SARS-CoV-2 Infection in Companion Animals: Prospective Serological Survey and Risk Factor Analysis in France
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
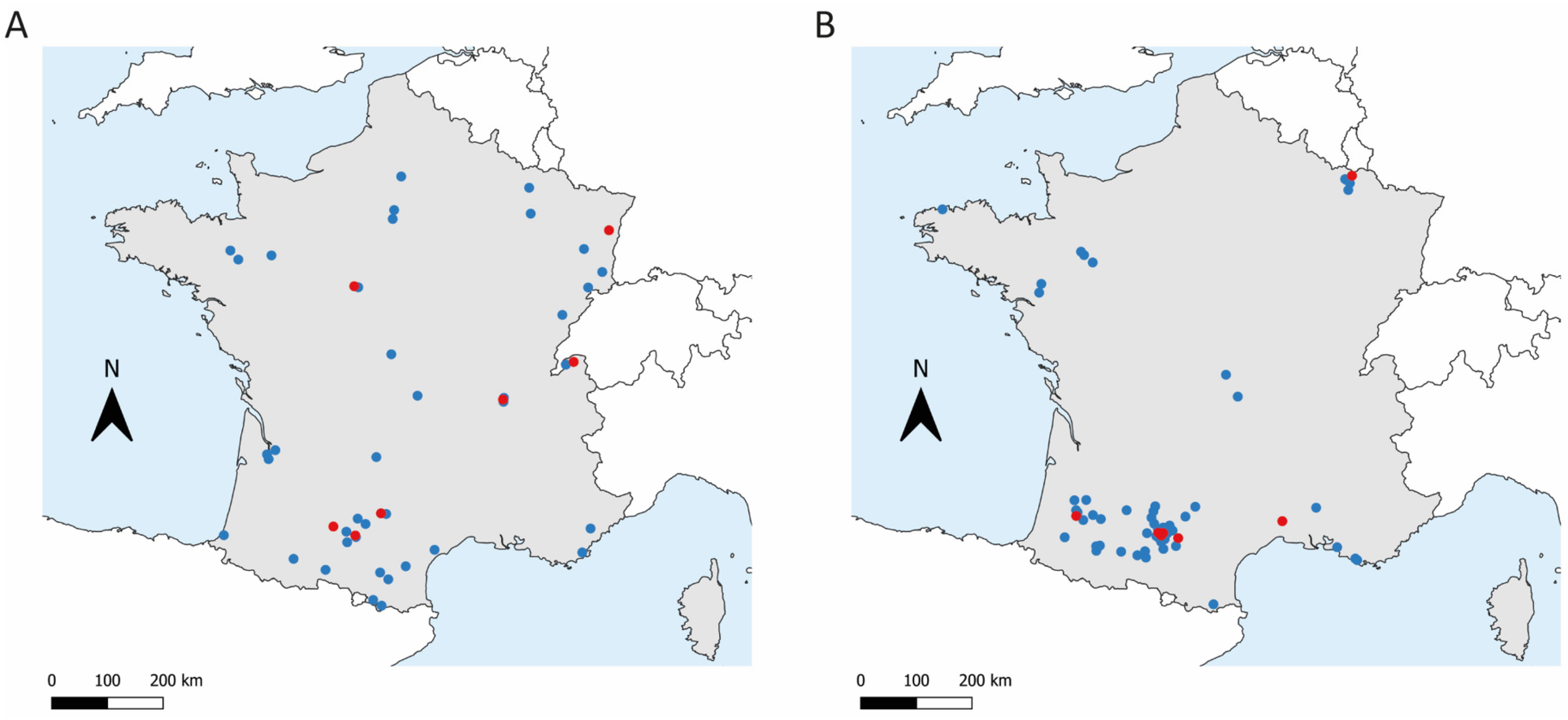
2.2. Sample Collection
2.3. Hemagglutination Test
2.4. ELISA
2.5. Serum Neutralization Assay
2.6. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, V.L.; Dennis, P.M.; McBride, D.S.; Nolting, J.M.; Madden, C.; Huey, D.; Ehrlich, M.; Grieser, J.; Winston, J.; Lombardi, D.; et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature 2022, 602, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Bessiere, P.; Fusade-Boyer, M.; Walch, M.; Lebre, L.; Brun, J.; Croville, G.; Boullier, S.; Cadiergues, M.C.; Guerin, J.L. Household Cases Suggest That Cats Belonging to Owners with COVID-19 Have a Limited Role in Virus Transmission. Viruses 2021, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Sit, T.H.C.; Brackman, C.J.; Ip, S.M.; Tam, K.W.S.; Law, P.Y.T.; To, E.M.W.; Yu, V.Y.T.; Sims, L.D.; Tsang, D.N.C.; Chu, D.K.W.; et al. Infection of dogs with SARS-CoV-2. Nature 2020, 586, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Barroso, R.; Vieira-Pires, A.; Antunes, A.; Fidalgo-Carvalho, I. Susceptibility of Pets to SARS-CoV-2 Infection: Lessons from a Seroepidemiologic Survey of Cats and Dogs in Portugal. Microorganisms 2022, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Arevalo, S.; Barneto, A.; Ramos, A.M.; Rivera, B.; Sanchez, R.; Sanchez-Morales, L.; Perez-Sancho, M.; Buendia, A.; Ferreras, E.; Ortiz-Menendez, J.C.; et al. Large-scale study on virological and serological prevalence of SARS-CoV-2 in cats and dogs in Spain. Transbound. Emerg. Dis. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Colitti, B.; Bertolotti, L.; Mannelli, A.; Ferrara, G.; Vercelli, A.; Grassi, A.; Trentin, C.; Paltrinieri, S.; Nogarol, C.; Decaro, N.; et al. Cross-Sectional Serosurvey of Companion Animals Housed with SARS-CoV-2-Infected Owners, Italy. Emerg. Infect. Dis. 2021, 27, 1919–1922. [Google Scholar] [CrossRef] [PubMed]
- Jairak, W.; Charoenkul, K.; Chamsai, E.; Udom, K.; Chaiyawong, S.; Hangsawek, A.; Waenkaew, S.; Mungaomklang, A.; Tangwangvivat, R.; Amonsin, A. Survey of SARS-CoV-2 in dogs and cats in high-risk areas during the second wave of COVID-19 outbreak, Thailand. Zoonoses Public Health, 2022; 1–9. [Google Scholar] [CrossRef] [PubMed]
- Michelitsch, A.; Schon, J.; Hoffmann, D.; Beer, M.; Wernike, K. The Second Wave of SARS-CoV-2 Circulation-Antibody Detection in the Domestic Cat Population in Germany. Viruses 2021, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.I.; Elia, G.; Grassi, A.; Giordano, A.; Desario, C.; Medardo, M.; Smith, S.L.; Anderson, E.R.; Prince, T.; Patterson, G.T.; et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nat. Commun. 2020, 11, 6231. [Google Scholar] [CrossRef] [PubMed]
- Pomorska-Mol, M.; Turlewicz-Podbielska, H.; Gogulski, M.; Ruszkowski, J.J.; Kubiak, M.; Kuriga, A.; Barket, P.; Postrzech, M. A cross-sectional retrospective study of SARS-CoV-2 seroprevalence in domestic cats, dogs and rabbits in Poland. BMC Vet. Res. 2021, 17, 322. [Google Scholar] [CrossRef] [PubMed]
- Townsend, A.; Rijal, P.; Xiao, J.; Tan, T.K.; Huang, K.A.; Schimanski, L.; Huo, J.; Gupta, N.; Rahikainen, R.; Matthews, P.C.; et al. A haemagglutination test for rapid detection of antibodies to SARS-CoV-2. Nat. Commun. 2021, 12, 1951. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Anderson, E.R.; Cansado-Utrilla, C.; Prince, T.; Farrell, S.; Brant, B.; Smyth, S.; Noble, P.M.; Pinchbeck, G.L.; Marshall, N.; et al. SARS-CoV-2 neutralising antibodies in dogs and cats in the United Kingdom. Curr. Res. Virol. Sci. 2021, 2, 100011. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, V.; Vilibic-Cavlek, T.; Tabain, I.; Benvin, I.; Kovac, S.; Hruskar, Z.; Mauric, M.; Milasincic, L.; Antolasic, L.; Skrinjaric, A.; et al. Seroprevalence of SARS-CoV-2 infection among pet animals in Croatia and potential public health impact. Transbound. Emerg. Dis. 2021, 68, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
| Serological Status for All Pets | p-Value * | Serological Status for Dogs | p-Value ** | Serological Status for Cats | p-Value ** | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |||||
| Species (n = 308) | dog | 9 | 156 | 0.42 | ||||||
| cat | 12 | 131 | ||||||||
| Relationship pet–owner (n = 305) | close | 16 | 199 | 0.73 | 5 | 95 | 0.74 | 11 | 104 | 0.69 |
| distant | 5 | 85 | 4 | 61 | 1 | 24 | ||||
| COVID-19 episode in the household (n = 308) | yes | 14 | 165 | 0.55 | 6 | 77 | 0.50 | 8 | 88 | 1 |
| no | 7 | 122 | 3 | 79 | 4 | 43 | ||||
| Severity of the COVID-19 episode (n = 308) | serious | 4 | 27 | 0.34 *** | 2 | 8 | 0.11 | 2 | 19 | 1 |
| mild | 10 | 138 | 4 | 69 | 6 | 69 | ||||
| none | 7 | 122 | 3 | 79 | 4 | 43 | ||||
| COVID-19 owner test (n = 292) | positive | 11 | 121 | 0.40 * | 6 | 65 | 0.41 | 5 | 56 | 0.71 |
| negative | 3 | 29 | 0 | 9 | 3 | 20 | ||||
| no test | 6 | 122 | 3 | 82 | 3 | 40 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessière, P.; Vergne, T.; Battini, M.; Brun, J.; Averso, J.; Joly, E.; Guérin, J.-L.; Cadiergues, M.-C. SARS-CoV-2 Infection in Companion Animals: Prospective Serological Survey and Risk Factor Analysis in France. Viruses 2022, 14, 1178. https://doi.org/10.3390/v14061178
Bessière P, Vergne T, Battini M, Brun J, Averso J, Joly E, Guérin J-L, Cadiergues M-C. SARS-CoV-2 Infection in Companion Animals: Prospective Serological Survey and Risk Factor Analysis in France. Viruses. 2022; 14(6):1178. https://doi.org/10.3390/v14061178
Chicago/Turabian StyleBessière, Pierre, Timothée Vergne, Matéo Battini, Jessie Brun, Julien Averso, Etienne Joly, Jean-Luc Guérin, and Marie-Christine Cadiergues. 2022. "SARS-CoV-2 Infection in Companion Animals: Prospective Serological Survey and Risk Factor Analysis in France" Viruses 14, no. 6: 1178. https://doi.org/10.3390/v14061178
APA StyleBessière, P., Vergne, T., Battini, M., Brun, J., Averso, J., Joly, E., Guérin, J.-L., & Cadiergues, M.-C. (2022). SARS-CoV-2 Infection in Companion Animals: Prospective Serological Survey and Risk Factor Analysis in France. Viruses, 14(6), 1178. https://doi.org/10.3390/v14061178







