Independent Evaluation of Cell Culture Systems for Hepatitis E Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Media
2.2. HEV Isolates
2.3. Propagation of HEV Strains in Cell Culture
2.4. HEV RNA Quantification
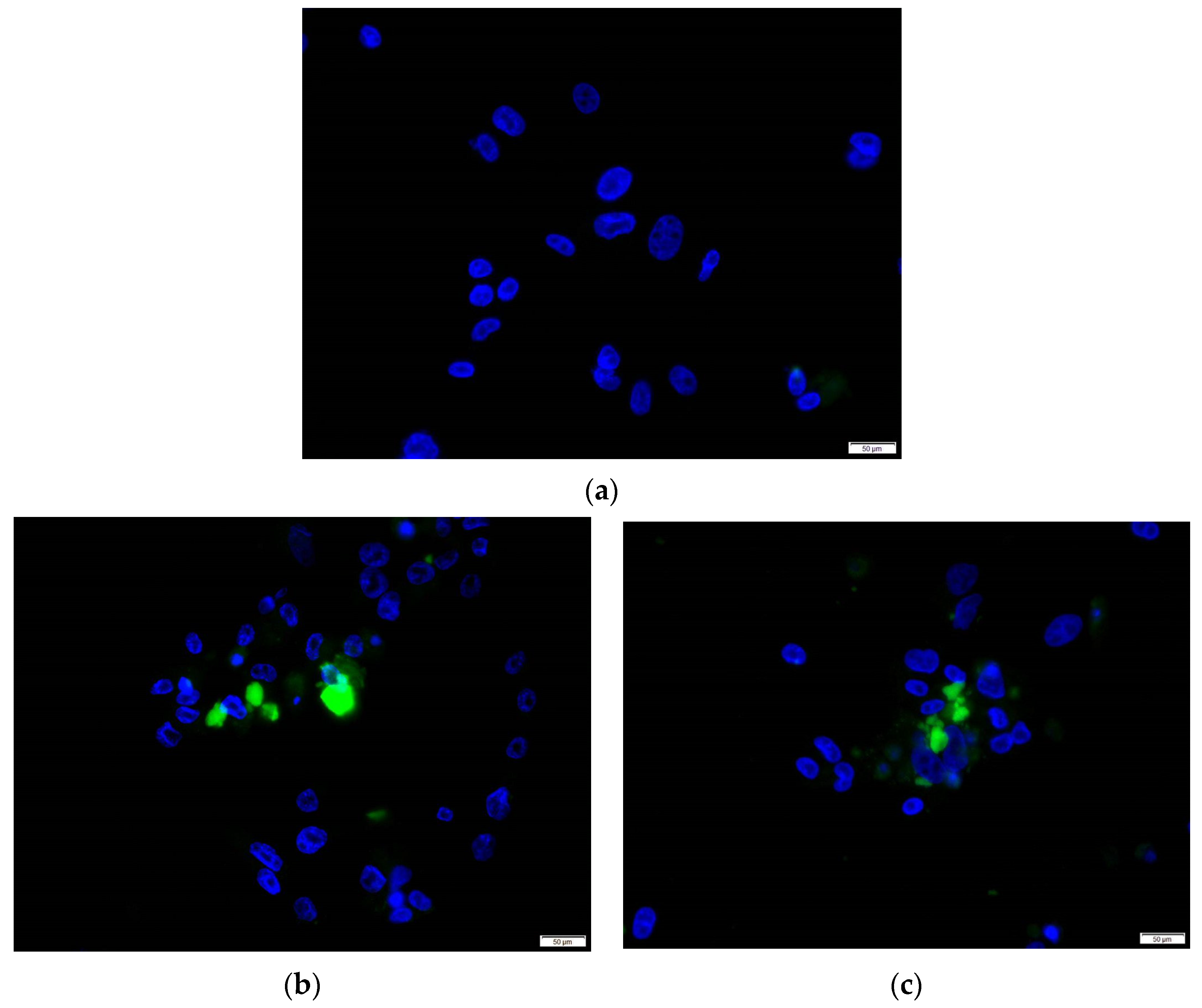
2.5. Immunofluorescence Assay
2.6. Detection of HEV ORF2 Antigen
2.7. Conversion of eHEV to nHEV
2.8. Ribavirin Inhibition Assay
2.9. Statistical Analysis
3. Results
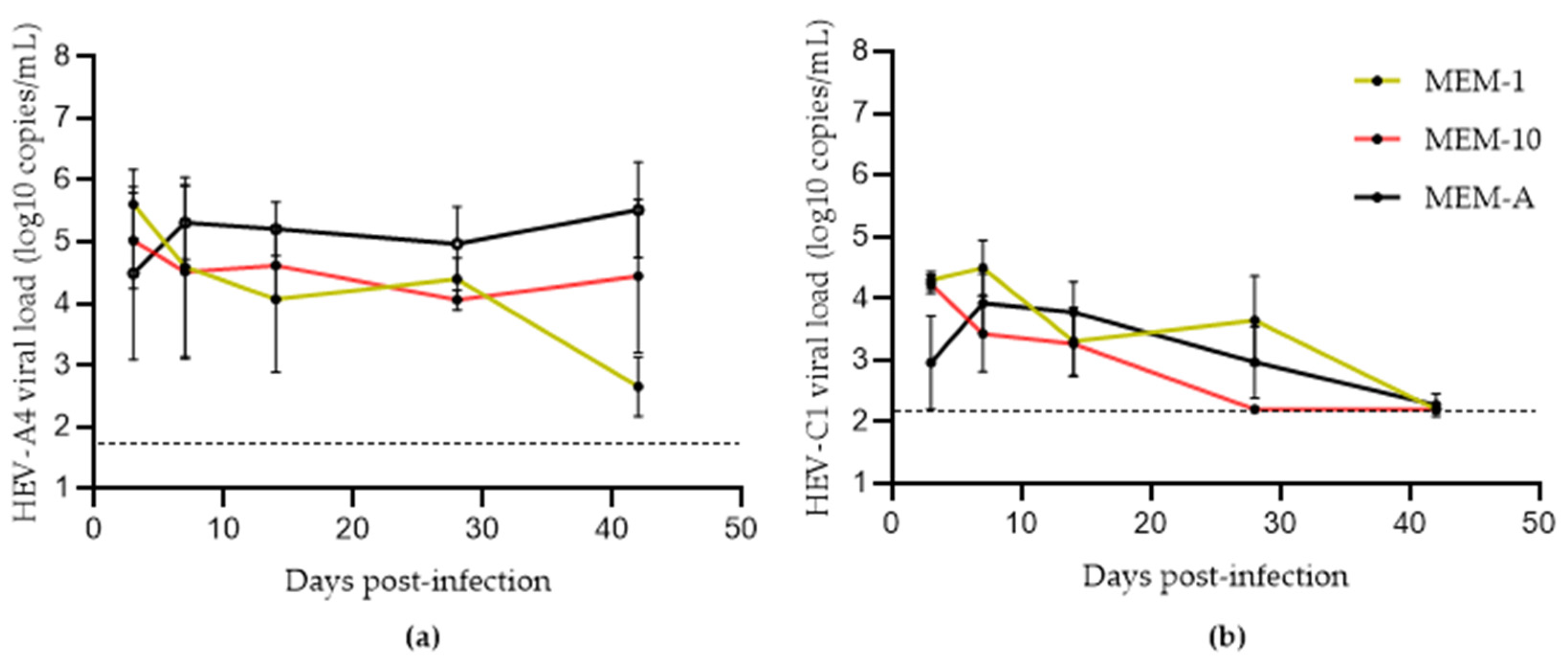
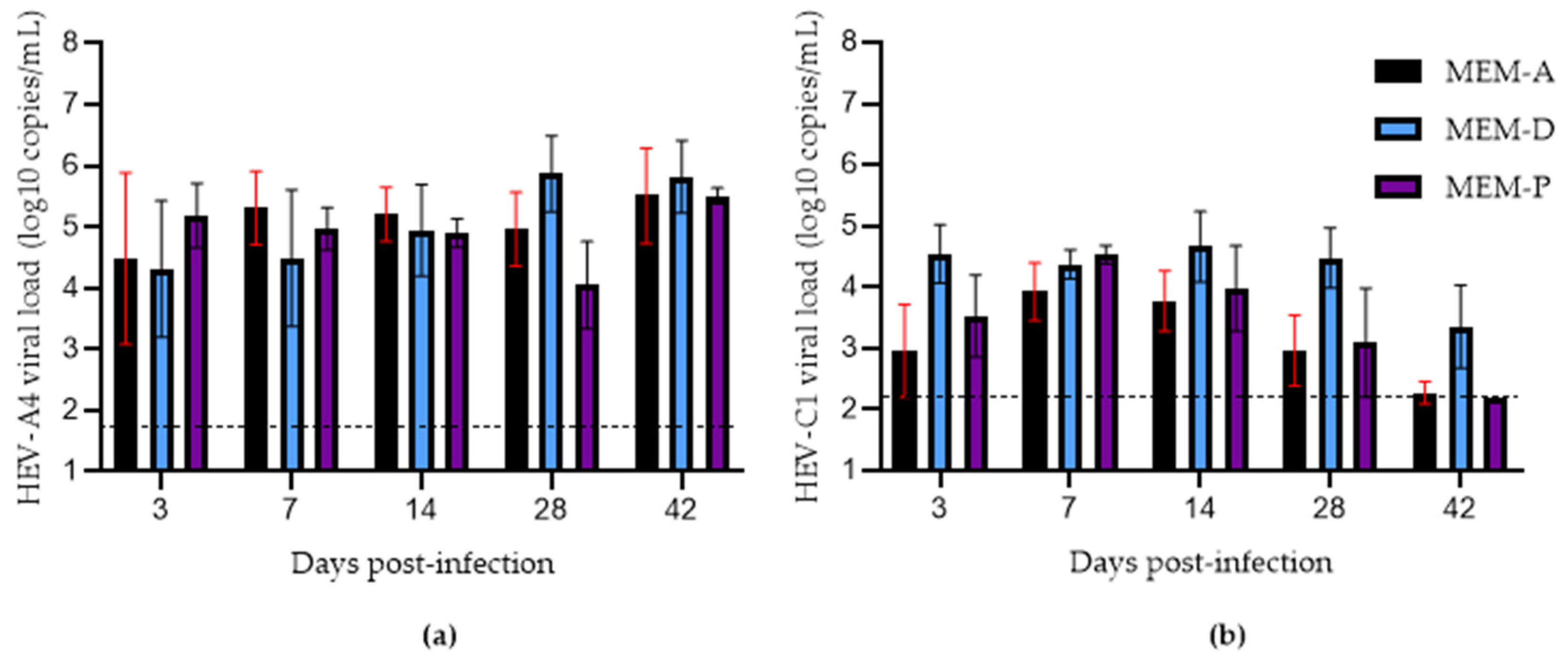
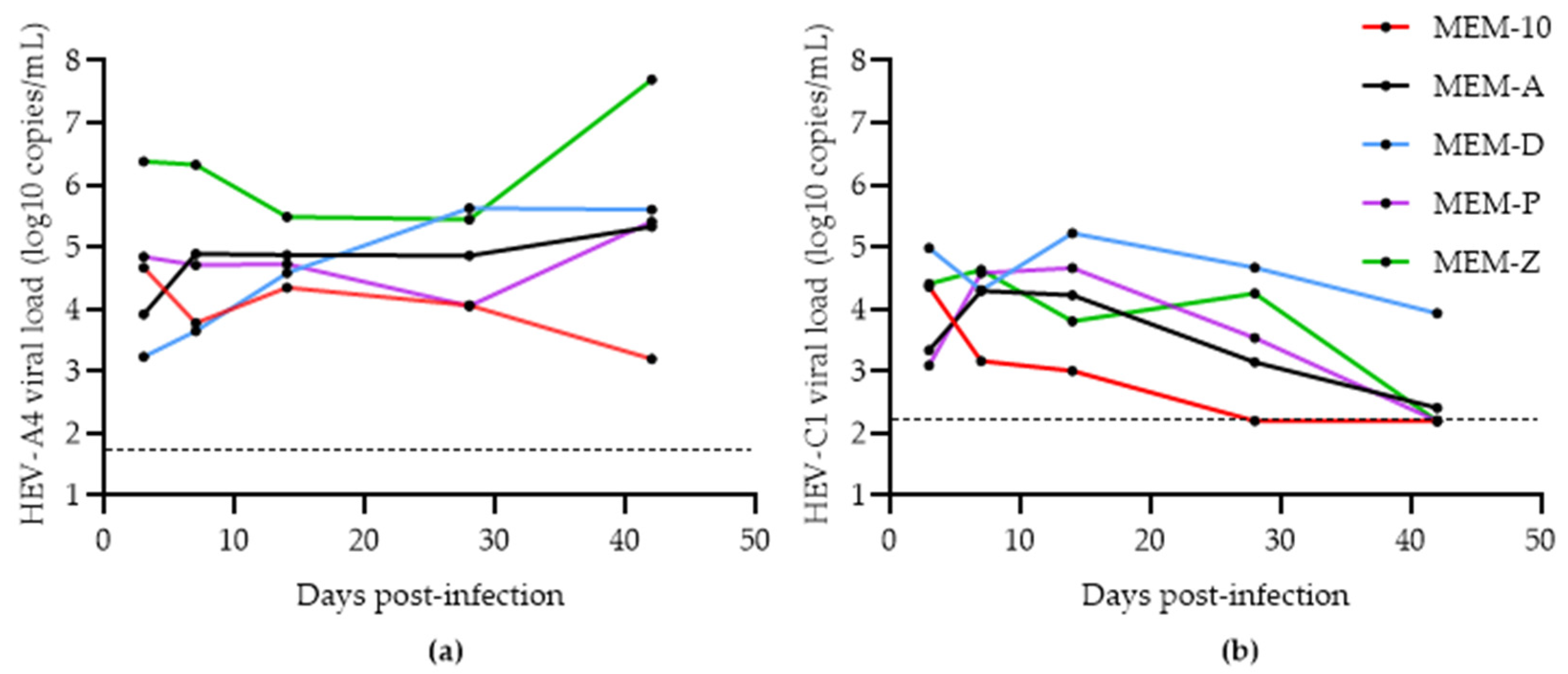
3.1. Assessing the Effects of Hyper-Confluence, Amphotericin B, and MgCl2 on Culture Yield of HEV-A4 and HEV-C1
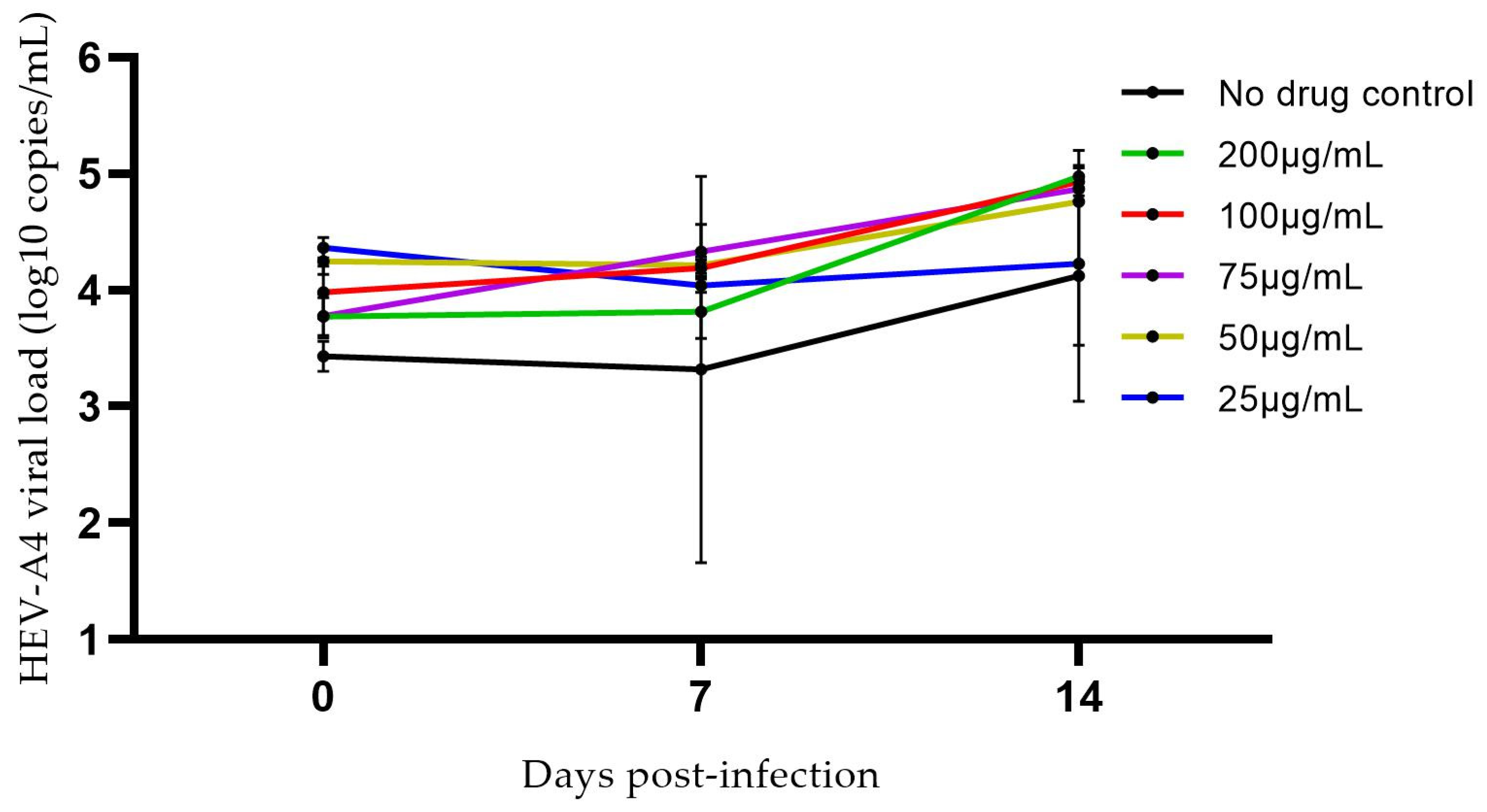
3.2. Effects of Progesterone and DMSO on Culture Yield
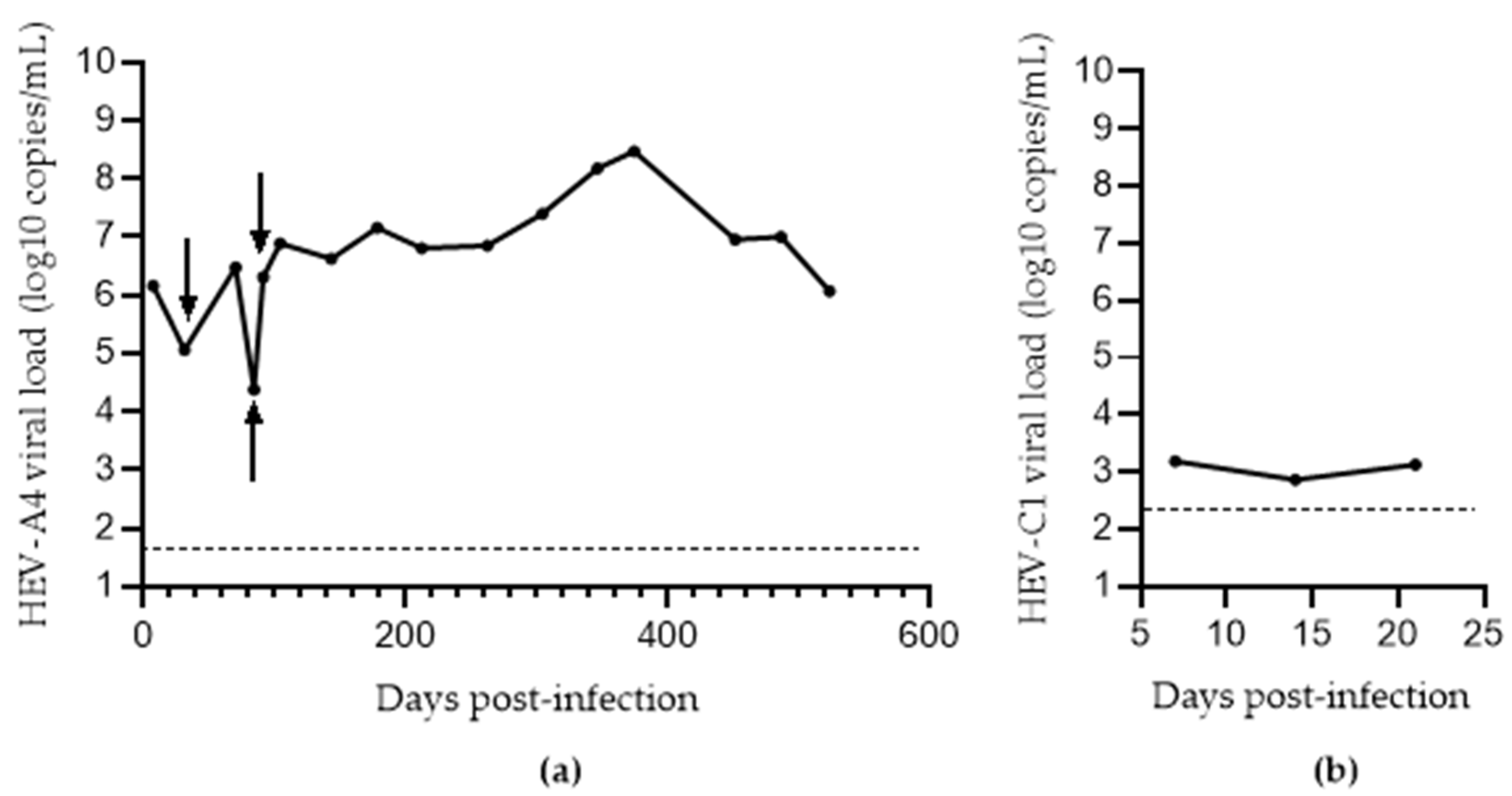
3.3. Comparison of Methods for Converting eHEV to nHEV
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marion, O.; Izopet, J.; Kamar, N. Hepatitis E virus infection. Rev. Prat. 2018, 68, 286–290. [Google Scholar] [PubMed]
- Cao, D.; Meng, X.-J. Molecular biology and replication of hepatitis E virus. Emerg. Microbes Infect. 2012, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, X.-L. Chirohepevirus from Bats: Insights into Hepatitis E Virus Diversity and Evolution. Viruses 2022, 14, 905. [Google Scholar] [CrossRef] [PubMed]
- Legrand-Abravanel, F.; Kamar, N.; Sandres-Saune, K.; Garrouste, C.; Dubois, M.; Mansuy, J.M.; Muscari, F.; Sallusto, F.; Rostaing, L.; Izopet, J. Characteristics of Autochthonous Hepatitis E Virus Infection in Solid—Organ Transplant Recipients in France. J. Infect. Dis. 2010, 202, 835–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johne, R.; Plenge-Bönig, A.; Hess, M.; Ulrich, R.G.; Reetz, J.; Schielke, A. Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J. Gen. Virol. 2010, 91 Pt 3, 750–758. [Google Scholar] [CrossRef]
- Sridhar, S.; Situ, J.; Cai, J.-P.; Yip, C.C.-Y.; Wu, S.; Zhang, A.J.-X.; Wen, L.; Chew, N.F.S.; Chan, W.M.; Poon, R.W.S.; et al. Multimodal investigation of rat hepatitis E virus antigenicity: Implications for infection, diagnostics, and vaccine efficacy. J. Hepatol. 2021, 74, 1315–1324. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Frias, M.; Lopez-Lopez, P.; de Los Angeles Risalde, M.; Brieva, T.; Machuca, I.; Camacho, A.; Martinez-Peinado, A.; Gomez-Villamandos, J.C.; Rivero, A. Hepatitis E Virus (HEV) Infection in Anti-HEV Immunoglobulin G-Carrying Patients after Successful Hepatitis C Virus Treatment: Reactivation or Reinfection? Clin. Infect. Dis. 2017, 64, 964–966. [Google Scholar]
- Andonov, A.; Robbins, M.; Borlang, J.; Cao, J.; Hatchette, T.; Stueck, A.; Deschambault, Y.; Murnaghan, K.; Varga, J.; Johnston, L. Rat Hepatitis E Virus Linked to Severe Acute Hepatitis in an Immunocompetent Patient. J. Infect. Dis. 2019, 220, 951–955. [Google Scholar] [CrossRef]
- Sridhar, S.; Yip, C.C.Y.; Wu, S.; Cai, J.; Zhang, A.J.-X.; Leung, K.-H.; Chung, T.W.; Chan, J.F.; Chan, W.M.; Teng, J.L.; et al. Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant. Emerg. Infect. Dis. 2018, 24, 2241–2250. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, S.; Yip, C.C.; Lo, K.H.; Wu, S.; Situ, J.; Chew, N.F.; Leung, K.H.; Chan, H.S.Y.; Wong, S.C.Y.; Leung, A.W.S.; et al. Hepatitis E virus species C infection in humans, Hong Kong. Clin. Infect. Dis. 2021, ciab919. [Google Scholar] [CrossRef]
- Sridhar, S.; Yip, C.C.Y.; Wu, S.; Chew, N.F.S.; Leung, K.H.; Chan, J.F.W.; Zhao, P.S.; Chan, W.M.; Poon, R.W.S.; Tsoi, H.W.; et al. Transmission of Rat Hepatitis E Virus Infection to Humans in Hong Kong: A Clinical and Epidemiological Analysis. Hepatology 2021, 73, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Meister, T.L.; Bruening, J.; Todt, D.; Steinmann, E. Cell culture systems for the study of hepatitis E virus. Antivir. Res. 2019, 163, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Ambardekar, C.; Lu, Y.; Feng, Z.; Ou, J.H.J. Distinct Entry Mechanisms for Nonenveloped and Quasi-Enveloped Hepatitis E Viruses. J. Virol. 2016, 90, 4232–4242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridhar, S. Use of S17 fragment containing hepatitis E virus infectious clones in cell culture experiments: The fine print does matter. J. Viral Hepat. 2018, 25, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, S.; Nicot, F.; Jeanne, N.; Dimeglio, C.; Roulet, A.; Lefebvre, C.; Carcenac, R.; Manno, M.; Dubois, M.; Peron, J.M.; et al. Insertions and Duplications in the Polyproline Region of the Hepatitis E Virus. Front. Microbiol. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp, C.P.; Biedermann, P.; Harms, D.; Wang, B.; Kebelmann, M.; Choi, M.; Helmuth, J.; Corman, V.M.; Thürmer, A.; Altmann, B.; et al. Advanced sequencing approaches detected insertions of viral and human origin in the viral genome of chronic hepatitis E virus patients. Sci. Rep. 2022, 12, 1720. [Google Scholar] [CrossRef] [PubMed]
- Capelli, N.; Dubois, M.; Pucelle, M.; Da Silva, I.; Lhomme, S.; Abravanel, F.; Chapuy-Regaud, S.; Izopet, J. Optimized Hepatitis E Virus (HEV) Culture and Its Application to Measurements of HEV Infectivity. Viruses 2020, 12, 139. [Google Scholar] [CrossRef] [Green Version]
- Sooryanarain, H.; Ahmed, S.A.; Meng, X.-J.; Patton John, T. Progesterone-Mediated Enhancement of Hepatitis E Virus Replication in Human Liver Cells. mBio 2021, 12, e01434-21. [Google Scholar] [CrossRef]
- Schemmerer, M.; Johne, R.; Erl, M.; Jilg, W.; Wenzel, J.J. Isolation of Subtype 3c, 3e and 3f-Like Hepatitis E Virus Strains Stably Replicating to High Viral Loads in an Optimized Cell Culture System. LID 2019, 11, 483. [Google Scholar] [CrossRef] [Green Version]
- Shiota, T.; Li, T.C.; Yoshizaki, S.; Kato, T.; Wakita, T.; Ishii, K. Establishment of hepatitis E virus infection-permissive and -non-permissive human hepatoma PLC/PRF/5 subclones. Microbiol. Immunol. 2015, 59, 89–94. [Google Scholar] [CrossRef]
- Jirintai, S.; Tanggis; Mulyanto; Suparyatmo, J.B.; Takahashi, M.; Kobayashi, T.; Nagashima, S.; Nishizawa, T.; Okamoto, H. Rat hepatitis E virus derived from wild rats (Rattus rattus) propagates efficiently in human hepatoma cell lines. Virus Res. 2014, 185, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Schemmerer, M.; Erl, M.; Wenzel, J.J. HuH-7-Lunet BLR Cells Propagate Rat Hepatitis E Virus (HEV) in a Cell Culture System Optimized for HEV. Viruses 2022, 14, 1116. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Cheng, V.C.C.; Wong, S.C.; Yip, C.C.Y.; Wu, S.; Lo, A.W.I.; Leung, K.H.; Mak, W.W.; Cai, J.; Li, X.; et al. Donor-Derived Genotype 4 Hepatitis E Virus Infection, Hong Kong, China, 2018. Emerg. Infect. Dis. 2019, 25, 425–433. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, F.; Zhang, L.; Harrison, T.J.; Huang, W.; Zhao, C.; Kong, W.; Jiang, C.; Wang, Y. Hepatitis E Virus Produced from Cell Culture Has a Lipid Envelope. PLoS ONE 2015, 10, e0132503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ideno, S.; Inoue, T.; Takahashi, K.; Urayama, T.; Maeno, H.; Takeuchi, K.; Sakai, K. Phenotypic characterization of cell culture-derived hepatitis E virus subjected to different chemical treatments: Application in virus removal via nanofiltration. J. Virol. Methods 2021, 296, 114244. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Tanaka, T.; Takahashi, H.; Hoshino, Y.; Nagashima, S.; Jirintai, N.; Mizuo, H.; Yazaki, Y.; Takagi, T.; Azuma, M.; et al. Hepatitis E Virus (HEV) Strains in Serum Samples Can Replicate Efficiently in Cultured Cells Despite the Coexistence of HEV Antibodies: Characterization of HEV Virions in Blood Circulation. J. Clin. Microbiol. 2010, 48, 1112–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montpellier, C.; Wychowski, C.; Sayed, I.M.; Meunier, J.-C.; Saliou, J.-M.; Ankavay, M.; Bull, A.; Pillez, A.; Abravanel, F.; Helle, F.; et al. Hepatitis E Virus Lifecycle and Identification of 3 Forms of the ORF2 Capsid Protein. Gastroenterology 2018, 154, 211–223.e8. [Google Scholar] [CrossRef]
- Nagashima, S.; Takahashi, M.; Jirintai, S.; Tanggis Kobayashi, T.; Nishizawa, T.; Okamoto, H. The membrane on the surface of hepatitis E virus particles is derived from the intracellular membrane and contains trans-Golgi network protein 2. Arch. Virol. 2014, 159, 979–991. [Google Scholar] [CrossRef]
- Nagashima, S.; Takahashi, M.; Kobayashi, T.; Null, N.; Nishizawa, T.; Nishiyama, T.; Primadharsini, P.P.; Okamoto, H. Characterization of the Quasi-Enveloped Hepatitis E Virus Particles Released by the Cellular Exosomal Pathway. J. Virol. 2017, 91, e00822-17. [Google Scholar] [CrossRef] [Green Version]
- Berto, A.; Van der Poel, W.H.M.; Hakze-van der Honing, R.; Martelli, F.; La Ragione, R.M.; Inglese, N.; Collins, J.; Grierson, S.; Johne, R.; Reetz, J.; et al. Replication of hepatitis E virus in three-dimensional cell culture. J. Virol. Methods 2013, 187, 327–332. [Google Scholar] [CrossRef]
- Roethl, E.; Gassner, M.; Krenn, B.M.; Romanovskaya-Romanko, E.A.; Seper, H.; Romanova, J.; Nakowitsch, S.; Sturlan, S.; Wolschek, M.; Sirotkin, A.; et al. Antimycotic-antibiotic amphotericin B promotes influenza virus replication in cell culture. J. Virol. 2011, 85, 11139–11145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.; Feng, Z. Hepatitis E Virus Entry. Viruses 2019, 11, 883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holla, P.; Ahmad, I.; Ahmed, Z.; Jameel, S. Hepatitis E Virus Enters Liver Cells through a Dynamin-2, Clathrin and Membrane Cholesterol-Dependent Pathway. Traffic 2015, 16, 398–416. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, D.; Wei, S.; Li, Q.; Yuan, X.; Geng, L.; Li, X.; Liu, M. Cell culture of sporadic hepatitis E virus in China. Clin. Diagn. Lab. Immunol. 1999, 6, 729–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.W.; Ho, C.L.; Cheng, S.W.; Lin, Y.J.; Chen, C.C.; Cheng, P.N.; Yen, C.J.; Chang, T.T.; Chiang, P.M.; Chan, S.H.; et al. Progesterone receptor membrane component 1 as a potential prognostic biomarker for hepatocellular carcinoma. World J. Gastroenterol. 2018, 24, 1152–1166. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, Y.; Wen, Z.; Zhang, F.; Qi, Y.; Huang, W.; Zhang, H.; Wang, Y. Asialoglycoprotein receptor facilitates infection of PLC/PRF/5 cells by HEV through interaction with ORF2. J. Med. Virol. 2016, 88, 2186–2195. [Google Scholar] [CrossRef]
- Todt, D.; Friesland, M.; Moeller, N.; Praditya, D.; Kinast, V.; Brüggemann, Y.; Knegendorf, L.; Burkard, T.; Steinmann, J.; Burm, R.; et al. Robust hepatitis E virus infection and transcriptional response in human hepatocytes. Proc. Natl. Acad. Sci. USA 2020, 117, 1731–1741. [Google Scholar] [CrossRef] [Green Version]

| Name | Composition |
|---|---|
| MEM-1 | Minimum Essential Medium (MEM) supplemented with 1% heat-inactivated fetal bovine serum (FBS) |
| MEM-10 | MEM supplemented with 10% FBS |
| MEM-A | MEM-10 supplemented with 2.5 µg/mL amphotericin B and 30 mM MgCl2 |
| MEM-D | MEM-A supplemented with 1% dimethyl sulfoxide (DMSO) |
| MEM-P | MEM-A supplemented with 80 nM progesterone |
| MEM-Z | MEM-D supplemented with 80 nM progesterone |
| Name | Treatment |
|---|---|
| No treatment | No treatment as a control for quasi-enveloped cell culture-derived HEV |
| F-T | Repeated freeze–thaw cycle (4 times) |
| NP-40 | 2% NP-40 in PBS for 2 h at 37 °C |
| NaDOC/T | Sonicated twice at 40 kHz for 4 min in ice water, filtered through a 0.22 µm membrane, and suspended in TB with 10% sodium deoxycholate (NaDOC/T) and 1% EDTA trypsin for 2 h at 37 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chew, N.; Situ, J.; Wu, S.; Yao, W.; Sridhar, S. Independent Evaluation of Cell Culture Systems for Hepatitis E Virus. Viruses 2022, 14, 1254. https://doi.org/10.3390/v14061254
Chew N, Situ J, Wu S, Yao W, Sridhar S. Independent Evaluation of Cell Culture Systems for Hepatitis E Virus. Viruses. 2022; 14(6):1254. https://doi.org/10.3390/v14061254
Chicago/Turabian StyleChew, Nicholas, Jianwen Situ, Shusheng Wu, Weiming Yao, and Siddharth Sridhar. 2022. "Independent Evaluation of Cell Culture Systems for Hepatitis E Virus" Viruses 14, no. 6: 1254. https://doi.org/10.3390/v14061254
APA StyleChew, N., Situ, J., Wu, S., Yao, W., & Sridhar, S. (2022). Independent Evaluation of Cell Culture Systems for Hepatitis E Virus. Viruses, 14(6), 1254. https://doi.org/10.3390/v14061254







