Abstract
Since 2016, frequent outbreaks of egg-reducing syndromes caused by an unknown virus in duck farms have resulted in huge economic losses in China. The causative virus was isolated and identified as a novel species in Avihepatovirus of the picornavirus family according to the current guidelines of the International Committee on Taxonomy of Viruses (ICVT), and was named the duck egg-reducing syndrome virus (DERSV). The DERSV was most closely related to wild duck avihepatovirus-like virus (WDALV) with 64.0%, 76.8%, 77.5%, and 70.7% of amino acid identities of P1, 2C, 3C, and 3D proteins, respectively. The DERSV had a typical picornavirus-like genomic structure, but with the longest 2A region in the reported picornaviruses so far. Importantly, the clinical symptoms were successfully observed by artificially infecting ducks with DERSV, even in the contact exposed ducks, which suggested that DERSV transmitted among ducks by direct contact. The antibody levels of DERSV were correlated with the emergence of the egg-reducing syndromes in ducks in field. These results indicate that DERSV is a novel emerging picornavirus causing egg-reducing syndrome in ducks.
1. Importance
Duck egg-reducing syndromes caused huge economic losses to the poultry industry in China recently, however, the causative agent had kept unknown since the disease broke out. In this study, the virus was isolated, identified, and named the duck egg-reducing syndrome virus, which was a novel virus species belonging to avihepatovirus in the picornavirus family. Interestingly, the virus possesses the longest 2A region in all reported picornaviruses and might produce seven putative polypeptides. Our findings enriched the knowledge of picornaviruses and provided the key information for prevention and control of duck egg-reducing syndromes.
2. Introduction
Picornaviruses are non-enveloped viruses with positive-sense, single-stranded RNA genomes [1,2] ranging in size from 6.7 to 10.1 kilobases (kb), which typically contain a single open reading frame (ORF) flanked by the highly structured 5′ and 3′ untranslated regions (UTRs). Most of the Picornaviruses encode a large polyprotein except for the members of the species in genus Dicipivirus that have two internal ribosome entry sites (IRES) and encode two ORFs [1,3]. Picornaviruses are ubiquitous and globally distributed, and the number of newly discovered picornaviruses has increased dramatically in the past decade [4]. The family Picornaviridae was composed of 80 species and was divided into 35 genera in 2017 [5]. Up to now, the family of Picornaviridae consists of 158 species grouped into 68 genera (https://www.picornaviridae.com/index.html, accessed on 8 March 2021).
Although only a few viruses in the Picornaviridae family cause infectious diseases in humans or animals, huge economic losses were caused in the national health care system [4] and animal husbandry each year. In duck farms, duck hepatitis A virus (DHAV) in genus Avihepatovirus causes a highly fatal infectious disease in ducks under 6 weeks old [6]. In addition, Avian sapelovirus (ASV) in genus Sapelovirus causes growth retard in ducklings. Duck megrivirus (DMV) in genus Megrivirus, aalivirus A GL/12 (AalV-A) in genus Aalivirus, and wild duck avihepatovirus-like virus (WDALV) in genus Avihepatovirus were detectable by the reverse transcription-polymerase chain reaction (RT-PCR) in ducks, but the failure of isolation and proliferation of those viruses kept us from understanding their pathogenicity in ducks [7,8,9]. Since 2016, outbreaks of duck infectious disease characterized as egg production reduction caused by unknown pathogens frequently occurred in duck farms in China. The investigation of duck farms with declining egg production in many different areas, we found that the positive rate of DERSV is very high. According to our study, the epidemic firstly occurred in Anhui, China, but quickly spread to many other provinces in China, including Zhejiang, Jiangsu, Guangdong, and Shandong. The disease was characterized by a slow decline in egg production from the peak of 90% of laying rates to 50% of that in ducks. The decline in duck egg production has brought huge economic losses to duck farms. Importantly, the disease could not be cured by multiple antibiotics treatments; the known common avian viruses such as Tembusu virus, Adenovirus, Avian influenza viruses, Newcastle disease virus, Duck reovirus, Muscovy duck reovirus, Duck hepatitis virus 1, Duck hepatitis virus 3, Infectious bursal disease virus, Avian leukosis virus, Duck circovirus, Duck virusenteritis, Duck astrovirus, Goose parvovirus and Muscovy duck parvovirus were ruled out by PCR or RT-PCR in the diseased duck samples. Finally, a novel causative virus belonging to genus Avihepatovirus in the family of Picornaviridae was isolated and identified, and its genetic characteristics and pathogenicity in ducks were reported in this study.
3. Materials and Methods
3.1. Virus Isolation
In a duck farm in Anhui (AH) provinces, China, an outbreak of a disease is characterized by a slow decline in egg production from the peak of 90% of laying rates to 50% of that in ducks and by a long recovery period. The tissues of follicles, kidneys, and spleen samples of diseased ducks were collected and homogenized with phosphate-buffered saline (PBS). The polymerase chain reaction (PCR) was used to identify the virus using the primers for diagnosis of different known avian viruses including Tembusu virus and adenovirus that cause egg-loss symptoms in ducks, avian influenza viruses, Newcastle disease virus, duck reovirus, muscovy duck reovirus, duck hepatitis virus 1, duck hepatitis virus 3, infectious bursal disease virus, avian leukosis virus, Duck Circovirus, duck virusenteritis, duck astrovirus, goose parvovirus, muscovy duck parvovirus. The supernatant was filtered with a 0.22 μm filter and inoculated into the allantoic cavity of 14-day-old goose embryonated eggs for virus isolation (100 μL per egg). The goose embryonated eggs were incubated in a humidified egg incubator at 37 °C, and their survival was checked every day. The harvested allantoic fluid or/and embryos of the eggs that died 24–144 h post-inoculation (hpi) was collected. Virus-infected goose embryo allantoic fluid was purified by the limited dilution method three times. After purification by the limited dilution method, we identified the known avian viruses by PCR and RT-PCR again. Ten-fold serial dilutions of the virus-infected embryo allantoic fluid (0.1 mL) were inoculated into the allantoic sacs of 9-day-old SPF embryonated duck eggs and incubated for 144 h at 37 °C. Three eggs were used for each dilution. The embryo allantoic fluid of duck eggs were collected at 144 h post-inoculation and detected by RT-PCR; the 50% egg infectious dose was calculated by using Reed and Muench’s method. The embryo allantoic fluid had an infectivity titer of 106.5 EID50/mL.
3.2. Observation under Electron Microscopy
The virus was enriched by ultracentrifugation and purified by 20%, 40% and 60% (w/v) discontinuous sucrose density gradient and centrifuged at 280,000× g (Break 0) for 5 h. The purified virus was stained with phosphotungstate acid, and observed under the transmission electron microscope (TEM, H-7500, Hitachi, Japan).
3.3. Nucleic Acid Extraction and Identification
Nucleic acid was extracted from purified virus by using the TIANamp virus RNA Kit (Tiangen Biotech, Co., Ltd., Beijing, China) or the Viral DNA Extraction Kit (Sangon Biotech, Co., Ltd., Shanghai, China), according to the manufacturer’s instructions. To determine the nucleic acid type, extracted nucleic acid was treated with RNase and DNase (Takara Biotechnology, Dalian, China), according to the manufacturer’s recommendations.
3.4. Construction of a CDNA Library of Viral Genes
Viral nucleic acid library was constructed by sequence-independent amplification using the viral RNA extracted by TIANamp virus RNA Kit. The first-strand cDNAs were synthesized with random reverse transcription primer of K-8N (GACCATCTAGCGACCTCCACNNNNNNNN) according to the previous description [10]. On the opposite strand of the cDNA, single round priming and extension was performed using Klenow fragment (TaKaRa Biotechnology, Dalian, China).
PCR of extension products was performed using 5 uL of the reaction described above in a total reaction volume of 50 uL containing 5U TaKaRa Ex Taq, 100 mM 10× Ex Taq Buffer (Mg2 + plus), 10 mM dNTPs (TaKaRa Biotechnology, Dalian, China), and 1 mM primer K (GAC CAT CTA GCG ACC TCC AC). Products were identified by agarose gel electrophoresis and the fragments larger than 250 bp were isolated and subcloned into pMD-19T vector for sequencing [10,11].
3.5. Nucleotide Sequencing and Phylogenetic Analysis
Plasmids extracted from the bacterial clones picked randomly from the cDNA library were directly sequenced. To compile and analyze the sequences, we used the SEQMAN program (DNASTAR, Madison, WI, USA). Putative ORF and deduced amino acid sequences of the viral genome was predicted using ORF finder (https://www.ncbi.nlm.nih.gov/orffinder/, accessed on 11 May 2018). The sequences of the reference viruses were obtained from GenBank by using the basic local alignment search tool (BLAST) at the National Center for Biotechnology Information (NCBI) (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 14 May 2018).The secondary structure of IRES was predicted by RNAstructure (http://rna.urmc.rochester.edu/RNAstructureWeb/Servers/Predict1/Predict1.html, accessed on 15 May 2018), and drawn with StructureEditor. The conserved domains in the structural and nonstructural proteins were predicted using the conserved domain database (CDD) search [12]. The nucleotide sequences were compared with the sequences of reference viruses (Table A1) with the MEGALIGN program (DNASTAR) by using the Clustal alignment algorithm [6,13]. The phylogenetic trees were generated with 68 genera from the family of Picornaviridae by MEGA software (MEGA version 7.0). The possible cleavage sites of the polyprotein of DERSV were mapped based on (i) NetPicoRNA predictions [14], and (ii) the amino acid alignment with the selected strains of the closest relatives WDALV, AalV-A, DHAV, and (iii) the preference of picornavirus 3Cpro for the small amino acid residue (e.g., Q, E, G, S, R, M, A, and N) at the P1 position [7,14].
3.6. Study of the Correlation of DERSV between DERSV Infection
To understand the relationship between DERSV and duck egg production decline, diseased duck samples with egg decline were collected in different duck farms distributed in AH, Shandong (SD), Jiangsu (JS), and Guangdong (GD), and DERSV was detected. Some of the DERSV positive samples were selected according to the areas and inoculated with 9-day-old duck eggs for virus isolation, whole-genome sequencing, and further analysis.
Serological surveillance was conducted in 5 h in a breeding duck farm from October to December 2020. The average egg production rate was recorded every day, and each of 20 serum samples in each duck house were collected randomly at three-time points. The specific DERSV antibodies in the sera were detected by a blocking enzyme-linked immunosorbent assay. Briefly, the purified DERSV was diluted to 0.11 μg/well with 0.1 M bicarbonate buffer (Ph 9.6) and coated on the enzyme-linked immunosorbent assay (ELISA) plate. After incubating at 4 °C overnight, it was washed with PBS (pH 7.4) containing 0.05% Tween-20 (PBST) for 3 times. The blocking buffer (phosphate buffer containing 5% skim milk, 100 μL/well) was added to the reaction plate and sealed at 37 °C for 1 h. Serum samples were diluted 10 times with PBS and incubated on the ELISA plate for 1 h at 37 °C. The wells were then washed 3 times with PBST and incubated with monoclonal antibody (MAb)3G11(1:10) for 1 h at 37 °C. The MAb 3G11 can specifically react with DERSV but does not cross-react with other potentially infected duck viruses such as avian influenza viruses, Newcastle disease virus, duck hepatitis virus 1, duck hepatitis virus 3, goose parvovirus, duck Tembusu virus, adenovirus, duck reovirus. After washing the plate 3 times, goat anti-mouse IgG (1:2000, Sigma, St. Louis, MO, USA) conjugated with horseradish peroxidase (HRP) was added and incubated for 1 h at 37 °C. After rinsing with PBST for 3 times, add 3,3′,5,5′-tetramethylbenzidine 100 μL and incubate at room temperature for 10 min. Then, 0.1N of sulfuric acid was added to stop the reaction. Measure the optical density (OD) of the wells at 450 nm, and the percent inhibition (PI) value was calculated according to the formula: PI (%) = [1 − (OD450 sample/OD450 negative-control sample)] × 100. The serum was considered positive for DERSV reactivity when the PI value was ≥22.1%.
3.7. Animal Study
To determine the pathogenicity and transmissibility of DERSV, eight laying shelducks were inoculated intramuscularly with 105.5 EID50 of DERSV in a volume of 200 μL. One day later, eight naive laying shelducks were introduced into the same isolator to test the transmissibility of DERSV. The inoculated ducks were euthanized on 6 dpi, and the exposed ducks were euthanized one day later, and the liver, kidney, and follicles samples were collected. The serum samples were collected from the remaining inoculated ducks at 2, 4, and 6 dpi and from the contact ducks at 2, 4, and 6 dpc for antibody detection. All samples were collected for virus isolation by using 9-day-old embryonated duck eggs and detected by RT-PCR. Additionally, we identified the known avian viruses by PCR and RT-PCR again. For histopathological analyses, samples were fixed with 4% paraformaldehyde, sectioned, and pathological observation of tissue sections after staining with hematoxylin and eosin. Alternatively, immunohistochemical analysis was conducted with a Mab(3G11) against the DERSV.
4. Results
4.1. Isolation of a Novel Virus
Since 2016, outbreaks of a duck egg-reduction syndrome caused by unknown pathogens were reported in AH, SD, JS, and GD provinces in China. Various antibiotics were used to mitigate the outbreak in ducks without any success, suggesting that the pathogen of the duck egg-reduction syndrome might be a viral pathogen. To identify the pathogen, the known avian viruses such as Tembusu virus and Adenovirus that cause egg drop symptoms in ducks, Avian influenza viruses, Newcastle disease virus, Duck reovirus, Muscovy duck reovirus, Duck hepatitis virus 1, Duck hepatitis virus 3, Infectious bursal disease virus, Avian leukosis virus, Duck circovirus, Duck virusenteritis, Duck astrovirus, Goose parvovirus, Muscovy duck parvovirus were detected by PCR or RT-PCR in the diseased duck samples, and all results were negative, which suggested that the pathogen might be a novel virus. To isolate this virus, a homogenate of the follicles, kidneys, and spleen samples collected from ducks with the egg-reduction syndrome were inoculated into the allantoic cavity of 14-day-old goose embryonated eggs. All the infected goose embryonated eggs died after 120 hpi. The allantoic fluid of the dead goose embryonated eggs was harvested. After three times of limited dilution purification in goose embryonated eggs, the purified virus was harvested and detected for the above-mentioned known viruses, which were all ruled out in the isolated virus. Therefore, the virus of the egg-reduction syndrome may be a novel unknown virus.
4.2. A Novel RNA Virus Was Identified and Named as DERSV
In order to further characterize the novel virus, the virus was purified by sucrose density gradient centrifugation and observed under a transmission electron microscope. The virion was spherical and unenveloped with a diameter of approximately 25–30 nm. Moreover, the nucleic acids extracted from the purified virus were resistant to DNase but not to RNase, which suggested that this virus is an RNA virus.
To determine the genome sequence of this RNA virus, the cDNA libraries were constructed by sequence-independent amplification using primer K-8N. A total of 100 positive clones were sequenced, and a continuous sequence (about 2700 bp) was obtained. The remaining sequence of the viral genome were amplified segmentally by RT-PCR using the primer walking process and by rapid amplification of the cDNA terminal [14,15]. The whole genome sequence was confirmed by seven overlapping DNA fragments amplified by RT-PCR with specific primers. Finally, the complete genome sequence of this virus was successfully obtained, which was in a length of 9026 bp (GenBank accession no. OL956952).
Using BLASTx searches, this new virus shared 45.2–66.6% nucleotide homology with that of the picornaviruses, which suggested that this virus was a kind of new picornavirus. Then, a phylogenetic tree was drawn to analyze the relationship between the new virus and the known picornaviruses including newly discovered picornavirus genuses such as Ailurivirus, Anativirus, Boosepivirus, Bopivirus, Caecilivirus, Tropivirus, etc. in recent years (Table A1). The result demonstrated that the virus was close to WDALV, AalV-A, and duck hepatitis A virus 1/2/3 that were picornaviruses from ducks (Figure 1). According to the new 2020 ICTV taxonomy (https://ictv.global/taxonomy, accessed on 8 March 2021), different members of a genus mean significant divergence (number of differences per site between sequences) of the orthologous proteins exceed 66% of P1 and 64% of 2C, 3C, and 3D. To further define the classification of the new virus, we analyzed the amino acid homology of P1, 2C, 3C, 3D between DERSV and DERSV-related picornavirus, such as WDALV, AalV-A and duck hepatitis A virus 1/2/3.Detailed analysis revealed that the new virus shared higher amino acid identities with WDALV (64%, 76.8%, 77.5%, and 70.7% in P1, 2C, 3C, and 3D proteins), AalV-A (35.6%, 62.2%, 59.3%, and 57.3% in P1, 2C, 3C, and 3D proteins), and duck hepatitis A virus 1 (35.1%, 52.1%, 45.0% and 47.6% in P1, 2C, 3C, and 3D proteins) (Table 1). Thus, the new virus in this study belongs to genus Avihepatovirus as well as duck hepatitis A virus described as previously [16,17]. However, this novel virus is different from WDALV, AalV-A and duck hepatitis A virus based on the large divergence in the amino acid and nucleotide sequence levels. Given this novel virus mainly causes egg drop in the ducks, this virus was named as duck egg-reducing syndrome virus (DERSV).
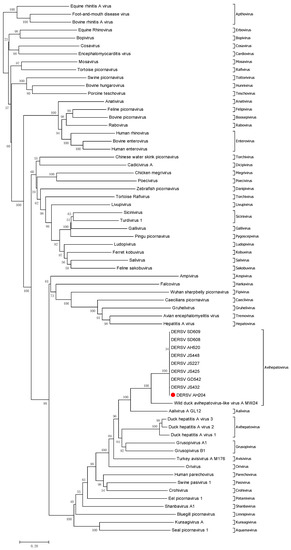
Figure 1.
Phylogenetic relationship between DERSV and other picornaviruses. The neighbor-joining tree was constructed according to the amino acid sequences of DERSV (AH204 strain). The numbers along the branches mark the bootstrap values percentage out of 1000 bootstrap resamplings. The scale bar indicates the number of substitutions per site.

Table 1.
Comparisons of Amino acid sequence homology of DERSV(AH204) with the closely related picornaviruses.
4.3. DERSV Had a Typical Picornavirus-Like Genomic Structure
The complete polyadenylated RNA of the DERSV comprised 9026 nt excluding the poly(A) tail and had a single 8394 nt ORF flanked by 5′UTR (419 nt) and 3′UTR (303 nt), encoding a putative polyprotein precursor of 2768 amino acids (Figure 2A).
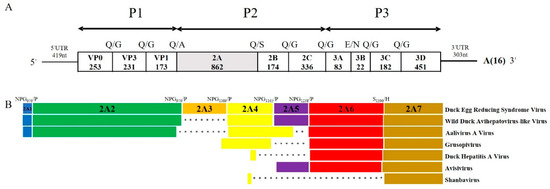
Figure 2.
Schematic diagram of whole-genome and 2A region. (A) Predicted genome organization of the DERSV. P1 represents viral structural proteins, and P2 and P3 represent non-structural proteins. The length of amino acid was indicated in each gene box. (B) Comparison of putative 2A peptides of Picornaviruses. The dot represents the absence of amino acids and the different colors in the box represent 2A1(blue), 2A2(green), 2A3(orange), 2A4(yellow), 2A5(purple), 2A6(red), 2A7(golden).
The virus had the typical picornavirus-like genome organization. The possible cleavage sites of the polyprotein of DERSV were mapped based on (i) the amino acid alignment with the selected strains of the closest relatives WDALV, AalV-A, DHAV, and (ii) NetPicoRNA predictions [14]. The Q/G (Gln/Gly), E/G (Glu/Gly), and Q/S (Gln/Ser) cleavage sites of 3Cpro were strongly supported by the polyprotein alignments and the NetPicoRNA predictions [6]. There was no evidence of the presence of the potential L-protein and the cleavage of VP0 into VP4 and VP2. Thus, the DERSV polyprotein might consist of capsid proteins P1 (253aa-VP0, 231aa-VP3, 173aa-VP1), and nonstructural proteins P2 (862aa-2A, 174aa-2B 336aa-2C) and P3 (83aa-3A, 22aa-3B 182aa-3C, 451aa-3D) (Figure 2A).
Distinguishingly, the 2A region of the DERSV had six putative cleavage sites suggested that the virus might produce up to seven 2A peptides (Figure 2B). Five canonical cleavage sites of DxExNPG|P at amino acid sites of 676, 976, 1069, 1165, and 1238 of precursor polyprotein might result in five putative 2A peptides, a 2A1 (19 aa), a 2A2 (300 aa), a 2A3 (93 aa), a 2A4 (96) aa and a 2A5 (73aa) and a 281 aa peptide which might be cleaved at S1396|H to generate an additional two putative 2A products, 2A6 (158 aa) and 2A7 (123 aa). The S/H cleavage sites were not common among picornavirus, but are found in some picornaviruses, e.g., WDALV, DHAV-1 [16].
The 2A1 of DERSV shared 78.9% of amino acid homology with WDALV and 50% of amino acid homology with Foot-and-mouth disease virus. In contrast, the 2A3 is a unique peptide for the DERSV compared with other viruses in the Picornaviridae. Due to the insertion of 2A3, the 2A region of the DERSV is the longest in all the reported picornaviruses. The 2A2, 2A4, 2A5, 2A6, and 2A7 of DERSV possessed 44%, 69.6%, 58.9%, 51.9%, and 60.2% amino acid homology with that of WDALV. In addition, another predictive autocleavage motifs, GxExNPG788P, was found on 2A2, which suggested that more 2A peptides might be available in DERSV. Together with three characteristic sequence motifs (G1-G3) and GTP binding site of Ras-like-GTPase superfamily of small guanosine triphosphatases, the conserved motif of GxxGxGKS (x = any residue) potentially responsible for a 2C-like NTPase function was found in 2A6 (Table 2) [6]. Based on sequence alignment, the 2A6 was related to P-loop-containing nucleoside triphosphate hydrolases, and the 2A7 of DERSV was a parechovirus-like 2A with an H-box/NC-motifs (Table 2) [18].

Table 2.
The seven 2A peptide of DERSV.
The predicted 419 nt 5′UTR of DERSV was significantly shorter than the most of picornaviruses. The secondary structure of the 3’-proximal portion of the DERSV 5′UTR was predicted by using RNA structure (http://rna.urmc.rochester.edu/RNAstructureWeb, accessed on 15 May 2018), which revealed that the region from nt145 to nt424 showed structural similarity to hepacivirus/pestivirus (HP)-like type IV IRES with stem-loop domains II and III and the predicted initiation codon was mapped at position 417 that was surrounded by the optimal Kozak context (GxxAUGG) (Figure 3) [19,20,21].The DERSV Domain II consisted of 67 nucleotides that contained the E-loop GAA173–175 and AGUA183–186 motifs present in most type IV IRES elements. Domain III in DERSV included a series of signature elements characteristic of HP-like IRES in domain IIId and domain IIIe. Interestingly, the DERSV domain IIIe consisted of a 3 nt stem (5′-CCU/5′-GGA) and five unpaired highly conserved GAUA motifs that were distinct from aalivirus (5′-CUC/5′-GAG) and all other those of other HP-like IRESs(5′-GGGU/5′-UCGGG) reported to date. Similar to classical swine fever virus, feline picornavirus 1, and bat picornaviruses 1 and 2 [19,22,23,24], the subdomain IIId of DERSV IRES contained an unpaired GGG motif within the terminal loop.
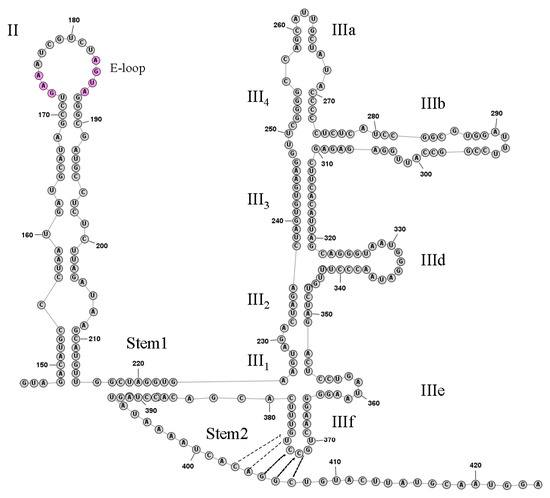
Figure 3.
Predicted secondary structure of the DERSV IRES. Domains II and III are labeled according to corresponding domains in the type IV IRESs [14]. In domain III, individual helical segments are labeled III1 and III2, etc.; and individual hairpins are labeled IIIa and IIIb, etc. The pseudoknot stem 1, stem 2 and domain IIIf helix are labeled.
Analysis of the capsid regions revealed that DERSV contained a potential myristylation sequence (GxxxxS/T) at positions 155–159. This location is not quite similar to those of AalV-A at positions 39–43, DHAV at positions 31–35 [16], canine picornavirus 1 at positions 50–54 [25] and feline picornavirus 1 at positions 51–55 [23]. Using the CDD search, two RHV-like domains were found at residues 64–252 and 346–480 in P1 polypeptides [12]. Similar to other picornaviruses, the nonstructural proteins of DERSV contained some conserved characteristic motifs including two characteristic motifs for helicase (GATGSGKS1251-1258) in 2A6 and 2C for nucleoside triphosphate (NTP) binding, (QEIHIFDDLGQ1873-1883) in 2C protein for putative helicase activity [26,27], a cysteine protease motif (GLCG2277-2280) in 3C protein [26], and RNA-dependent RNA polymerase(RdRp) motifs (KDELR2466-2470, GGMCSGSPCTTVLNT2593-2607, YGDD2633-2636 and FLKR2681-2684) in 3D protein.
4.4. DERSV Infection Is Correlated with the Decline of Egg Production in Ducks
To understand the relationship between DERSV and duck egg production decline in field, a total of 379 samples were collected from diseased ducks with egg decline syndromes in different duck farms distributed in AH, SD, JS, and GD for DERSV detection. The positivity rate of DERSV was 61.7% (234/379) detected by RT-PCR from diseased ducks’ tissues including, livers, spleens, pancreases, ovaries, intestines, oviducts, kidneys, which suggested that this virus has been widely spreading in the main duck-raising areas of China. The positive ratio of the virus was highest in tissues kidneys (75%, 69/92), ovaries (69.6%, 39/56). Then, the positive rate of the virus was also higher in livers (56.0%, 51/91), intestines (64.7%, 22/34), oviducts (55.9%, 19/34) spleens (50%, 24/48), pancreases (41.7%, 10/24). All these results show that DERSV is closely related to the decline of egg production in ducks. Eight tissue samples were selected from 234 of DERSV positive tissues from different regions and different times for virus isolation and sequencing, and compared with the early DERSV isolates. Sequence comparison showed that the nucleotide homology and amino acid homology between these DERSV isolates (GenBank accession no. OM259401, OM259402, OM259403, OM259404, OM259405, OM259406, OM259407, OM259408) were 99.9–100% and 99.7–100%, respectively, indicating that although the variation of duck egg reduction syndrome virus isolate was small, it was distributed in different duck breeds in many provinces.
4.5. The Close Relationship between DERSV Antibody Level and Egg Decline Syndromes in the Clinic
To further confirm whether DERSV caused the clinical symptoms of duck egg-reducing syndromes, the average egg production rate and the serological surveillance in 5 h in a breeding duck farm during the outbreaks of egg-reducing syndromes were monitored from October to December 2020. In the early stage, the egg-reducing syndromes were found only in house #16, whereas the egg production rates kept on a high level in the other four duck houses. The antibody against DERSV was detectable in a part of serum samples from ducks raised in the house where egg drop happened, whereas no positive samples were found in the other four duck houses. Twenty-five days later, the serum samples were collected when the egg-reducing syndromes happened in houses #13, #14. The antibody against DERSV turned positive in some serum samples collected in those two duck houses. At the same time, all of the serum samples collected in the house occurred the egg drop disease firstly convert positive to DERSV antibody, whereas the serum samples collected in the remaining two houses with normal egg production showed negative to DERSV antibody. Sixteen days later, the serum samples were collected when the egg drop disease happened in another duck house (8#). The DERSV antibody showed a similar covert pattern in the new outbreak house as in those three houses broke out early. At that time, the egg production rates in those three early outbreak houses kept at a lower level and showed no signs of recovery (Figure 4). The emergence of the egg reducing syndrome and the development of DERSV antibodies were correlated in this farm, suggesting that DERSV was a causative agent of the duck egg-reducing syndrome.
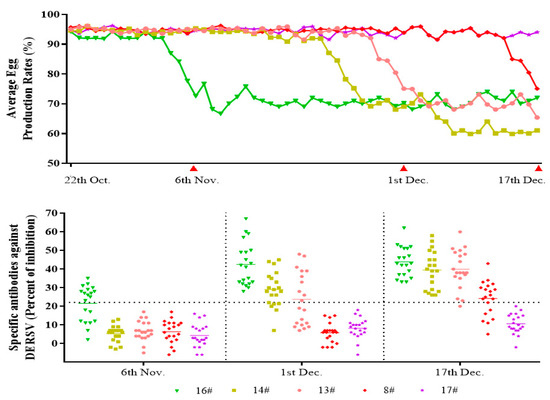
Figure 4.
The correlation survey between the outbreaks of egg-reducing syndromes and the appearance of the specific antibodies against DERSV. In the early stage, the egg-reducing syndromes happened in one duck house. Meanwhile, the antibodies against DERSV were detectable in some serum samples, and seroconversion was found later in all the serum samples. In the other three duck houses, the egg-reducing syndromes happened one by one, and the antibodies against DERSV changed in a similar dynamic pattern as in the first duck house. The DERSV antibodies were not detected in the house without the egg-reducing syndrome.
4.6. Pathogenicity of DERSV in Ducks
To evaluate the pathogenicity of the DERSV in ducks, we infected eight laying shelducks by inoculating them intramuscularly (i.m.) with 105.5 EID50 of DERSV in a volume of 200 μL. One day after inoculation, eight naive laying shelducks were introduced into the isolator where the i.m.-inoculated ducks were housed. The inoculated ducks or contact exposed ducks showed a certain percentage of tissue damage including significant follicular hemorrhage and rupture (3/8 inoculated ducks, 2/8 exposed ducks), liver hemorrhage (5/8 inoculated ducks, 4/8 exposed ducks), and kidney (7/8 inoculated ducks, 6/8 exposed ducks) (Figure 5A). After hematoxylin and eosin staining of impaired ovary, liver and kidney sections, the result demonstrated that necrosis of follicular epithelial cells(ovary), hyperemia of hepatic venules and sinuses(liver), hyperemia of the renal interstitium, and degeneration of renal tubular epithelial cells(kidney) (Figure 5A). In addition, viral antigens were detected in ovary, liver, and kidney sections with pathological changes.
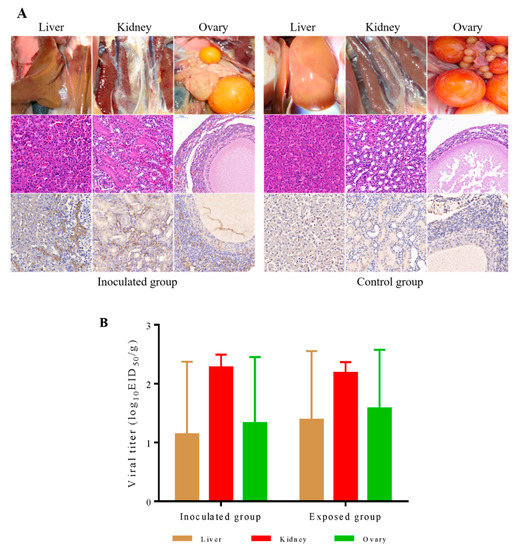
Figure 5.
Pathogenicity of DERSV ducks. (A) Infected ducks show liver and renal hemorrhage, follicular hemorrhage, and rupture. Congestion of hepatic venules and sinusoid, congestion of renal interstitium, degeneration of renal tubular epithelial cells, and Necrosis of follicular epithelial cells were detectable on the tissue sections accordingly. In addition, viral antigens could be observed in those tissues of infected ducks. (B) Virus titer in tissues of livers, kidneys, and ovaries of inoculated and exposed ducks was determined on 9-day-old embryonated duck eggs. The lower limit of virus detection was 0.5log10 EID50 per gram tissue.
To determine the distribution of the DERSV in ducks, we detected the virus in the ovary, liver, kidney tissues, and serum samples. In the inoculated ducks, DERSV replicated best in the kidneys with a titer of 102.28 ± 0.22 EID50/g, followed by the ovaries (a titer of 101.33 ± 1.12 EID50/g) and liver (a titer of 101.14 ± 1.23 EID50/g) (Figure 5B). In the contact exposed ducks, the replication level of DERSV in kidney (a titer of 102.18 ± 0.19 EID50/g), ovary (a titer of 101.58 ± 0.99 EID50/g) and liver (a titer of 101.39 ± 1.17 EID50/g) were similar to those in inoculated ducks (Figure 5B). Moreover, DERSV was first isolated (1/8) from blood on the 2 dpi, the infected group had a positive rate of 50% (4/8) on the 4 dpi and the highest rate of 75% (6/8) on the 6 dpi in the blood samples of inoculated ducks. In the contact exposed ducks, this virus started to be isolated on 4 dpc with a positive rate of 25% (2/8) and 62.5% (5/8) on 6 dpc (Table 3). All these results suggested that this virus was transmitted among ducks by contact. Finally, we identified the known virus by RT-PCR again. The known viruses could not be detected by PCR or RT-PCR again. These suggest that these clinical symptoms of ducks were caused by infection with DERSV and were not mixed with known viruses.

Table 3.
Virus isolation and detection from serum.
5. Discussion
A stunning increase in newly identified viruses in the past decade has shown that picornaviruses have an extremely wide range of hosts distributed globally [4,28]. Moreover, picornaviruses exhibit a surprising diversity of genome sequences and genome layouts, challenging the definition of taxonomic relevant criteria. In this study, we identified a novel virus species named as DERSV, which caused duck-egg-drop syndrome. The virus was most closely related to WDALV and AalV-A. Based on current guidelines defined by ICTV, the amino acid identities in P1, 2C, 3C, and 3D regions among members of a genus should ≥34%, ≥36%, ≥36%, and ≥36%, respectively. Based on the rules, DERSV could be classified into the genus Avihepatovirus or the genus Aalivirus. However, DERSV shared higher amino acid identities with WDALV, which belongs to the genus Avihepatovirus. As a result, we proposed that DERSV was a novel species in the genus Avihepatovirus. So far, both AalV-A and WDALV were failed to be isolated, although they were detected in ducks [8,29], and the pathogenicity of those viruses in ducks remains unknown for the lack of animal infection experiment.
The 2A protein is the least conserved non-structural protein in picornavirus, involved in the virus assembly and virulence [30,31,32,33]. AalV-A has six putative 2A peptides, which is the picornavirus with the largest number of 2A peptides reported so far [8,32]. Interestingly, DERSV possesses a total of up to seven putative 2A peptides. The first 6 2A peptides were separated by the “DxExNPGP” motif and might induce a co-translational “cleavage” event called ribosome “skipping,” to be separated at the “G/P” site. There are three possibilities for 2A-mediated ‘ribosome-skipping’ after the release of the nascent polypeptide: (1) jump successfully and continue to translate 2A downstream protein; (2) jump successfully, but ribosomes terminate translation; or occasionally, producing only 2A upstream protein; (3) jump failure, continue translation to produce fusion protein [34]. DxExNPGP motif induces the “cleavage” event of co-translation, enabling P1-2A to be released from P2 polyproteins [33]. There have been reported that the D mutation in the “DxExNPGP” motif of the foot-and-mouth disease virus lost this function, but some studies have exhibited that the key amino acids in this motif are NPGP and the “GxExNPGP” in DHAV-1 2A1 can still function successfully [33]. In DERSV 2A2, we found an additional GxExNPG788P motif, which might result in up to 8 2A peptides of DERSV. Interestingly, a unique peptide 2A3 of DERSV was found firstly in this study. 2A proteins or peptides can resist host immune response and promote virus replication [33]. Most of the avian picornaviruses contained multiple mature 2A peptides, suggesting that the mechanism by which picornaviruses infect avian hosts may be more complex than other picornaviruses [35]. The function of DERSV 2A3 in virus replication need to be explored further.
To date, five distinct types (I, II, III, IV, and V) of picornavirus IRES elements have been recognized. The picornavirus type IV IRES structures resemble HP IRESs in key respects, notably in comprising domains II and III, and containing a series of signature elements in domain III [7,19,36]. According to previous reports, it was found that picornavirus HP-like IRESs were divided into three groups A, B and C, and most of the picornavirus HP-like IRESs belonged to group B [6,7,19,22,37,38,39]. It is worth noting that the secondary structure model of the inferential domain III of DERSV S is highly conservative in Aalivirus, pigeon picornaviruses B, Quail picornavirus, Mesivirus, duck megrivirus [7,8,38,39].
The picornaviruses are important pathogens in humans and animals, causing diseases that affect the central nervous system, respiratory and gastrointestinal tract, heart, liver, pancreas, skin, and eyes [4]. Although many new picornaviruses were found in animals through high-throughput sequencing recently, little is known about the pathogenicity of most of those viruses in their natural hosts for lack of virus isolation methods [9,40]. So far, only two duck picornavirus species the DHAV and the ASV-1 were isolated from domestic duck, whereas three duck picornavirus DMV, AalV-A, and WDALV were detected but failed to isolate so far [8,29]. The virus reported in this study was a novel species belonging to the genus of the Avihepatovirus genus. However, the main clinical signs and symptoms of the disease caused by the virus were the duck egg-reducing and follicular hemorrhage and rupture. To distinguish with duck hepatitis virus, we suggest naming the novel virus as duck egg-reducing syndrome virus according to the main clinical signs caused by the virus in ducks.
Both DERSV and Duck egg drop syndrome virus (DEDSV) lead to a drop in egg production, but they are quite different: DERS is a disease caused by picornavirus infection. The main clinical symptoms are that the laying rate slowly decreases from a peak of 90% to 50%, and the laying rate will rise after a period of time, and infected ducks will not die. DEDS is a disease caused by DEDSV (flavivirus) infection, and its main clinical symptoms are anorexia, diarrhea, ataxia, paralysis and, most importantly, a significant decline in egg production, which rapidly dropped to less than 10% [41]. Some of the affected ducks showed neurological disorders characterized by progressive paralysis, with a mortality rate of between 5% and 30% [41].
At present, in the absence of specific DERSV treatment, vaccination will be the most feasible strategy to control DERSV. In view of the successful experience of picornavirus vaccines such as foot-and-mouth disease virus vaccine [42], poliovirus vaccine [43], and hepatitis virus vaccine [44], DERSV vaccine may become the main means to control the disease in future.
Taken together, our results indicated that a novel virus named was DERSV was isolated and identified from egg-reducing syndrome ducks. This virus belongs to the avian hepatitis virus family of picornavirus and has a typical picornavirus-like genomic structure. In the field, the antibody level of DERSV was closely related to the egg-reducing syndrome.
Author Contributions
Conceptualization, X.S. and Z.L.; data curation, X.S., D.S. and Y.L.; formal analysis, X.P. and D.Y.; funding acquisition, X.S., D.S. and Z.L.; investigation, X.L., W.L. and D.H.; methodology, X.S. and D.S.; software, J.Y.; supervision, Z.L.; validation, X.S., Q.L. and C.Y.; writing—original draft, X.S.; writing—review and editing, Q.T. and Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shanghai Agriculture Applied Technology Development Program, China (2021-02-08-00-12-F00746) and the Agricultural Science and Technology Innovation Project, CAAS, China.
Institutional Review Board Statement
All animal experiments were carried out according to the recommendations in the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of the People’s Republic of China. The protocol (SHVRI-SZ-20191028-01) in the study was approved by the Animal Care Committee of the Shanghai Veterinary Research Institute.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
The list of some representative viruses in the picornavirus family.
Table A1.
The list of some representative viruses in the picornavirus family.
| Picornavirus Genus | Viruses | GenBank Accession No. | Genome Size (nt) |
|---|---|---|---|
| Aalivirus | Aalivirus A GL/12 | KJ000691 | 8959 |
| Ailurivirus | Aimelvirus | MF327529 | 8029 |
| Ampivirus | Ampivirus | KP770140 | 9246 |
| Anativirus | Anativirus | AY563023 | 8289 |
| Aphthovirus | Foot-and-mouth disease virus | AF308157 | 8134 |
| Bovine rhinitis A virus | JN936206 | 7250 | |
| Equine rhinitis A virus | NC_003982 | 7734 | |
| Aquamavirus | Seal picornavirus | NC_009891 | 6693 |
| Avihepatovirus | Wild duck avihepatovirus-like virus | MH453803 | 8742 |
| Duck hepatitis A virus 1 | DQ864514 | 7689 | |
| Duck hepatitis A virus 2 | EF067924 | 7769 | |
| Duck hepatitis A virus 3 | EU352805 | 7791 | |
| Avisivirus | Turkey avisivirus A M176 | KC465954 | 7532 |
| Avisivirus B1 | KF979333 | 7310 | |
| Boosepivirus | Bovine picornavirus | LC006971 | 7570 |
| Bopivirus | Bopivirus | KM589358 | 7018 |
| Caecilivirus | Caecilians picornavirus | MG600103 | 8318 |
| Cardiovirus | Encephalomyocarditis virus | MH191297 | 7734 |
| Cosavirus | Cosavirus | FJ438903 | 7335 |
| Crahelivirus | Crahelivirus | KY312540 | 7443 |
| Crohivirus | Crohivirus | AB937989 | 7321 |
| Danipivirus | Zebrafish picornavirus | MH368041 | 8298 |
| Diresapivirus | Diresapivirus | KJ641685 | 6624 |
| Dicipivirus | Cadicivirus A | JN819202 | 8755 |
| Bovine enterovirus | D00214 | 7414 | |
| Enterovirus | Human enterovirus | AY697458 | 7438 |
| Human rhinovirus | JX074057 | 6979 | |
| Erbovirus | Equine Rhinovirus | X96871 | 8828 |
| Felipivirus | Feline picornavirus | JN572115 | 7391 |
| Gallivirus | Gallivirus | JQ691613 | 8496 |
| Gruhelivirus | Gruhelivirus | KY312541 | 7073 |
| Grusopivirus | Grusopivirus A1 | KY312544 | 7917 |
| Grusopivirus B1 | KY312545 | 7104 | |
| Harkavirus | Falcovirus | KP230449 | 8003 |
| Hemipivirus | Hemipivirus | MG600089 | 7939 |
| Hepatovirus | Hepatitis A virus | NC_001489 | 7478 |
| Hunnivirus | Bovine hungarovirus | JQ941880 | 7583 |
| Kobuvirus | Ferret kobuvirus | KF006985 | 8052 |
| Kunsagivirus | Kunsagivirus A | KC935379 | 7272 |
| Limnipivirus | Bluegill picornavirus 1 | NC_018506 | 8050 |
| Livupivirus | Livupivirus | KX463670 | 7768 |
| Ludopivirus | Ludopivirus | MF358731 | 8051 |
| Marsupivirus | Burpengary virus | MK882499 | 6821 |
| Malagasivirus | Lesavirus | KM396707 | 7687 |
| Megrivirus | Chicken megrivirus | KF961186 | 9560 |
| Mosavirus | Mosavirus | JF973687 | 6964 |
| Mischivirus | Miniopterus schreibersii picornavirus | JQ814851 | 8468 |
| Mupivirus | Mupivirus | KY432924 | 8422 |
| Orivirus | Orivirus. | KM203656 | 7037 |
| Myrropivirus | Myrropivirus | MG600081 | 7994 |
| Oscivirus | Turdivirus | GU182408 | 7641 |
| Parechovirus | Human parechovirus | L02971 | 7339 |
| Parabovirus | Parabovirus | KY432927 | 8315 |
| Pasivirus | Swine pasivirus 1 | NC_018226 | 6916 |
| Passerivirus | Turdivirus 1 | NC_014411 | 8035 |
| Pemapivirus | Pemapivirus | MG600106 | 9196 |
| Potamipivirus | Eel picornavirus 1 | KC843627 | 7496 |
| Poecivirus | Poecivirus | KU977108 | 7653 |
| Pygoscepivirus | Pingu picornavirus | MH255796 | 7601 |
| Rabovirus | Rabovirus | KP233897 | 7853 |
| Rafivirus | Tortoise Rafivirus | KJ415177 | 7204 |
| Rajidapivirus | Rajidapivirus | MG600093 | 7947 |
| Rosavirus | Rosavirus | JF973686 | 8749 |
| Rohelivirus | Rohelivirus | KX156153 | 7780 |
| Sapelovirus | Porcine sapelovirus | NC_003987 | 7491 |
| Sakobuvirus | Feline sakobuvirus A | KF387721 | 7807 |
| Salivirus | Salivirus | GQ179640 | 7982 |
| Shanbavirus | Shanbavirus A1 | KJ641698 | 7264 |
| Senecavirus | Senecavirus | KR063107 | 7270 |
| Sicinivirus | Sicinivirus | KF741227 | 9276 |
| Symapivirus | Symapivirus | MG600076 | 8591 |
| Teschovirus | Porcine teschovirus | LC386154 | 7138 |
| Tottorivirus | Swine picornavirus 1 | LC113907 | 7062 |
| Torchivirus | Tortoise picornavirus | KM873611 | 7093 |
| Tremovirus | Avian encephalomyelitis virus | NC_003990 | 7032 |
| Tropivirus | Chinese water skink picornavirus | MG600091 | 8049 |
References
- Pankovics, P.; Boros, Á.; Bíró, H.; Horváth, K.B.; Phan, T.G.; Delwart, E.; Reuter, G. Novel picornavirus in domestic rabbits (Oryctolagus cuniculus var. domestica). Infect. Genet. Evol. 2016, 37, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Ao, Y.; Zhou, Y.; Li, D.; Duan, Z. A novel picornavirus identified in wild Macaca mulatta in China. Arch. Virol. 2020, 165, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Lau, S.K.; Choi, G.K.; Huang, Y.; Teng, J.L.; Tsoi, H.W.; Tse, H.; Yeung, M.L.; Chan, K.H.; Jin, D.Y.; et al. Natural occurrence and characterization of two internal ribosome entry site elements in a novel virus, canine picodicistrovirus, in the picornavirus-like superfamily. J. Virol. 2012, 86, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Zell, R. Picornaviridae-the ever-growing virus family. Arch. Virol. 2018, 163, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef]
- Boros, Á.; Nemes, C.; Pankovics, P.; Kapusinszky, B.; Delwart, E.; Reuter, G. Genetic characterization of a novel picornavirus in turkeys (Meleagris gallopavo) distinct from turkey galliviruses and megriviruses and distantly related to the members of the genus Avihepatovirus. J. Gen. Virol. 2013, 94, 1496–1509. [Google Scholar] [CrossRef][Green Version]
- Liao, Q.; Zheng, L.; Yuan, Y.; Shi, J.; Zhang, D. Genomic characterization of a novel picornavirus in Pekin ducks. Vet. Microbiol. 2014, 172, 78–91. [Google Scholar] [CrossRef]
- Wang, X.; Liu, N.; Wang, F.; Ning, K.; Li, Y.; Zhang, D. Genetic characterization of a novel duck-origin picornavirus with six 2A proteins. J. Gen. Virol. 2014, 95, 1289–1296. [Google Scholar] [CrossRef]
- Wille, M.; Eden, J.S.; Shi, M.; Klaassen, M.; Hurt, A.C.; Holmes, E.C. Virus-virus interactions and host ecology are associated with RNA virome structure in wild birds. Mol. Ecol. 2018, 27, 5263–5278. [Google Scholar] [CrossRef]
- Victoria, J.G.; Kapoor, A.; Dupuis, K.; Schnurr, D.P.; Delwart, E.L. Rapid identification of known and new RNA viruses from animal tissues. PLoS Pathog. 2008, 4, e1000163. [Google Scholar] [CrossRef]
- Stang, A.; Korn, K.; Wildner, O.; Uberla, K. Characterization of virus isolates by particle-associated nucleic acid PCR. J. Clin. Microbiol. 2005, 43, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Lu, S.; Anderson, J.B.; Chitsaz, F.; Derbyshire, M.K.; DeWeese-Scott, C.; Fong, J.H.; Geer, L.Y.; Geer, R.C.; Gonzales, N.R.; et al. CDD: A Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011, 39, D225–D229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Z.; Wang, S.; Liu, J.; Paul, N.; He, T.; Liu, C.; Zhang, H.; Lv, Y.; Cao, R.; et al. Hepatitis C virus genotype/subtype distribution and evolution among Chinese blood donors: Revealing recent viral expansion. PLoS ONE 2020, 15, e0235612. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.; Hansen, J.; Blaas, D.; Brunak, S. Cleavage site analysis in picornaviral polyproteins: Discovering cellular targets by neural networks. Protein Sci. 1996, 5, 2203–2216. [Google Scholar] [CrossRef]
- Ozawa, T.; Kondo, M.; Isobe, M. 3’ rapid amplification of cDNA ends (RACE) walking for rapid structural analysis of large transcripts. J. Hum. Genet. 2004, 49, 102–105. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Rapid Amplification of 5’ cDNA Ends (5’-RACE). CSH Protoc. 2006, 2006, 633–640. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, D. Molecular analysis of duck hepatitis virus type 1. Virology 2007, 361, 9–17. [Google Scholar] [CrossRef]
- Kim, M.C.; Kwon, Y.K.; Joh, S.J.; Lindberg, A.M.; Kwon, J.H.; Kim, J.H.; Kim, S.J. Molecular analysis of duck hepatitis virus type 1 reveals a novel lineage close to the genus Parechovirus in the family Picornaviridae. J. Gen. Virol. 2006, 87, 3307–3316. [Google Scholar] [CrossRef]
- Hughes, P.J.; Stanway, G. The 2A proteins of three diverse picornaviruses are related to each other and to the H-rev107 family of proteins involved in the control of cell proliferation. J. Gen. Virol. 2000, 81, 201–207. [Google Scholar] [CrossRef]
- Hellen, C.U.; de Breyne, S. A distinct group of hepacivirus/pestivirus-like internal ribosomal entry sites in members of diverse picornavirus genera: Evidence for modular exchange of functional noncoding RNA elements by recombination. J. Virol. 2007, 81, 5850–5863. [Google Scholar] [CrossRef]
- Arhab, Y.; Bulakhov, A.G.; Pestova, T.V.; Hellen, C.U.T. Dissemination of Internal Ribosomal Entry Sites (IRES) Between Viruses by Horizontal Gene Transfer. Viruses 2020, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Liu, L. Fields Virology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Kapoor, A.; Victoria, J.; Simmonds, P.; Wang, C.; Shafer, R.W.; Nims, R.; Nielsen, O.; Delwart, E. A highly divergent picornavirus in a marine mammal. J. Virol. 2008, 82, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.; Woo, P.C.; Yip, C.C.; Choi, G.K.; Wu, Y.; Bai, R.; Fan, R.Y.; Lai, K.K.; Chan, K.H.; Yuen, K.Y. Identification of a novel feline picornavirus from the domestic cat. J. Virol. 2012, 86, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.; Woo, P.C.; Lai, K.K.; Huang, Y.; Yip, C.C.; Shek, C.T.; Lee, P.; Lam, C.S.; Chan, K.H.; Yuen, K.Y. Complete genome analysis of three novel picornaviruses from diverse bat species. J. Virol. 2011, 85, 8819–8828. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Choi, G.K.; Yip, C.C.; Huang, Y.; Tsoi, H.W.; Yuen, K.Y. Complete genome sequence of a novel picornavirus, canine picornavirus, discovered in dogs. J. Virol. 2012, 86, 3402–3403. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Koonin, E.V. Viral proteins containing the purine NTP-binding sequence pattern. Nucleic Acids Res. 1989, 17, 8413–8440. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Koonin, E.V. Superfamily of UvrA-related NTP-binding proteins. Implications for rational classification of recombination/repair systems. J. Mol. Biol. 1990, 213, 583–591. [Google Scholar] [CrossRef]
- Rueckert, R.R.; Wimmer, E. Systematic nomenclature of picornavirus proteins. J. Virol. 1984, 50, 957–959. [Google Scholar] [CrossRef]
- Tseng, C.H.; Tsai, H.J. Molecular characterization of a new serotype of duck hepatitis virus. Virus Res. 2007, 126, 19–31. [Google Scholar] [CrossRef]
- Emerson, S.U.; Huang, Y.K.; Nguyen, H.; Brockington, A.; Govindarajan, S.; St Claire, M.; Shapiro, M.; Purcell, R.H. Identification of VP1/2A and 2C as virulence genes of hepatitis A virus and demonstration of genetic instability of 2C. J. Virol. 2002, 76, 8551–8559. [Google Scholar] [CrossRef]
- Gullberg, M.; Polacek, C.; Bøtner, A.; Belsham, G.J. Processing of the VP1/2A junction is not necessary for production of foot-and-mouth disease virus empty capsids and infectious viruses: Characterization of “self-tagged” particles. J. Virol. 2013, 87, 11591–11603. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, X.; Zeng, Q.; Wang, M.; Cheng, A.; Pan, K.; Zhu, D.; Liu, M.; Jia, R.; Yang, Q.; Wu, Y.; et al. DHAV-1 2A1 Peptide—A Newly Discovered Co-expression Tool That Mediates the Ribosomal “Skipping” Function. Front. Microbiol. 2018, 9, 2727. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, A.; Wang, M.; Jia, R.; Sun, K.; Pan, K.; Yang, Q.; Wu, Y.; Zhu, D.; Chen, S.; et al. Structures and Corresponding Functions of Five Types of Picornaviral 2A Proteins. Front. Microbiol. 2017, 8, 1373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, O.; Wall, J.B.J.; Zheng, M.; Zhou, Y.; Wang, L.; Vaseghi, H.R.; Qian, L.; Liu, J. Systematic comparison of 2A peptides for cloning multi-genes in a polycistronic vector. Sci. Rep. 2017, 7, 2193. [Google Scholar] [CrossRef]
- Boros, Á.; Pankovics, P.; Reuter, G. Avian picornaviruses: Molecular evolution, genome diversity and unusual genome features of a rapidly expanding group of viruses in birds. Infect. Genet. Evol. 2014, 28, 151–166. [Google Scholar] [CrossRef]
- Sweeney, T.R.; Dhote, V.; Yu, Y.; Hellen, C.U. A distinct class of internal ribosomal entry site in members of the Kobuvirus and proposed Salivirus and Paraturdivirus genera of the Picornaviridae. J. Virol. 2012, 86, 1468–1486. [Google Scholar] [CrossRef]
- Boros, Á.; Pankovics, P.; Knowles, N.J.; Nemes, C.; Delwart, E.; Reuter, G. Comparative complete genome analysis of chicken and Turkey megriviruses (family picornaviridae): Long 3’ untranslated regions with a potential second open reading frame and evidence for possible recombination. J. Virol. 2014, 88, 6434–6443. [Google Scholar] [CrossRef]
- Kofstad, T.; Jonassen, C.M. Screening of feral and wood pigeons for viruses harbouring a conserved mobile viral element: Characterization of novel Astroviruses and Picornaviruses. PLoS ONE 2011, 6, e25964. [Google Scholar] [CrossRef]
- Pankovics, P.; Boros, A.; Reuter, G. Novel picornavirus in domesticated common quail (Coturnix coturnix) in Hungary. Arch. Virol. 2012, 157, 525–530. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.D.; Chen, X.; Tian, J.H.; Chen, L.J.; Li, K.; Wang, W.; Eden, J.S.; Shen, J.J.; Liu, L.; et al. The evolutionary history of vertebrate RNA viruses. Nature 2018, 556, 197–202. [Google Scholar] [CrossRef]
- Liu, P.; Lu, H.; Li, S.; Wu, Y.; Gao, G.F.; Su, J. Duck egg drop syndrome virus: An emerging Tembusu-related flavivirus in China. Sci. China Life Sci. 2013, 56, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Diaz-San Segundo, F.; Medina, G.N.; Stenfeldt, C.; Arzt, J.; de Los Santos, T. Foot-and-mouth disease vaccines. Vet. Microbiol. 2017, 206, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Macleod, D.R. Current Status of Measles and Oral Poliovirus Vaccines. Can. Med. Assoc. J. 1964, 91, 1118–1122. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).