remodeling in Ischemia and Infarction. Cardiovasc. Res. 1999, 42, 284–297. [Google Scholar] [CrossRef] [Green Version]
- Galán, M.; Vigón, L.; Fuertes, D.; Murciano-Antón, M.A.; Casado-Fernández, G.; Domínguez-Mateos, S.; Mateos, E.; Ramos-Martín, F.; Planelles, V.; Torres, M.; et al. Persistent Overactive Cytotoxic Immune Response in a Spanish Cohort of Individuals with Long-COVID: Identification of Diagnostic Biomarkers. Front. Immunol. 2022, 13, 848886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Richards, A.; Barrasa, M.I.; Hughes, S.H.; Young, R.A.; Jaenisch, R. Reverse-Transcribed SARS-CoV-2 RNA Can Integrate into the Genome of Cultured Human Cells and Can Be Expressed in Patient-Derived Tissues. Proc. Natl. Acad. Sci. USA 2021, 118, e2105968118. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, X.; Su, Y.; Ma, J.; Hong, K. Prevalence and Impact of Cardiac Injury on COVID-19: A Systematic Review and Meta-Analysis. Clin. Cardiol. 2021, 44, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and Cardiovascular Disease: From Basic Mechanisms to Clinical Perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Mirò, Ò.; Sabaté, M.; Jiménez, S.; Mebazaa, A.; Martínez-Nadal, G.; Piñera, P.; Burillo-Putze, G.; Martín, A.; Martín-Sánchez, F.J.; Jacob, J.; et al. A Case-Control, Multicentre Study of Consecutive Patients with COVID-19 and Acute (Myo)Pericarditis: Incidence, Risk Factors, Clinical Characteristics and Outcomes. Emerg. Med. J. 2022, 39, 402–410. [Google Scholar] [CrossRef]
- Rathore, S.S.; Rojas, G.A.; Sondhi, M.; Pothuru, S.; Pydi, R.; Kancherla, N.; Singh, R.; Ahmed, N.K.; Shah, J.; Tousif, S.; et al. Myocarditis Associated with Covid-19 Disease: A Systematic Review of Published Case Reports and Case Series. Int. J. Clin. Pract. 2021, 75, e14470. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of Myocarditis, Pericarditis, and Cardiac Arrhythmias Associated with COVID-19 Vaccination or SARS-CoV-2 Infection. Nat. Med. 2022, 28, 410–422. [Google Scholar] [CrossRef]
- Shah, R.M.; Shah, M.; Shah, S.; Li, A.; Jauhar, S. Takotsubo Syndrome and COVID-19: Associations and Implications. Curr. Probl. Cardiol. 2021, 46, 100763. [Google Scholar] [CrossRef]
- Omerovic, E.; Citro, R.; Bossone, E.; Redfors, B.; Backs, J.; Bruns, B.; Ciccarelli, M.; Couch, L.S.; Dawson, D.; Grassi, G.; et al. Pathophysiology of Takotsubo Syndrome—A Joint Scientific Statement from the Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of the European Society of Cardiology—Part 1: Overview and the Central Role for Catecholamines and Sympathetic Nervous System. Eur. J. Heart Fail. 2022, 24, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Omerovic, E.; Citro, R.; Bossone, E.; Redfors, B.; Backs, J.; Bruns, B.; Ciccarelli, M.; Couch, L.S.; Dawson, D.; Grassi, G.; et al. Pathophysiology of Takotsubo Syndrome—A Joint Scientific Statement from the Heart Failure Association Takotsubo Syndrome Study Group and Myocardial Function Working Group of the European Society of Cardiology—Part 2: Vascular Pathophysiology, Gender and Sex Hormones, Genetics, Chronic Cardiovascular Problems and Clinical Implications. Eur. J. Heart Fail. 2022, 24, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Couch, L.S.; Fiedler, J.; Chick, G.; Clayton, R.; Dries, E.; Wienecke, L.M.; Fu, L.; Fourre, J.; Pandey, P.; Derda, A.A.; et al. Circulating MicroRNAs Predispose to Takotsubo Syndrome Following High-Dose Adrenaline Exposure. Cardiovasc. Res. 2021, cvab210. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Chiarito, M.; Ferrante, G.; Cannata, F.; Azzolini, E.; Viggiani, G.; De Marco, A.; Briani, M.; Bocciolone, M.; Bragato, R.; et al. Early Detection of Elevated Cardiac Biomarkers to Optimise Risk Stratification in Patients with COVID-19. Heart 2020, 106, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-J.; Cheng, X.; Zhou, F.; Lei, F.; Akolkar, G.; Cai, J.; Zhang, X.-J.; Blet, A.; Xie, J.; Zhang, P.; et al. Redefining Cardiac Biomarkers in Predicting Mortality of Inpatients With COVID-19. Hypertension 2020, 76, 1104–1112. [Google Scholar] [CrossRef]
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Salvo, G.D.; Sade, L.E.; Pearce, K.; et al. Global Evaluation of Echocardiography in Patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 949–958. [Google Scholar] [CrossRef]
- Szekely, Y.; Lichter, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Gal Oz, A.; Rothschild, E.; Baruch, G.; Peri, Y.; et al. Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation 2020, 142, 342–353. [Google Scholar] [CrossRef]
- Giustino, G.; Croft, L.B.; Stefanini, G.G.; Bragato, R.; Silbiger, J.J.; Vicenzi, M.; Danilov, T.; Kukar, N.; Shaban, N.; Kini, A.; et al. Characterization of Myocardial Injury in Patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2043–2055. [Google Scholar] [CrossRef]
- Smith, C.; Odd, D.; Harwood, R.; Ward, J.; Linney, M.; Clark, M.; Hargreaves, D.; Ladhani, S.N.; Draper, E.; Davis, P.J.; et al. Deaths in Children and Young People in England after SARS-CoV-2 Infection during the First Pandemic Year. Nat. Med. 2022, 28, 185–192. [Google Scholar] [CrossRef]
- Meyerowitz-Katz, G.; Merone, L. A Systematic Review and Meta-Analysis of Published Research Data on COVID-19 Infection Fatality Rates. Int. J. Infect. Dis. 2020, 101, 138–148. [Google Scholar] [CrossRef]
- Irfan, O.; Muttalib, F.; Tang, K.; Jiang, L.; Lassi, Z.S.; Bhutta, Z. Clinical Characteristics, Treatment and Outcomes of Paediatric COVID-19: A Systematic Review and Meta-Analysis. Arch. Dis. Child. 2021, 106, 440–448. [Google Scholar] [CrossRef] [PubMed]
- CDC COVID-19 Response Team. Coronavirus Disease 2019 in Children—United States, February 12-April 2, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An Outbreak of Severe Kawasaki-like Disease at the Italian Epicentre of the SARS-CoV-2 Epidemic: An Observational Cohort Study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef]
- Ramcharan, T.; Nolan, O.; Lai, C.Y.; Prabhu, N.; Krishnamurthy, R.; Richter, A.G.; Jyothish, D.; Kanthimathinathan, H.K.; Welch, S.B.; Hackett, S.; et al. Paediatric Inflammatory Multisystem Syndrome: Temporally Associated with SARS-CoV-2 (PIMS-TS): Cardiac Features, Management and Short-Term Outcomes at a UK Tertiary Paediatric Hospital. Pediatr. Cardiol. 2020, 41, 1391–1401. [Google Scholar] [CrossRef]
- Penner, J.; Abdel-Mannan, O.; Grant, K.; Maillard, S.; Kucera, F.; Hassell, J.; Eyre, M.; Berger, Z.; Hacohen, Y.; Moshal, K.; et al. 6-Month Multidisciplinary Follow-up and Outcomes of Patients with Paediatric Inflammatory Multisystem Syndrome (PIMS-TS) at a UK Tertiary Paediatric Hospital: A Retrospective Cohort Study. Lancet Child Adolesc. Health 2021, 5, 473–482. [Google Scholar] [CrossRef]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory Shock in Children during COVID-19 Pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Webb, K.; Abraham, D.R.; Faleye, A.; McCulloch, M.; Rabie, H.; Scott, C. Multisystem Inflammatory Syndrome in Children in South Africa. Lancet Child Adolesc. Health 2020, 4, e38. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Belot, A.; Antona, D.; Renolleau, S.; Javouhey, E.; Hentgen, V.; Angoulvant, F.; Delacourt, C.; Iriart, X.; Ovaert, C.; Bader-Meunier, B.; et al. SARS-CoV-2-Related Paediatric Inflammatory Multisystem Syndrome, an Epidemiological Study, France, 1 March to 17 May 2020. Eurosurveillance 2020, 25, 2001010. [Google Scholar] [CrossRef]
- Moraleda, C.; Serna-Pascual, M.; Soriano-Arandes, A.; Simó, S.; Epalza, C.; Santos, M.; Grasa, C.; Rodríguez, M.; Soto, B.; Gallego, N.; et al. Multi-Inflammatory Syndrome in Children Related to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Spain. Clin. Infect. Dis. 2021, 72, e397–e401. [Google Scholar] [CrossRef]
- Toubiana, J.; Poirault, C.; Corsia, A.; Bajolle, F.; Fourgeaud, J.; Angoulvant, F.; Debray, A.; Basmaci, R.; Salvador, E.; Biscardi, S.; et al. Kawasaki-like Multisystem Inflammatory Syndrome in Children during the Covid-19 Pandemic in Paris, France: Prospective Observational Study. BMJ 2020, 369, m2094. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.Y.; Oster, M.E.; Godfred-Cato, S.E.; Bryant, B.; Datta, S.D.; Campbell, A.P.; Leung, J.W.; Tsang, C.A.; Pierce, T.J.; Kennedy, J.L.; et al. Factors Linked to Severe Outcomes in Multisystem Inflammatory Syndrome in Children (MIS-C) in the USA: A Retrospective Surveillance Study. Lancet Child Adolesc. Health 2021, 5, 323–331. [Google Scholar] [CrossRef]
- Dionne, A.; Mah, D.Y.; Son, M.B.F.; Lee, P.Y.; Henderson, L.; Baker, A.L.; de Ferranti, S.D.; Fulton, D.R.; Newburger, J.W.; Friedman, K.G. Atrioventricular Block in Children with Multisystem Inflammatory Syndrome. Pediatrics 2020, 146, e2020009704. [Google Scholar] [CrossRef]
- Matsubara, D.; Kauffman, H.L.; Wang, Y.; Calderon-Anyosa, R.; Nadaraj, S.; Elias, M.D.; White, T.J.; Torowicz, D.L.; Yubbu, P.; Giglia, T.M.; et al. Echocardiographic Findings in Pediatric Multisystem Inflammatory Syndrome Associated With COVID-19 in the United States. J. Am. Coll. Cardiol. 2020, 76, 1947–1961. [Google Scholar] [CrossRef]
- Godfred-Cato, S.; Bryant, B.; Leung, J.; Oster, M.E.; Conklin, L.; Abrams, J.; Roguski, K.; Wallace, B.; Prezzato, E.; Koumans, E.H.; et al. COVID-19–Associated Multisystem Inflammatory Syndrome in Children—United States, March–July 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1074–1080. [Google Scholar] [CrossRef]
- Flood, J.; Shingleton, J.; Bennett, E.; Walker, B.; Amin-Chowdhury, Z.; Oligbu, G.; Avis, J.; Lynn, R.M.; Davis, P.; Bharucha, T.; et al. Paediatric Multisystem Inflammatory Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS): Prospective, National Surveillance, United Kingdom and Ireland, 2020. Lancet Reg. Health Eur. 2021, 3, 100075. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.L.; Harwood, R.; Smith, C.; Kenny, S.; Clark, M.; Davis, P.J.; Draper, E.S.; Hargreaves, D.; Ladhani, S.; Linney, M.; et al. Risk Factors for PICU Admission and Death among Children and Young People Hospitalized with COVID-19 and PIMS-TS in England during the First Pandemic Year. Nat. Med. 2022, 28, 193–200. [Google Scholar] [CrossRef]
- Chua, G.T.; Wong, J.S.C.; Lam, I.; Ho, P.P.K.; Chan, W.H.; Yau, F.Y.S.; Rosa Duque, J.S.; Ho, A.C.C.; Siu, K.K.; Cheung, T.W.Y.; et al. Clinical Characteristics and Transmission of COVID-19 in Children and Youths During 3 Waves of Outbreaks in Hong Kong. JAMA Netw. Open 2021, 4, e218824. [Google Scholar] [CrossRef]
- Chou, J.; Platt, C.D.; Habiballah, S.; Nguyen, A.A.; Elkins, M.; Weeks, S.; Peters, Z.; Day-Lewis, M.; Novak, T.; Armant, M.; et al. Mechanisms Underlying Genetic Susceptibility to Multisystem Inflammatory Syndrome in Children (MIS-C). J. Allergy Clin. Immunol. 2021, 148, 732–738.e1. [Google Scholar] [CrossRef]
- Taribagil, P.; Creer, D.; Tahir, H. ‘Long COVID’ Syndrome. BMJ Case Rep. CP 2021, 14, e241485. [Google Scholar] [CrossRef]
- Omololu, A.; Ojelade, B.; Ajayi, O.; Adesomi, T.; Alade, O.; Adebisi, S.; Nwadike, V. “Long COVID”: A Case Report of Persistent Symptoms in a Patient with Prolonged SARS-CoV-2 Shedding for over 110 Days. SAGE Open Med. Case Rep. 2021, 9, 2050313X211015494. [Google Scholar] [CrossRef] [PubMed]
- Malkova, A.; Kudryavtsev, I.; Starshinova, A.; Kudlay, D.; Zinchenko, Y.; Glushkova, A.; Yablonskiy, P.; Shoenfeld, Y. Post COVID-19 Syndrome in Patients with Asymptomatic/Mild Form. Pathogens 2021, 10, 1408. [Google Scholar] [CrossRef]
- Overview|COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 18 April 2022).
- Miglis, M.G.; Prieto, T.; Shaik, R.; Muppidi, S.; Sinn, D.-I.; Jaradeh, S. A Case Report of Postural Tachycardia Syndrome after COVID-19. Clin. Auton. Res. 2020, 30, 449–451. [Google Scholar] [CrossRef]
- Vallejo, N.; Teis, A.; Mateu, L.; Bayés-Genís, A. Persistent Chest Pain after Recovery of COVID-19: Microvascular Disease-Related Angina? Eur. Heart J. Case Rep. 2021, 5, ytab105. [Google Scholar] [CrossRef] [PubMed]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan Impairment in Low-Risk Individuals with Post-COVID-19 Syndrome: A Prospective, Community-Based Study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyère, F.; Stefic, K.; et al. Follow-up of Adults with Noncritical COVID-19 Two Months after Symptom Onset. Clin. Microbiol. Infect. 2021, 27, 258–263. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. for the Gemelli Against COVID-19 Post-Acute Care Study Group Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Lo, Y.L.; Leong, H.N.; Hsu, L.Y.; Tan, T.T.; Kurup, A.; Fook-Chong, S.; Tan, B.H. Autonomic Dysfunction in Recovered Severe Acute Respiratory Syndrome Patients. Can. J. Neurol. Sci. 2005, 32, 264. [Google Scholar] [CrossRef] [Green Version]
- Hammami, R.; Bahloul, A.; Charfeddine, S.; Gargouri, R.; Abid, L. The Long COVID: Findings of 24 Rhythmic Holter in Patients Suffering from Palpitations. Arch. Cardiovasc. Dis. Suppl. 2022, 14, 82–83. [Google Scholar] [CrossRef]
- Nurek, M.; Rayner, C.; Freyer, A.; Taylor, S.; Järte, L.; MacDermott, N.; Delaney, B.C. Recommendations for the Recognition, Diagnosis, and Management of Long COVID: A Delphi Study. Br. J. Gen. Pract. 2021, 71, e815–e825. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From COVID-19 Infection. JAMA Cardiol. 2021, 6, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhang, Z.; Luo, L.; Wu, W.; Jia, T.; Lu, L.; Liu, W.V.; Qin, Y.; Hu, F.; Ding, X.; et al. Cardiac T1 and T2 Mapping Showed Myocardial Involvement in Recovered COVID-19 Patients Initially Considered Devoid of Cardiac Damage. J. Magn. Reason. Imaging 2021, 54, 421–428. [Google Scholar] [CrossRef]
- Huang, Y.; Pinto, M.D.; Borelli, J.L.; Mehrabadi, M.A.; Abrihim, H.; Dutt, N.; Lambert, N.; Nurmi, E.L.; Chakraborty, R.; Rahmani, A.M.; et al. COVID Symptoms, Symptom Clusters, and Predictors for Becoming a Long-Hauler: Looking for Clarity in the Haze of the Pandemic. medRxiv 2021. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male Sex Identified by Global COVID-19 Meta-Analysis as a Risk Factor for Death and ITU Admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Sigfrid, L.; Drake, T.M.; Pauley, E.; Jesudason, E.C.; Olliaro, P.; Lim, W.S.; Gillesen, A.; Berry, C.; Lowe, D.J.; McPeake, J.; et al. Long Covid in Adults Discharged from UK Hospitals after Covid-19: A Prospective, Multicentre Cohort Study Using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg. Health Eur. 2021, 8, 100186. [Google Scholar] [CrossRef]
- Bai, F.; Tomasoni, D.; Falcinella, C.; Barbanotti, D.; Castoldi, R.; Mulè, G.; Augello, M.; Mondatore, D.; Allegrini, M.; Cona, A.; et al. Female Gender Is Associated with Long COVID Syndrome: A Prospective Cohort Study. Clin. Microbiol. Infect. 2022, 28, 611.e9–611.e16. [Google Scholar] [CrossRef]
- Evans, R.A.; McAuley, H.; Harrison, E.M.; Shikotra, A.; Singapuri, A.; Sereno, M.; Elneima, O.; Docherty, A.B.; Lone, N.I.; Leavy, O.C.; et al. Physical, Cognitive, and Mental Health Impacts of COVID-19 after Hospitalisation (PHOSP-COVID): A UK Multicentre, Prospective Cohort Study. Lancet Respir. Med. 2021, 9, 1275–1287. [Google Scholar] [CrossRef]
- Wormser, G.P.; Shapiro, E.D. Implications of Gender in Chronic Lyme Disease. J. Womens Health (Larchmt) 2009, 18, 831–834. [Google Scholar] [CrossRef] [Green Version]
- Cervia, C.; Zurbuchen, Y.; Taeschler, P.; Ballouz, T.; Menges, D.; Hasler, S.; Adamo, S.; Raeber, M.E.; Bächli, E.; Rudiger, A.; et al. Immunoglobulin Signature Predicts Risk of Post-Acute COVID-19 Syndrome. Nat. Commun. 2022, 13, 446. [Google Scholar] [CrossRef] [PubMed]
| PIMS | Kawasaki disease | |
|---|---|---|
| Age | School-age children (~7–8 years) | Infants and young children (~3 years) |
| Ethnicity | Predominantly Black and Hispanic | Asian |
| Gastrointestinal involvement | Common | Uncommon |
| Cardiovascular involvement | Common | Less common |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivasan, A.; Wong, F.; Couch, L.S.; Wang, B.X. Cardiac Complications of COVID-19 in Low-Risk Patients. Viruses 2022, 14, 1322. https://doi.org/10.3390/v14061322
Srinivasan A, Wong F, Couch LS, Wang BX. Cardiac Complications of COVID-19 in Low-Risk Patients. Viruses. 2022; 14(6):1322. https://doi.org/10.3390/v14061322
Chicago/Turabian StyleSrinivasan, Akash, Felyx Wong, Liam S. Couch, and Brian X. Wang. 2022. "Cardiac Complications of COVID-19 in Low-Risk Patients" Viruses 14, no. 6: 1322. https://doi.org/10.3390/v14061322
APA StyleSrinivasan, A., Wong, F., Couch, L. S., & Wang, B. X. (2022). Cardiac Complications of COVID-19 in Low-Risk Patients. Viruses, 14(6), 1322. https://doi.org/10.3390/v14061322






